Abstract
HER2/neu is an efficient target for cancer therapy. However, reports about its overexpression rate in colorectal carcinomas showed wide variability. This study aims to investigate HER2/neu expression in colorectal carcinomas using these two rabbit monoclonal HER2/neu antibodies, and to clarify the relationship between protein overexpression and gene amplification of HER2/neu and their clinicopathologic importance. Tissue microarray was performed from sections of 106 cases colorectal carcinomas. Their clinical data, including gender, age, stage, recurrence, lymph node metastasis, and follow-ups were collected. Immunohistochemistry for rabbit monoclonal antibody SP3 and 4B5 were performed, Fluorescent in situ hybridization was applied to detect the amplification of HER2/neu gene. The HER2/neu overexpression of (2+ and 3+) in our results were seen in 7.5% (8/106) for 4B5 and 3.8% (4/106) for SP3 respectively, the HER2/neu amplification was in 2.8% (3/106). All cases of overexpression for SP3 were included by those for 4B5. Both antibodies stained 3 cases of HER2/neu 3+, and FISH confirmed HER2/neu amplification did occurred in these cases. In our study, 4B5 was more sensitive to detect HER2/neu of colorectal carcinoma than SP3. 2.8% patients with colorectal patients might benefit from anti-HER2/neu therapy.
Keywords: HER2/neu, rabbit monoclonal antibody, colorectal carcinomas
Introduction
Colorectal carcinoma is a leading cause of cancer-related deaths worldwide. Although chemotherapy has shown to be an efficient management, ongoing improvement is needed, especially for advanced stage. Targeted cancer therapy provide a promising way to tailor cancer treatment with more selective for cancer cells than normal cells. Monoclonal antibodies target against vascular endothelial growth factor receptor (such as bevacizumab) [1] and epidermal growth factor receptor (such as cetuximab) [2] have been introduced for colorectal carcinoma therapy.
The human epidermal growth factor receptor 2/neu ( HER2/neu) gene is located on chromosomal region 17q12. It encodes a transmembrane glycoprotein which belongs to the EGF/erbB growth factor receptor family [3]. HER2/neu protein has been shown to be overexpressed in breast cancer and gastric cancer and an effective target for adjuvant therapy. Its monoclonal antibody, Trastuzumab, has been used as routine drug to treat HER2/neu positive breast and gastric cancer. There have been several studies evaluating HER2/neu expression in colorectal carcinomas by immunohistochemical staining. The results of them were conflict with expression rate range from zero to 84% [4-11], as well as the relationship between prognosis and HER2/neu overexpression.
Recently developed rabbit monoclonal HER2/neu antibodies have higher affinity and specificity [12,13]. The 4B5 antibody is directed against the extracellular domain of the HER2-receptor, and the SP3 antibody is directed against intracellular domain [14]. This study aims to investigate HER2/neu expression in colorectal carcinomas using these two rabbit monoclonal HER2/neu antibodies, and to clarify the relationship between protein overexpression and gene amplification of HER2/neu and their clinicopathologic importance.
Materials and methods
Patients and tissue samples
We examined 106 cases colorectal carcinomas obtained from 2003 to 2007 from the surgical pathological database of the First Affiliated Hospital of Wenzhou Medical University. The patients were composed of 39 men and 52 women with a median age of 60.09 (34-81 years). In all cases colectomy was performed and their clinical data, including gender, age, stage, recurrence, lymph node metastasis, and follow-ups were collected (Table 1, Figure 3). None of the patient received irradiation or chemotherapy prior to surgery. Tumor grades were defined according to the criteria of 2010 WHO. The pathological TNM status was assessed according to the criteria of the sixth edition of the TNM classification of the International Union Against Cancer [15]. Patients who died of other than colorectal cancer were excluded from the study. The study was approved by the Ethical Committee of Wenzhou Medical University.
Table 1.
Clinical and pathological features of colorectal carcinomas
| Clinical/pathological features | n | |
|---|---|---|
| Gender | Female | 61 |
| Male | 45 | |
| Age | <40 | 4 |
| 40-70 | 75 | |
| >70 | 27 | |
| Tumor grade | G1 | 22 |
| G2 | 64 | |
| G3 | 20 | |
| Tumor stage | pT1 | 26 |
| pT2 | 35 | |
| pT3 | 61 | |
| Nodal status | pN0 | 51 |
| pN1 | 31 | |
| pN2 | 44 | |
| Tumor type | Tubular carcinoma | 99 |
| Mucinous carcinoma | 7 | |
| Total number | 106 | |
Figure 3.
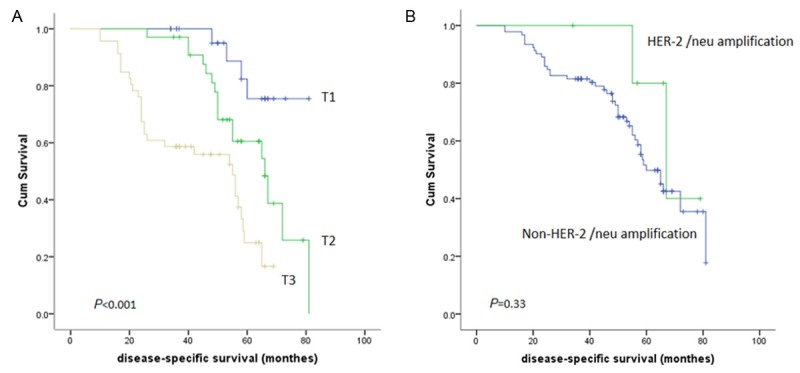
Kaplan-Meier plot for: (A) Disease-specific survival and pT-stage in 103 colorectal carcinoma; (B) Disease-specific survival and HER-2/neu amplification in colorectal carcinoma.
All surgical specimens were fixed in neutral-buffered formalin (10%) in 20 min after surgical removal of the tissue. After overnight fixation, tissues were sampled for processing to make paraffin embedded blocks.
Tissue microarray (TMA)
TMA was constructed from formalin-fixed and paraffin embedded blocks. One tissue section was chosen for each case on which three random representative locations of cancer foci and one location of normal mucosa were marked. Having matched the marked foci with the tissue paraffin block, 4 cores of tissue per case were embedded into the recipient paraffin blocks using a tissue arrayer (Boyikang Company, Beijing). Sections were then cut for immunostaining.
Immunohistochemistry (IHC) fluorescent in situ hybridization (FISH)
The rabbit Monoclonal antibody SP3 for HER2/neu (Labvision, Fremnot, CA, USA) was stained according to manufacturer recommendation. In brief, the 4 µm-thick sections were performed (10 mmol/L citrate buffer, pH 6.0) in presser cooker for heat-induced antigen retrieval after dewaxed and rehydrated through xylene and ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 minutes. Primary antibody (dilution 1:100) was incubated for 40 minutes at room temperature, and was detected by EnVision Plus System (DAKO) for 30 minutes at room temperature. The reaction product was detected with 3, 3-diaminobenzidine chromogen. For negative controls, the tissue sections were incubated with phosphate-buffered saline in the absence of primary antibody. Counter staining was carried out with hematoxylin.
Another rabbit Monoclonal antibody 4B5 for HER2/neu was immunostained on the Benchmark XT automated stainer (Ventana medical systems, Tucson, AZ, United States). Antigen retrieval was a standard automated process at 37°C for 16 minutes on the stainer. The antibody was used as received from Ventana prediluted at a stated final concentration of 6 ìg/mL of specific antibody.
On all slides for the two antibodies, three breast cancer samples with an immunoscore of 0, 1+ and 3+ according to ASCO/CAP guideline [16] were used as control.
The IHC positive cases and 10 negative cases were chosen to preform FISH. FISH was performed using the Pathvysion HER-2 DNA probe kit (Abbott Laboratiories, Abbott Park, IL, USA). The procedure FISH was performed using procedures provided by manufacture. In brief, 4mm sections were dewaxed and rehydrated through xylene and ethanol, and heated in pretreatment reagent at 80°C for 10 min. After treatment in 0.2% pepsin/0.01 N HCl for 30 min at 37°C, the slides were incubated in a ThermoBrite hybridizer (Abbott Molecular, Wiesbaden, Germany) at 75°C for 5 minutes and 37°C for 18 hours. After hybridization, slides were rinsed in 2 × SSC/0.3 NP-40 at 72°C for 2 minutes, and counterstained with 4,6-diamidino-2-phenylindole (DAPI).
Evaluation of IHC and FISH staining results
Both the immunostaining of membrane and cytoplasm were scored. The membrane staining was evaluated on a score of 0 to 3 using the score system suggested by Hoffman et al [17]. Scoring of membrane was as follows: 0, no reactivity or FISH analysis shows homogenous (A) and heterogenous (B) amplification of HER-2 (clusters of red signals) in CRC. Green signals represent centromere 17. in less than 10% of cells; 1+, faint/barely perceptible membranous reactivity in 10% of cells or higher or reactivity in only part of the cell membrane; 2+, weak to moderate complete or basolateral membranous reactivity in 10% of tumor cells or higher; 3+, strong complete or basolateral membranous reactivity in 10% of tumor cells or higher. Scoring of cytoplasm was graded as negative, weak (score as 1) and strong (score as 2).
FISH was scored according to ASCO/CAP guideline [16]. FISH results are reported as the average HER2/neu count to average CEP (chromosome enumeration probe) 17. The average number of HER2 signals per nucleus and the average number of CEP17 chromosome probes per nucleus were counted in 20 non-overlapping cells. A HER2/CEP17 ratio of more than 2.2 was reported as amplified and a ratio of less than 1.8 as not amplified. An additional 20 cells were counted if the ratio was in the equivocal range (1.8-2.2).
Statistical analysis
HER-2 protein were correlated with clinicopathological parameters and 5-year cancer-related survival using Pearson’s χ2 testing. Patients were followed up for a median of 48.5 months (Range=10-81 months). The Kaplan-Meier method was usedto obtain the survival curve. For all tests, P<0.05 was considered to be statistically significant.
Results
IHC of HER2/neu expression
Immunostaining results and clinicopathologic data of related cases are listed in Table 1. In cases with immunoscores of 1+ and 2+ (both of immunoreactivity of SP3 and 4B5), most cancer cells showed week lateral or basolateral (U-shaped) membranous staining. All 3 cases with immunoscores of 3+ showed intensive complete membranous staining. The staining intensity of 4B5 was stronger than that of SP3 (Figure 1). In 5 cases scored 2+ for 4B5 staining, only 1 scored 1+ and 1 scored 2+ for SP3 staining. There was no notable background staining noted for both antibodies.
Figure 1.
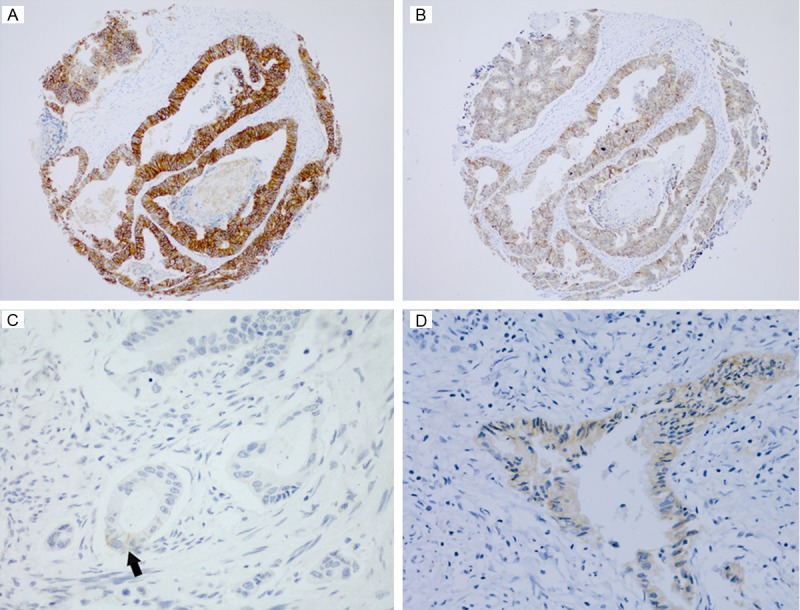
Immunohistochemical staining for HER-2 in colorectal carcinomas. HER-2/neu staining scored 3+ of 4B5 (A) was stronger than that of SP3 (B) in the same case (× 100); (C) An example of HER-2/neu staining scored as 1+ (× 200), membranous reactivity occurred in part of the cell membrane ( arrowed); (D) Cytoplasmic HER-2/neu positive in a colorectal carcinoma (× 200).
In positive cases for 4B5 antibody, 89% (16/18) showed cytoplasmic staining coexist with membranous staining, and all of the positive cells showed weak immunoreactivity (Figure 1). Of 7 SP3 cytoplasmic positive cases, 2 cases showed strong staining.
There were 3 signet ring cell carcinomas and 4 mucinous adenocarcinomas in our series of cases. None of them overexpressed 4B5 and SP3 in either membrane or cytoplasm. Three cases of surrounding normal glands showed 4B5 weak membranous staining (1+).
HER2/neu FISH versus immunoscore
FISH was undertaken for all TMA slides, and all HER2/neu 2+ and 3+ staining sections were analyzed. All 3 cases of HER2/neu 3+ also exhibited HER2/neu amplification (Figure 2). For the cases scored 2+, only 1 case with 4B5 immunoreactivity was amplified. None of the 2 cases of strong cytoplasmic staining showed amplification.
Figure 2.
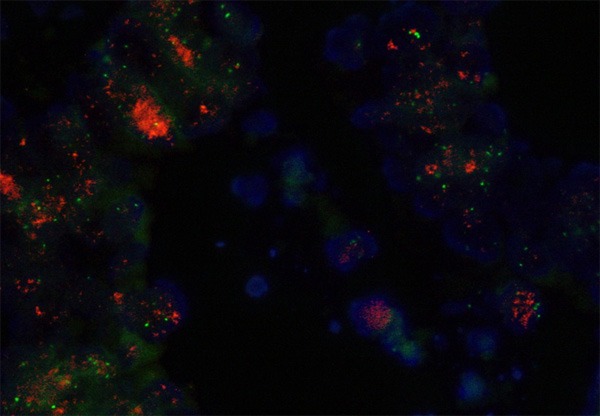
FISH analysis shows amplification of HER-2 /neu (clusters of red signals) in colorectal carcinoma. Green signals represent centromere 17.
Clinicopathological correlation
We defined HER2/neu 2+ and 3+ as overexpression, and there was no correlation between HER2/neu membranous and cytoplasmic overexpression and gender, age, tumor size, TNM stage (p>0.05). There was no correlation between HER2/neu (for 4B5 staining) overexpression and overall survival (p=0.33, Figure 3).
Discussion
After HER2/neu has been shown to be an effective target for adjuvant therapy for breast cancer and gastric cancer, many studies have been evaluated HER2/neu state in other tumor types. Of them, the studies of detection of HER2/neu in colorectal cancer by IHC reported expression varying in a great degree. It is well known that many factors can affect the positive rates of IHC. Alongside of different tissue fixation, paraffin blocks and section storage, antigen retrieval, one of major factors is the variety in antibodies.
Rabbit monoclonal antibodies were developed from c-myc/v-abl transgenic rabbits [12]. This model can produce rabbit plasmacytoma cell lines and allow the establishment of rabbit-rabbit hybridomas which is necessary to develop monoclonal antibodies [13,18]. Compared to mouse monoclonal antibodies, Rabbit monoclonal antibodies have higher sensitivity and more consistency [13]. Up till now, most of immunohistochemical HER2/neu studies about colorectal carcinomas were based on mouse polyclonal or monoclonal antibodies [19]. We tested two commercially available HER2/neu rabbit monoclonal antibodies to explore their reaction against colorectal carcinomas.
The HER2/neu overexpression of (2+ and 3+) in our results were seen in 7.5% (8/106) for 4B5 and 3.8% (4/106) for SP3 respectively, the HER2/neu amplification was in 2.8% (3/106). All cases of overexpression for SP3 were included by those for 4B5. Both antibodies stained 3 cases of HER2/neu 3+, and FISH confirmed HER2/neu amplification did occurred in these cases. FISH also showed an amplification of case of HER2/neu 2+ for 4B5 (case 4 in Table 2) which was HER2/neu 1+ for SP3. For the same section, the staining intensity of 4B5 was stronger than that of SP3. These results suggested that, in our study, 4B5 was more sensitive to detect HER2/neu of colorectal carcinoma than SP3.
Table 2.
HER2/neu positive cases and related clinical information
| 4B5 | SP3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Case No. | Gender | Age | Grade | pT | pN | Stage | membrane | cytoplasm | membrane | cytoplasm |
| 1 | M | 60 | 2 | 2 | 1 | IIA | 3 | 1 | 3 | 1 |
| 2 | F | 59 | 1 | 2 | 1 | IIA | 3 | 1 | 3 | 2 |
| 3 | F | 55 | 2 | 2 | 1 | IIIA | 3 | 1 | 3 | 1 |
| 4 | F | 60 | 1 | 2 | 1 | IIIB | 2 | 1 | 1 | 0 |
| 5 | F | 80 | 2 | 2 | 1 | IIA | 2 | 1 | 0 | 0 |
| 6 | F | 57 | 1 | 1 | 1 | I | 2 | 1 | 0 | 0 |
| 7 | M | 76 | 2 | 2 | 1 | IIA | 2 | 1 | 0 | 0 |
| 8 | F | 56 | 2 | 1 | 1 | I | 2 | 1 | 2 | 0 |
| 9 | M | 74 | 1 | 1 | 1 | I | 1 | 1 | 0 | 0 |
| 10 | M | 74 | 2 | 2 | 2 | IIIB | 1 | 0 | 0 | 0 |
| 11 | F | 51 | 2 | 2 | 1 | IIA | 1 | 1 | 0 | 0 |
| 12 | M | 49 | 2 | 1 | 2 | IIIA | 1 | 1 | 0 | 2 |
| 13 | F | 59 | 3 | 2 | 1 | IIIB | 1 | 1 | 0 | 0 |
| 14 | F | 58 | 1 | 2 | 2 | IIIB | 1 | 0 | 0 | 0 |
| 15 | F | 56 | 1 | 1 | 2 | IIIA | 1 | 1 | 0 | 0 |
| 16 | F | 57 | 2 | 2 | 2 | IIIC | 1 | 1 | 0 | 0 |
| 17 | F | 36 | 1 | 2 | 2 | IIIB | 1 | 1 | 0 | 0 |
| 18 | F | 34 | 1 | 2 | 1 | IIA | 1 | 1 | 0 | 0 |
| 19 | F | 59 | 1 | 3 | 1 | IIIB | 0 | 0 | 1 | 1 |
| 20 | M | 67 | 1 | 3 | 2 | IIIC | 0 | 0 | 1 | 1 |
| 21 | M | 55 | 1 | 3 | 0 | IIA | 0 | 0 | 0 | 1 |
Nunes et al. [20] compared overexpression of SP3 and 2 rabbit polyclonal antibodies (HercepTest, A0485), 3 mouse monoclonal antibodies (NCL-CB11, CM-CB11 and 4D5) in breast carcinomas, found SP3 was more sensitive, but less specific than others. Our study showed that, with more sensitivity and strong staining intensity, 4B5 could be used to detect HER2/neu overexpression with less false negative, but with more cost for subsequent FISH test.
There were no evidence supporting overexpression of HER2/neu related to tumor grading, pT, pN or survival. Some studies showed Cytoplasmic HER2/neu staining was associated with survival, but we did not observe that in our results.
Although studies for anti-HER2/neu therapy in colorectal carcinoma are rare, based on the successful treatment of gastric cancer, we view a small group patients (2.8% in our data) with colorectal carcinoma will have a favorable response to anti-HER2/neu therapy.
Acknowledgements
This work was supported by Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2011).
Disclosure of conflict of interest
None.
References
- 1.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez C, Schiff R. HER2: biology, detection, and clinical implications. Arch Pathol Lab Med. 2011;135:55–62. doi: 10.1043/2010-0454-RAR.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Half E, Broaddus R, Danenberg KD, Danenberg PV, Ayers GD, Sinicrope FA. HER-2 receptor expression, localization, and activation in colorectal cancer cell lines and human tumors. Int J Cancer. 2004;108:540–548. doi: 10.1002/ijc.11599. [DOI] [PubMed] [Google Scholar]
- 5.Kavanagh DO, Chambers G, O’Grady L, Barry KM, Waldron RP, Bennani F, Eustace PW, Tobbia I. Is overexpression of HER-2 a predictor of prognosis in colorectal cancer? BMC Cancer. 2009;9:1. doi: 10.1186/1471-2407-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JY, Lim SJ, Park K. Cyclooxygenase-2 and c-erbB-2 expression in colorectal carcinoma assessed using tissue microarrays. Appl Immunohistochem Mol Morphol. 2004;12:67–70. doi: 10.1097/00129039-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Kountourakis P, Pavlakis K, Psyrri A, Rontogianni D, Xiros N, Patsouris E, Pectasides D, Economopoulos T. Clinicopathologic significance of EGFR and Her-2/neu in colorectal adenocarcinomas. Cancer J. 2006;12:229–236. doi: 10.1097/00130404-200605000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Wang D, Li J, Chen P. Clinicopathological and prognostic significance of HER-2/neu and VEGF expression in colon carcinomas. BMC Cancer. 2011;11:277. doi: 10.1186/1471-2407-11-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathanson DR, Culliford ATt, Shia J, Chen B, D’Alessio M, Zeng ZS, Nash GM, Gerald W, Barany F, Paty PB. HER 2/neu expression and gene amplification in colon cancer. Int J Cancer. 2003;105:796–802. doi: 10.1002/ijc.11137. [DOI] [PubMed] [Google Scholar]
- 10.Ooi A, Takehana T, Li X, Suzuki S, Kunitomo K, Iino H, Fujii H, Takeda Y, Dobashi Y. Protein overexpression and gene amplification of HER-2 and EGFR in colorectal cancers: an immunohistochemical and fluorescent in situ hybridization study. Mod Pathol. 2004;17:895–904. doi: 10.1038/modpathol.3800137. [DOI] [PubMed] [Google Scholar]
- 11.Marx AH, Burandt EC, Choschzick M, Simon R, Yekebas E, Kaifi JT, Mirlacher M, Atanackovic D, Bokemeyer C, Fiedler W, Terracciano L, Sauter G, Izbicki JR. Heterogenous high-level HER-2 amplification in a small subset of colorectal cancers. Hum Pathol. 2010;41:1577–1585. doi: 10.1016/j.humpath.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Spieker-Polet H, Sethupathi P, Yam PC, Knight KL. Rabbit monoclonal antibodies: generating a fusion partner to produce rabbit-rabbit hybridomas. Proc Natl Acad Sci U S A. 1995;92:9348–9352. doi: 10.1073/pnas.92.20.9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi S, Laurino L, Furlanetto A, Chinellato S, Orvieto E, Canal F, Facchetti F, Dei Tos AP. Rabbit monoclonal antibodies: a comparative study between a novel category of immunoreagents and the corresponding mouse monoclonal antibodies. Am J Clin Pathol. 2005;124:295–302. doi: 10.1309/NR8H-N08G-DPVE-MU08. [DOI] [PubMed] [Google Scholar]
- 14.Manion E, Hornick JL, Lester SC, Brock JE. A comparison of equivocal immunohistochemical results with anti-HER2/neu antibodies A0485 and SP3 with corresponding FISH results in routine clinical practice. Am J Clin Pathol. 2011;135:845–851. doi: 10.1309/AJCPIP5LOO3NGDJG. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton SRB, Boffetta P, et al. WHO Clasification of Tumours of the Digestive System. Lyon: IARC; 2010. [Google Scholar]
- 16.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, Ochiai A, Ruschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 18.Verbanac KM, Gross U, Rebellato LM, Thomas JM. Generation of rabbit anti-lymphocyte monoclonal antibodies. Transplant Proc. 1993;25:837–838. [PubMed] [Google Scholar]
- 19.Blok EJ, Kuppen PJ, van Leeuwen JE, Sier CF. Cytoplasmic Overexpression of HER2: a Key Factor in Colorectal Cancer. Clin Med Insights Oncol. 2013;7:41–51. doi: 10.4137/CMO.S10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunes CB, Rocha RM, Reis-Filho JS, Lambros MB, Rocha GF, Sanches FS, Oliveira FN, Gobbi H. Comparative analysis of six different antibodies against Her2 including the novel rabbit monoclonal antibody (SP3) and chromogenic in situ hybridisation in breast carcinomas. J Clin Pathol. 2008;61:934–938. doi: 10.1136/jcp.2007.053892. [DOI] [PubMed] [Google Scholar]


