Abstract
Solitary fibrous tumor (SFT) is rare mesenchymal neoplasm that has been originally and most often documented in the pleura. Recently, the ubiquitous nature of the SFT has been recognized with reports of involvement of numerous sites all over the body such as: upper respiratory tract, somatic tissue, mediastinum, head, and neck. Less than 10 cases SFT of breast have been reported. Herein, we presented a 52-year-old Asian female with SFT of breast, this tumor showed predominant malignant features. To our knowledge, SFT of breast with such malignant evidence is extremely rare.
Keywords: Solitary fibrous tumor, morphology, immunochemistry
Introduction
Solitary fibrous tumor (SFT) is rare mesenchymal neoplasm that has been originally and most often documented in the pleura. Recently, the ubiquitous nature of the SFT has been recognized with reports of involvement of numerous sites all over the body, i.e, upper respiratory tract, somatic tissue, mediastinum, head, and neck, etc [1,2]. SFT is extremely rare in breast. Less than 10 cases SFT of breast have been reported [3-5]. Here, we present a SFT of breast with malignant features such as: large tumor size (diameter 10.5 cm), increased mitotic index (≥ 4 mitoses per 10 high-power fields) infiltrative margins [6].
Case presentation
Clinical history
A 52-year-old Asian female accidentally found a small nodule in the left breast about one year ago, this nodule is about 2-3 cm3, right below the nipple. The patient did not take appropriate treatment because the lesion was lack of clinical symptoms. Over the past year, the nodule grew promptly. When the patient went to the hospital, the lesion occupied almost the entire left breast. The patient claimed for the pain in the left breast without nipple discharge. The skin of the left breast is slightly reddish. The histories of chronic disease, tumor, breast trauma and the family history of breast cancer were denied. Breast palpation presented that the skin of left breast is slightly reddish, without ulceration, the appearance of nipple is normal. A giant hard mass (approximate size: 10.0 × 10.0 × 4.0 cm3) with smooth surface and moderate activity was touched right blew the nipple. No obvious mass was found in the right breast or bilateral axillary. The clinical physical and imaging examination showed no obvious abnormalities in other organs. Mammography and ultrasound were performed on bilateral breast. Radiologists classify the lesion as BIRADS category 4C (Figure 1). The tumor was strongly advised to be surgically removed.
Figure 1.
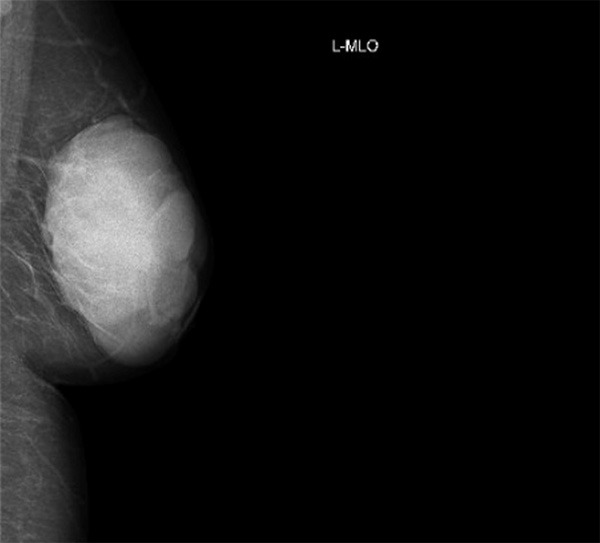
Mammography presentation of breast malignant SFT. The BIRADS category 4C lesion showed clear boundary and focal lobulated appearance.
Gross features
The circular incision was performed at the central area of the left breast by the surgeon. Tumor was completely removed, grossly, the tumor (size: 10.0 × 9.5 × 4.2 cm3) with complete capsule, the section of the tumor was white-gray, with tough texture and local lobulated appearance.
Microscopic features
The resected specimens were fixed with 10% neutral-buffered formalin and embedded in paraffin blocks. The 4-μm slides from the tissue blocks were used for hematoxylin-eosin (HE) staining. Generally, normal histological features of normal mammary gland could not be seen in the tumor. The tumor showed a patternless architecture characterized by a combination of alternating hypocellular and hypercellular areas. In hypocellular area, a small mount of spindle-shaped tumor cells scattered in collagen fibers, extremely rare mammary gland-like structure could be seen among the cells (Figure 2A). In hypercellular area, Ovoid-spindle shaped tumor cells surrounded branching and staghorn vasculature (Figure 2B), round shape tumor cells showed hemangiopericytoma-like growth pattern (Figure 2C), the two areas have a relative clear boundary (Figure 2D). Focally, tumor cell presented the infiltrated growth pattern, infiltrative margins could be identified (Figure 2E). At higher magnification, tumor cells showed mild to moderated cytological atypia, numerous mitoses (≥ 4 mitoses per ten high-power fields) could also be found as a feature (Figure 2F).
Figure 2.
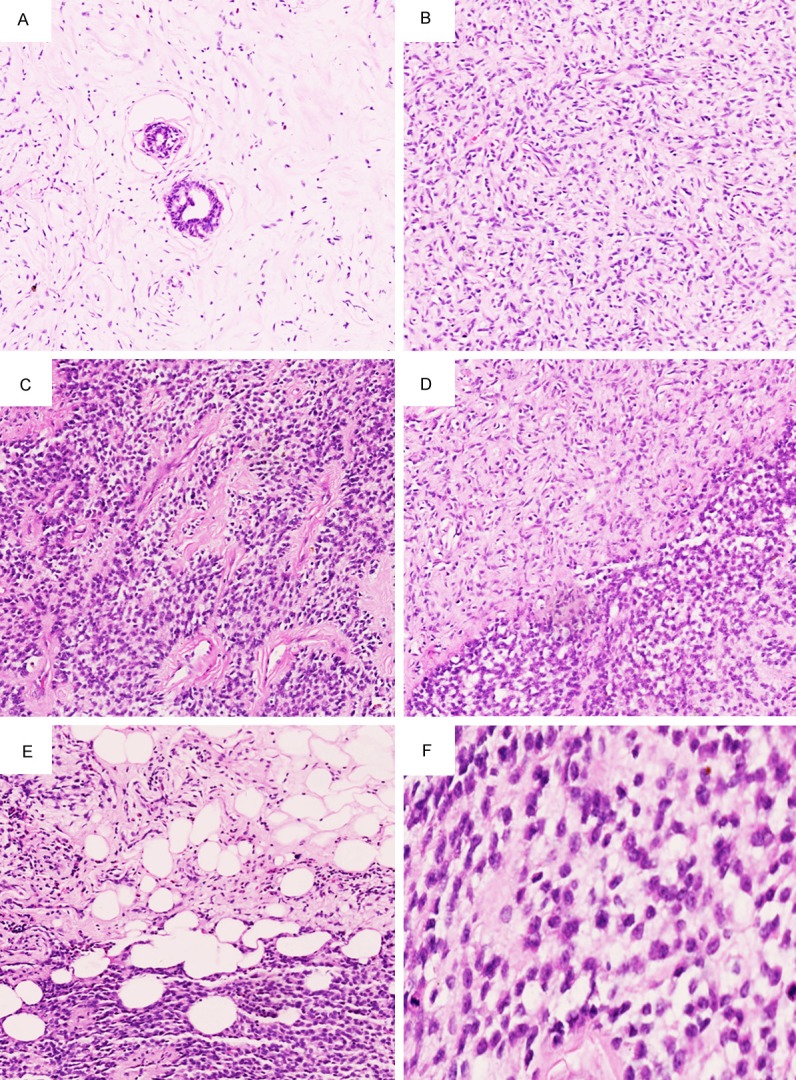
Microscopic features of breast malignant SFT. A. In hypocellular area, a small mount of spindle-shaped tumor cells scattered in collagen fibers, extremely rare mammary gland-like structure could be seen, HE 100 ×. B. In hypercellular area, Ovoid-spindle shaped tumor cells surrounded branching and staghorn vasculature, HE 100 ×. C. Round shaped tumor cells showed hemangiopericytoma-like growth pattern, HE 100 ×. D. There is a relative clear boundary between hypocellular and hypercellular areas, HE 100 ×. E. Focally, infiltrative margins could be identified, HE 100 ×. F. Tumor cells showed mild to moderated cytological atypia and active mitoses, HE 400 ×.
Immunohistochemistry
The immunohistochemical study showed that the tumor cells were strongly and diffusely positive for vimentin (Figure 3A), BCL-2 (Figure 3B); positive for CD34 (Figure 3C) and CD99; negative for p63, AE1/AE3, EMA, desmin, calrentinin, actin (SM), β-catenin and S-100. Ki67 index was about 15% (Figure 3D).
Figure 3.
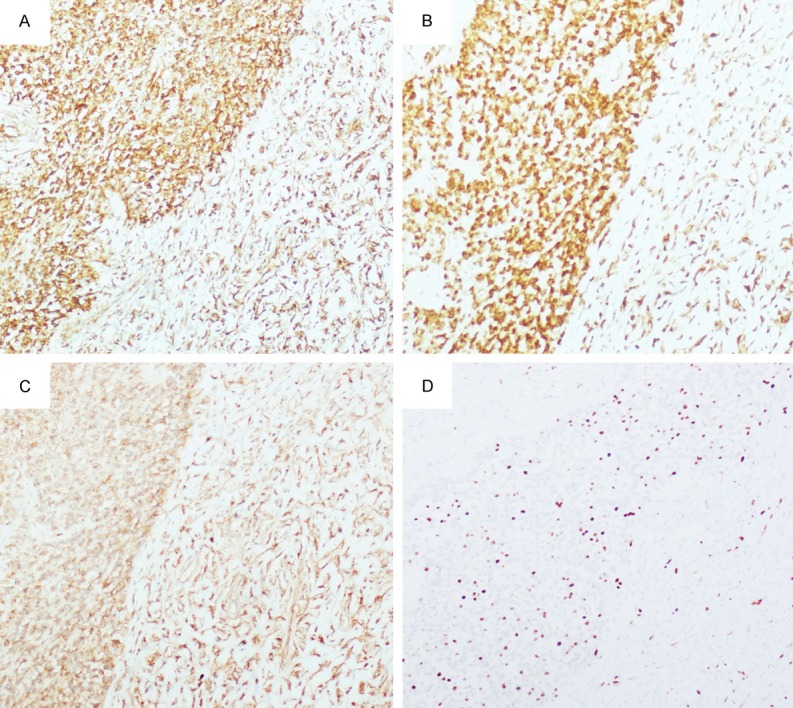
Immunohistochemistry studies of breast malignant SFT. A. Diffusely positive for vimentin, 100 ×. B. Diffusely positive for BCL-2, 100 ×. C. Positive for CD34, 100 ×. D. Ki67 index was about 15%, 100 ×.
Discussion
SFT is a ubiquitous mesenchymal tumor of probable fibroblastic type which shows a prominent hemangiopericytoma-like branching vascular pattern [1,2]. Morphologically, SFT is generally characterized by spindle cell proliferation showing a pattern-less architecture and staghorn vasculature. Our case showed typical morphological presentation of SFT, such as: patternless architecture characterized by a combination of alternating hypocellular and hypercellular areas, focal hemangiopericytoma-like growth pattern. These characteristics were very helpful to make a correct diagnosis of SFT. Malignant SFT are usually hypercellular lesions, showing at least focally moderate to marked cytological atypia, tumour necrosis, numerous mitoses (≥ 4 mitoses per ten high-power fields) and/or infiltrative margins [3-6]. While our case showed important malignant evidence such as: mild to moderated cytological atypia, numerous mitoses (≥ 4 mitoses per ten high-power fields) and infiltrative margins. These histological features were consistent with malignant SFT.
Immunohistochemistry is important to confirm the final diagnosis of malignant SFT. According to the previous reports, tumors cells in SFT are characteristically immunoreactive for vimentin, CD34, BCL-2, and CD99 [7], and the immunophenotype our case is consistent with the past reports. The positive expression of AE1/AE3, EMA, actin (SM) and S-100 in SFT were rare [7]. Our case showed negative expression p63, AE1/AE3, EMA, desmin, calrentinin, actin (SM), β-catenin and S-100, which is typical immunophenotype of SFT.
Although both morphological and immunohistochemistry studies support the diagnosis of breast malignant SFT well, some tumors characterized by spindle shaped cell hyperplasia should be considered in differential diagnosis. For example, the spindle cell variant of squamous cell carcinoma in breast might be a challenge of differential diagnosis. As the tumor cells emanate out to infiltrate the surrounding stroma, they become spindle shaped and lose their squamous features. A pronounced stromal reaction is often admixed with the spindled squamous carcinoma which could be distinguished from SFT [8]. Spindle cell variant of squamous cell carcinoma is also lack of hypercellular and hypocellular areas, as well as hemangiopericytoma-like growth pattern. Immunohistochemistry studies showed that spindle cell variant of squamous cell carcinoma always positive for AE1/AE3 and negative for CD34. The immunophenotype of our case is very helpful to exclude the diagnosis of spindle cell variant of squamous cell carcinoma. Malignant myoepithelioma is a rare tumor composed predominantly spindled myoepithelial cells, which should be distinguished from malignant SFT. According to previous reports, proliferation of branched vascular and typical hemangiopericytoma-like area is not the feature of malignant myoepithelial. In addition, the tumor cells were supposed to positive for action (SM), S-100 and negative for CD34 [9]. Myofibroblastoma is a benign spindle cell tumour of the mammary stroma composed of myofibroblasts, sometimes it showed infiltrating margins and prominent epithelioid cell component which could mimic with SFT [10], but the tumor is always lack of the changes in cell destiny and hemangiopericytoma growth. Although the tumor cells show the variable expression of CD34, BCL-2 and CD99, they are always positive for desmin and actin (SM) [10]. Malignant peripheral nerve sheath tumor showed long spindle tumor cells with lightly stained cytoplasm and slender nucleus, surrounded vascular and formed the vortex-like, bundle-like arrangement. The alternating of hypocellular area and hypercellular area could also be seen as a character which could mimic with malignant SFT. However, positive expression of S-100 and negative expression of CD34 were always helpful to distinguish from malignant SFT [11].
Malignant SFT positively correlated with local recurrence and metastatic disease. Some studies demonstrated a very low rate of recurrence and distant metastasis, whereas other investigators indicated a relatively increased relapse rate with extended follow-up periods [12]. Therefore, complete resection at an early stage should be the main goal of surgical treatment and follow-up should be maintained for more than 10 years [12,13]. The patient in our case was treated with simple mastectomy because of large tumor size and recent evidence of fast growing rate.
Tumor relapse occurred after up to 168 months, but most local recurrences or metastases were diagnosed within the first 2 years after surgery [12,13]. Nowadays, evolving therapies inhibiting specific angiogenic pathways show promising activities in SFT. The rich vascular features of SFT have been long recognized, but the effective value of the vascular endothelial growth factor (VEGF) and its receptor (VEGFR) in SFT still remains indistinct [14].
Acknowledgements
This work was partly supported by grants from the National Natural Science Foundation of China (No. 81272606, No. 81071905) to Dr. En-Hua Wang and National Natural Science Foundation of China to Dr. Lian-He Yang (No. 81301930).
Disclosure of conflict of interest
None.
References
- 1.Lee JC, Fletcher CD. Malignant fat-forming solitary fibrous tumor (so-called “lipomatous hemangiopericytoma”): clinicopathologic analysis of 14 cases. Am J Surg Pathol. 2011;35:1177–85. doi: 10.1097/PAS.0b013e318219cd0b. [DOI] [PubMed] [Google Scholar]
- 2.Masuda Y, Kurisaki-Arakawa A, Hara K, Arakawa A, Oh S, Suzuki K, Yao T, Saito T. A case of dedifferentiated solitary fibrous tumor of the thoracic cavity. Int J Clin Exp Pathol. 2013;7:386–93. [PMC free article] [PubMed] [Google Scholar]
- 3.Falconieri G, Lamovec J, Mirra M, Pizzolitto S. Solitary fibrous tumor of the mammary gland: a potential pitfall in breast pathology. Ann Diagn Pathol. 2004;8:121–5. doi: 10.1016/j.anndiagpath.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Dragoumis D, Desiris K, Kyropoulou A, Malandri M, Assimaki A, Tsiftsoglou A. Hemangiopericytoma/solitary fibrous tumor of pectoralis major muscle mimicking a breast mass. Int J Surg Case Rep. 2013;4:338–41. doi: 10.1016/j.ijscr.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rovera F, Imbriglio G, Limonta G, Marelli M, La Rosa S, Sessa F, Dionigi G, Boni L, Dionigi R. Solitary fibrous tumor of the male breast: a case report and review of the literature. World J Surg Oncol. 2008;6:16. doi: 10.1186/1477-7819-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vimi S, Punnya VA, Kaveri H, Rekha K. An aggressive solitary fibrous tumor with evidence of malignancy: a rare case report. Head Neck Pathol. 2008;2:236–41. doi: 10.1007/s12105-008-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magro G, Bisceglia M, Michal M, Eusebi V. Spindle cell lipoma-like tumor, solitary fibrous tumor and myofibroblastoma of the breast: a clinico-pathological analysis of 13 cases in favor of a unifying histogenetic concept. Virchows Arch. 2002;440:249–60. doi: 10.1007/s00428-001-0572-y. [DOI] [PubMed] [Google Scholar]
- 8.Saito T, Ryu M, Fukumura Y, Asahina M, Arakawa A, Nakai K, Miura H, Saito M, Yao T. A case of myxoid liposarcoma of the breast. Int J Clin Exp Pathol. 2013;6:1432–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Terada T. CD10-positive malignant spindle cell tumor of the lip in a child: a malignant myoepithelioma? Int J Clin Exp Pathol. 2013;6:978–81. [PMC free article] [PubMed] [Google Scholar]
- 10.Meguerditchian AN, Malik DA, Hicks DG, Kulkarni S. Solitary fibrous tumor of the breast and mammary myofibroblastoma: the same lesion? Breast J. 2008;14:287–92. doi: 10.1111/j.1524-4741.2008.00588.x. [DOI] [PubMed] [Google Scholar]
- 11.Dhingra KK, Mandal S, Roy S, Khurana N. Malignant peripheral nerve sheath tumor of the breast: case report. World J Surg Oncol. 2007;5:142. doi: 10.1186/1477-7819-5-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol. 1998;22:1501–11. doi: 10.1097/00000478-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, Brennan MF, Coit DG. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94:1057–68. [PubMed] [Google Scholar]
- 14.Park MS, Ravi V, Araujo DM. Inhibiting the VEGF-VEGFR pathway in angiosarcoma, epithelioid hemangioendothelioma, and hemangiopericytoma/solitary fibrous tumor. Curr Opin Oncol. 2010;22:351–5. doi: 10.1097/CCO.0b013e32833aaad4. [DOI] [PubMed] [Google Scholar]


