Abstract
Primary splenic lymphoma is rare as non-Hodgkin lymphomas. Splenic infiltration of lymphoma cells may cause splenomegaly in many cases. However, splenomegaly is caused not only by tumor involvement but also by non-tumorous disorders. One of the most prevalent non-neoplastic causes is portal hypertension mostly due to liver cirrhosis. On the other hand, liver cirrhosis may underlie various extrahepatic manifestations including development of B-cell non-Hodgkin lymphomas. Here, we report a case of primary follicular lymphoma of the spleen in a patient with liver cirrhosis related to hepatitis C and alcohol. The lymphoma was incidentally found in an enlarged spleen resected palliatively to alleviate symptomatic pancytopenia of the patient. The main characteristic of our case is an incidental finding of a rare situation brought by careful pathological examination. Our case illustrates the importance to recognize a possibility of co-occurrence of chronic liver disease and extrahepatic lymphoma.
Keywords: Follicular lymphoma, spleen, alcohol, hepatitis C, liver cirrhosis
Introduction
Primary splenic lymphoma is rare, occurring in no more than 1% of cases of non-Hodgkin lymphoma [1]. Among primary splenic lymphoma in Japanese patients, follicular lymphoma (FL) comprises 5.98% of them [2].
FL is a subtype of low grade B-cell lymphoma [3]. Its postulated normal counterparts are centrocytes inside germinal centers (GC) of secondary lymphoid follicles in lymph nodes. FL consists of follicle-like structures composedof centrocyte-like tumor cells expressing CD10 and BCL2 protein. Expression of BCL2 proteins in FLs is a consequence of t(14;18)(q32;q21) chromosomal translocation, in which BCL2 locus is under the control of IGH promoter. FL manifests itself not only as nodal but also as extranodal lesions including splenic one. Pure extranodal presentations are uncommon (9% in one survey) [4].
Splenic infiltration of lymphoma cells may cause splenomegaly in many cases. However, splenomegaly is caused not only by primary or secondary involvement of tumors, but also by non-tumorous disorders. One of the prevalent causes for splenomegaly is portal hypertension associated with liver cirrhosis or other hepatic disorders. Splenomagaly may cause symptomatic pancytopenia, in which case palliative splenectomy may be chosen. On the other hand, liver cirrhosis may underlie various extrahepatic manifestations including development of B-cell non-Hodgkin lymphomas.
Here, we report a case of primary follicular lymphoma of the spleen in a patient with liver cirrhosis related to hepatitis C and alcohol. The lymphoma was incidentally found in an enlarged spleen resected palliatively to alleviate symptomatic pancytopenia of the patient.
Case report
A 56-year-old Japanese female was admitted to our hospital for splenectomy. She had been suffering from liver cirrhosis related to alcohol and infection with hepatitis C virus (HCV) (Figure 1). Physical examination and abdominal computerized tomography (CT) with contrast enhancement revealed cirrhotic liver and enlarged spleen (around 17 × 8 cm in size) with vague nodularity (Figure 2A). Peripheral blood examination showed pancytopenia with 39.7 × 102/μL of white blood cell count (normal range: 40-90), 10.3 g/dL of hemoglobin (normal range: 11.5-15), and 6.7 × 104/μL of platelet count (normal range: 15-35). Her pancytopenia was considered to be attributed to hypersplenism caused by portal hypertension due to liver cirrhosis. Palliative splenectomy was chosen to relieve her symptomatic pancytopenia.
Figure 1.
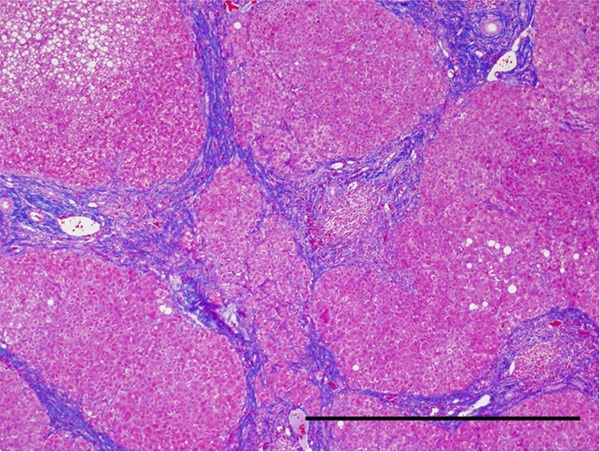
Histological image of the liver cirrhosis. Regenerative nodules are completely encircled by bridging fibrosis, which is stained blue with Azan Mallory method. Original magnification: × 40, Bar: 1 mm.
Figure 2.
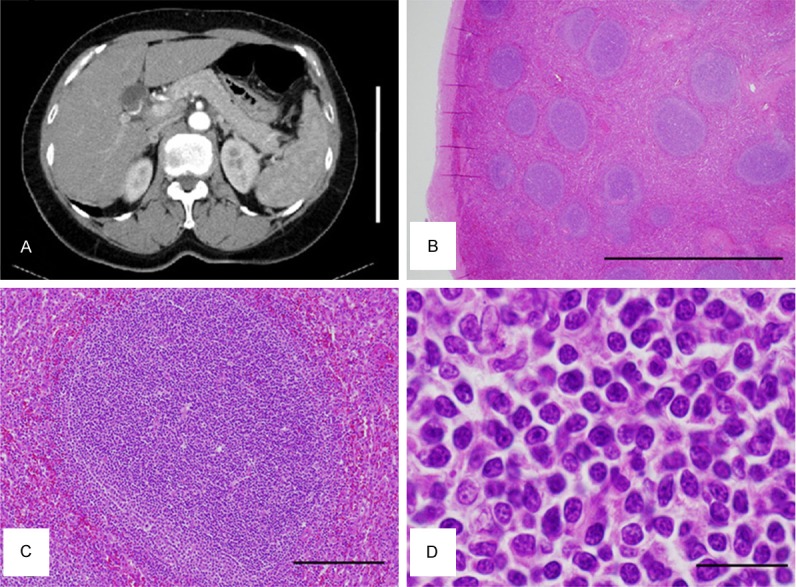
Radiological and histological views of the spleen. A. Abdominal CT with contrast enhancement. An enlarged spleen is shown in the right half of the figure. Bar: 20 cm. B-D. Representative HE images of the resected spleen B. At low power magnification, multiple follicle-like structures are observed. Original magnification: × 20, Bar: 2 mm. C. At high power magnification, the follicle-like structure is composed of homogeneous proliferation of smalllymphoid cells. Polarization observed in the normal germinal center (GC) appears unclear. Original magnification: × 100, Bar: 200 μm. D. Tumor cells are centrocytes-like in normal GCs. Apparently no centroblast-like cells are observed. Original magnification: × 1000, Bar: 20 μm.
Pathological examination of the resected spleen revealed apparent increase in number of lymphoid follicles (white pulps). At low power magnification, multiple follicle-like structures were observed (Figure 2B). The density of the follicle-like structrures appeared to be increased compared with follicles observed in the normal spleen. This was not typical as the spleen of cirrhotic patients, where lymphoid follicles in the white pulp are often atrophieddue to chronic congestion. Careful examination revealed slightly ambiguous polarity in GC-like structures in these follicle-like lesions (Figure 2C). That is, centrocyte-like cells in the follicle-like lesions were homogeneous in morphology and size, compared with centrocytes observed in the normal GCs (Figure 2D). Moreover, tingible body macrophages were not apparent in the GC-like structures (Figure 2C).
Immunohistochemistry revealed that centrocyte-like cells in the GC-like structures with the unclear polarity were stained positive for CD20 (Figure 3A), CD79a (data not shown), CD10 (Figure 3C), and BCL2 (Figure 3D), while they were negative for CD3 (Figure 3B), CD23 (data not shown), and Cyclin D1 (data not shown). Centroblast-like cells were not apparent. Preservation of normal reactive GCs was not evident. Diagnosis of FL, grade 1, in the spleen was made. 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT examination after the splenectomy revealed several para-aortic lymph nodes up to 8 mm in size, but no significant uptake of FDG was detected (data not shown). No other hotspots of FDG uptake were detected, including the bone marrow (data not shown). The patient was carefully followed up without chemotherapy. During 2-year follow-up by FDG-PET/CT after the diagnosis, there have been no significant changes in size of these lymph nodes without significant uptake of FDG. These results confirmed that the final diagnosis of the splenic lesion was primary FL of the spleen at clinical stage I.
Figure 3.
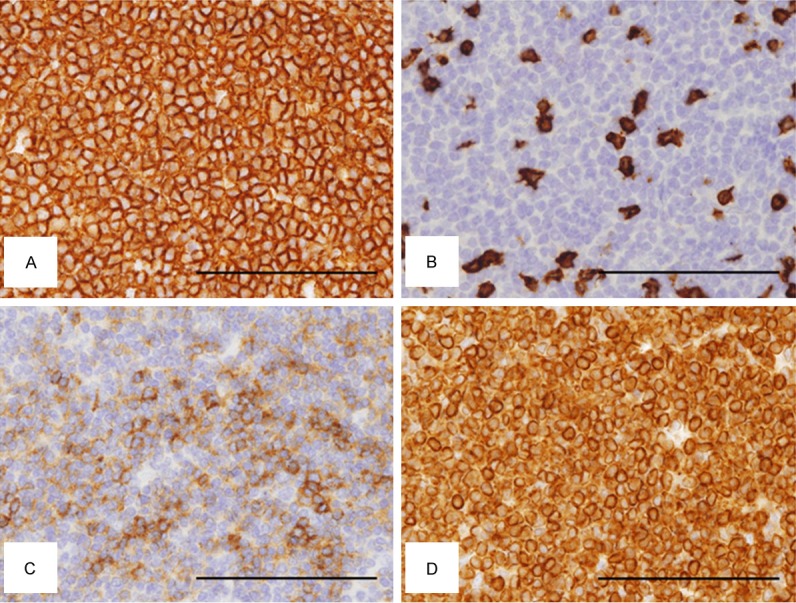
Immunohistochemical analysis of the tumor cells in the spleen. (A) CD20, (B) CD3, (C) CD10, (D) BCL2. Tumor cells are positive for CD20 (A), CD10 (C), and BCL2 (D), but negative for CD3 (B). Original magnification: × 400, Bar: 100 μm.
After the diagnosis of follicular lymphoma, interferon therapy for the liver cirrhosis was initiated. During the course of the therapy, multiple space-occupying lesions were pointed out in the liver by gadoxetic acid-enhanced magneticresonance imaging. They were diagnosed as hepatocellular carcinomas by needle biopsy (data not shown), and were treated by radiofrequency ablation and/or transcatheter arterial chemoembolization.
Discussion
In this paper, we reported a case of primary FL of the spleen incidentally found in a patient with alcohol- and HCV-related liver cirrhosis. Mollejo M et al. reported clinicopathological characteristics of 32 cases of primary splenic FL [5]. They described that splenic FL consists of 2 subgroups with different clinicopathological characteristics: (a) classical FL with t(14; 18) and CD10 expression, usually diagnosed at advanced stages, and (b) BCL2-negative FL cases, with higher histologic grade and more frequently initially seen as disease restricted to the spleen [5]. The lymphoma in our case is histopathologically designated as classical FL, but is considered to be localized to the spleen. The cause of the discrepancy may be related to a particular clinical background of this patient, that is, alcohol- and HCV-related liver cirrhosis, which was not described by Mollejo M et al. [5].
It is generally known that liver cirrhosis is not only a risk factor for hepatocellular carcinoma but also associated with a variety of extrahepatic abnormalities. There have been several retrospective cohort studies on malignancies associated with liver cirrhosis [6,7]. According to one study, the frequency of lymphomas was ranked as the third, following lung and breast cancers [6]. In the case of HCV-related liver disease, marginal zone lymphoma (in particular splenic marginal zone lymphoma), lymphoplasmacytic lymphoma, diffuse large B-cell lymphoma were reported to be frequently associated [8,9]. Other than these lymphomas and FL, Mollejo M et al. recently described previously underrecognized histological patterns of HCV-associated lymphoproliferative disorders [10]. In addition, there are case reports on primary effusion lymphoma-like lymphomas associated with HCV-related liver cirrhosis [11].
In the case of HCV-associated B-cell lymphomas, three pathological mechanisms have been elaborated for lymphomagenesis [9]. First, continuous stimulation of B cells by viral antigen leads to their consecutive excessive proliferation, just as illustrated by mucosa associated lymphoid tissue (MALT) lymphoma cells stimulated by Helicobacter pylori. Immunological abnormalities may play primary roles in extrahepatic lymphoma development [9]. Second, since HCV is potentially B-lymphotropic [12,13], HCV replication in B cells may mediate oncogenic effects by intracellular viral proteins, which has been controversial [9]. Third, it has been proposed that HCV infection may induce a high mutation frequency of cellular genes [14], which has also been controversial. Interestingly, one of the most convincing evidences for causal relationships between these lymphomas and HCV is the observation that eradication of HCV by antiviral and/or interferon therapy leads to remission of these lymphomas [9,15]. In our case, no relapse of the lymphoma was apparently observed during interferon therapy against HCV-related liver cirrhosis. It is tempting to speculate that the FL in our case responded to the interferon therapy. On the other hand, in anthracycline-based chemotherapy coupled with rituximab for B-cell non-Hodgkin lymphomas, care should be taken not only to reactivation of clinically silent HCV but also to hepatotoxicity of the drugs [9].
In conclusion, to our knowledge, our paper is the first report on primary FL of the spleen incidentally found in splenectomy specimen of a patient with alcohol- and hepatitis C-related liver cirrhosis. The low-power view of the spleen mimicked reactive lymphoid hyperplasia; however this was not typical as the spleen of cirrhotic patients, where lymphoid follicles in the white pulp are often atrophied due to chronic congestion. Careful examination ledto the correct diagnosis of FL of the spleen. One of the lessons from this case will be that it is clinically important to recognize a possibility of co-occurrence of chronic liver disease and extrahepatic lymphoma. Close collaboration between hepatologists and hematologists will be desired for appropriate management of patients with liver cirrhosis.
Acknowledgements
We thank all the colleagues in the Department of Surgical Pathology, Hyogo College of Medicine for preparation of pathological specimen.
Disclosure of conflict of interest
None.
References
- 1.Brox A, Shustik C. Non-Hodgkin’s lymphoma of the spleen. Leuk Lymphoma. 1993;11:165–171. doi: 10.3109/10428199309086992. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu-Kohno K, Kimura Y, Kiyasu J, Miyoshi H, Yoshida M, Ichikawa R, Niino D, Ohshima K. Malignant lymphoma of the spleen in Japan: a clinicopathological analysis of 115 cases. Pathol Int. 2012;62:577–582. doi: 10.1111/j.1440-1827.2012.02844.x. [DOI] [PubMed] [Google Scholar]
- 3.Harris NL, Swerdlow SH, Jaffe ES, Ott G, Nathwani BN, de Jong D, Yoshino T, Spagnolo D. Follicular lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th edition. Lyon: International Agency for Research on Cancer (IARC); 2008. pp. 220–226. [Google Scholar]
- 4.Harris NL, de Leval L, Ferry JA. Follicular lymphoma. In: Jaffe ES, Harris NL, Vardiman JW, Campo E, Arber DA, editors. Hematopathology. Philadelphia, PA: Saunders/Elsevier; 2011. pp. 267–290. [Google Scholar]
- 5.Mollejo M, Rodríguez-Pinilla MS, Montes-Moreno S, Algara P, Dogan A, Cigudosa JC, Juarez R, Flores T, Forteza J, Arribas A, Piris MA. Splenic follicular lymphoma: clinicopathologic characteristics of a series of 32 cases. Am J Surg Pathol. 2009;33:730–8. doi: 10.1097/PAS.0b013e318193fcef. [DOI] [PubMed] [Google Scholar]
- 6.Berman K, Tandra S, Vuppalanchi R, Ghabril M, Sandrasegaran K, Nguyen J, Caffrey H, Liangpunsakul S, Lumeng L, Kwo P, Chalasani N. Hepatic and extrahepatic cancer in cirrhosis: a longitudinal cohort study. Am J Gastroenterol. 2011;106:899–906. doi: 10.1038/ajg.2010.477. [DOI] [PubMed] [Google Scholar]
- 7.Kodama K, Tokushige K, Hashimoto E, Taniai M, Shiratori K. Hepatic and extrahepatic malignancies in cirrhosis caused by nonalcoholic steatohepatitis and alcoholic liver disease. Alcohol Clin Exp Res. 2013;37(Suppl 1):E247–52. doi: 10.1111/j.1530-0277.2012.01900.x. [DOI] [PubMed] [Google Scholar]
- 8.Hartridge-Lambert SK, Stein EM, Markowitz AJ, Portlock CS. Hepatitis C and non-Hodgkin lymphoma: the clinical perspective. Hepatology. 2012;55:634–41. doi: 10.1002/hep.25499. [DOI] [PubMed] [Google Scholar]
- 9.Peveling-Oberhag J, Arcaini L, Hansmann ML, Zeuzem S. Hepatitis C-associated B-cell non-Hodgkin lymphomas. Epidemiology, molecular signature and clinical management. J Hepatol. 2013;59:169–77. doi: 10.1016/j.jhep.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Mollejo M, Menárguez J, Guisado-Vasco P, Bento L, Algara P, Montes-Moreno S, Rodriguez-Pinilla MS, Cruz MA, Casado F, Montalbán C, Piris MA. Hepatitis C virus-related lymphoproliferative disorders encompass a broader clinical and morphological spectrum than previously recognized: a clinicopathological study. Mod Pathol. 2014;27:281–93. doi: 10.1038/modpathol.2013.120. [DOI] [PubMed] [Google Scholar]
- 11.Ascoli V, Lo-Coco F. Body cavity lymphoma. Curr Opin Pulm Med. 2002;8:317–22. doi: 10.1097/00063198-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Zignego AL, Giannini C, Monti M, Gragnani L. Hepatitis C virus lymphotropism: lessons from a decade of studies. Dig Liver Dis. 2007;39:S38–S45. doi: 10.1016/s1590-8658(07)80009-0. [DOI] [PubMed] [Google Scholar]
- 13.Conca P, Tarantino G. Hepatitis C virus lymphotropism and peculiar immunological phenotype: effects on natural history and antiviral therapy. World J Gastroenterol. 2009;15:2305–2308. doi: 10.3748/wjg.15.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machida K, Cheng KT, Sung VM, Shimodaira S, Lindsay KL, Levine AM, Lai MY, Lai MM. Hepatitis C virus induces a mutator phenotype: enhanced mutations of immunoglobulin and protooncogenes. Proc Natl Acad Sci U S A. 2004;101:4262–4267. doi: 10.1073/pnas.0303971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallisa D, Bernuzzi P, Arcaini L, Sacchi S, Callea V, Marasca R, Lazzaro A, Trabacchi E, Anselmi E, Arcari AL, Moroni C, Bertè R, Lazzarino M, Cavanna L. Role of anti-hepatitis C virus (HCV) treatment in HCV-related, low-grade, B-cell, non-Hodgkins lymphoma: a multicenter Italian experience. J Clin Oncol. 2005;23:468–473. doi: 10.1200/JCO.2005.06.008. [DOI] [PubMed] [Google Scholar]


