Abstract
Acute fibrinous and organizing pneumonia (AFOP) is a histological pattern characterized by intra-alveolus fibrinous deposition accompanied with a spectrum of clinical condition. It also presents in other types of lung lesions, thus renders risks to its diagnosis with small biopsies. Here we present 2 cases of lung consolidation and occupying lesions with typical histological presentation of AFOP. One case is tuberculosis presented as massive lung consolidation, initially treated as AFOP, and eventually progressed to bilateral military tuberculosis. The other case presented an occupying mass in the lung which was initially suspected to be an inflammatory mass with AFOP. Lobectomy revealed a poorly-differentiated adenocarcinoma, with AFOP pattern present in the peripheral tissues of the neoplastic mass. In conclusion, we suggest that it is not preferable to diagnose idiopathic AFOP in lung consolidation and occupying lesions before excluding other types of lesions. The diagnostic significance of AFOP should be deliberated.
Keywords: AFOP, tuberculosis, lung cancer, misdiagnosis
Introduction
In 2002, Beasely et al [1] described an unusual pattern of diffuse infiltrative lung disease termed acute fibrinous and organizing pneumonia (AFOP). It is accepted as an under recognized variant of acute lung injury, associated with a wide spectrum of clinical conditions, such as collagen vascular diseases [1-3], adverse drug reactions or environmental exposures [1], as well as pulmonary infections [4-6]. The histological feature of AFOP is the presenceof prominent intra-alveolar fibrinous deposition in the form of “fibrin balls” and organizing pneumonia with patchy distribution. Importantly, this microscopic presentation is also commonly observed in DAD, OP and EP [7-9], as well as in pulmonary vascular thrombosis [3,10], vacuities and proliferation of type II alveolar epithelium [1]. This feature of AFOP brings about a diagnostic problem that sampling limitation may lead to misdiagnosis because small biopsy tissues might not represent the intrinsic lesion. In this manuscript, we present 2 misdiagnosed cases of consolidation and occupying lung lesions with typical histological pattern of AFOP, to discuss the diagnostic problems of AFOP.
Case 1
A 64-year-old man had a complaint of an intermittent fever 39°C at highest point, accompanied with dry cough and breathlessness with 10 days duration. 1 month before admitted to hospital, he had been hospitalized due to cerebralinfarction and was diagnosed as diabetes and hypertension. On examination, he was noted to be in slight respiratory distress. Computed tomography scan (CT-scan) of the chest revealed bilateral massive lung consolidation lesion with scattered nodular opacities in the peripheral areas in the upper-lobe of the left lung, and bilateral pleural effusion (Figure 1A). Sputum culture and blood biomarker tests were unremarkable. Percutaneous needle lung biopsy (PNLB) was performed and histological examination revealed prominent fibrinous exudation within most the alveolar spaces with “fibrin balls” formation (Figure 1C). No necrosis or granulomas were observed; neither any evidence of diffuse alveolar damage, alveolitis or eosinophilic infiltration. Grocott methenamine silver (GMS) and Ziehl-Neelsen staining were performed, but no evidence of special infections were detected. A diagnosi of “organizing pneumonia with intra-alveolus deposition, which inclined to AFOP” was made. The patient was therefore started with methylprednisolone 80mg twice daily intravenous drip. In the following 8 days, his symptom of cough was lessened and his temperature was back to normal. He also showed a prominent improvement in radiological conditions. The patient discharged on a tapering schedule of methylprednisolone 12 mg twice daily.
Figure 1.

A: CT-scan image of Case 1 showed air-space consolidation with peripheral nodular opacities in the left lung with bilateral pleural effusion. B: 47 days after discharge, radiological re-examination exhibited bilateral military nodules in both side of the lung, with prominent spread of the previous consolidation lesion. C: Intra-alveolus lesions filling with eosinophilic “fibrin balls” and loose connective tissues were observed microscopically in the first PNLB (HE, original magnification *200). D: The second PNLB examination revealed typical caseous necrosis (HE, original magnification *200) and areas with positive acid fast bacilli (red arrows in the upper right square pointing at acid fast bacilli, Ziehl-Neelsen staining, oil immersion lens, original magnification *1000).
47 days later, the patient was admitted to hospital again for severe acute fever and dyspnea. CT-scan examination presented prominent spread of the previous lesion, accompanied with diffuse military nodules in both of the lungs (Figure 1B). PNLB microscopically showed typical caseous necrosis and epithelioid cell granulomas with positive Ziehl-Neelsen staining result (Figure 1D), which confirm to the diagnosis of tuberculosis. Anti-tuberculosis therapies were given and the patient markedly relieved from his symptoms. Follow-up of 9 months showed a stable condition without any recurrence of severe respiratory symptoms.
Case 2
An 84-year-old man was admitted to a local hospital and presented with a 3-week history of fever and dry cough. Chest CT-scan revealed an occupying mass measuring 7.5 cm * 5.5 cm in the lower-lobe of the right lung (Figure 2A). Sputum test and other laboratory examinations were unremarkable. PNLB was applied and histological examination revealed massive Masson bodies with eosinophilous intra-alveolus fibrinous deposition, accompanied with severe acute and chronic inflammatory cells infiltration (Figure 2C). The final diagnosis of “inflammatory lung nodule with organization pneumonia” was made, and the patient was treated with steroids and antibiotics therapies in the following 2 weeks. He was gradually relieved from symptoms of cough and his temperature also fall close to normal.
Figure 2.
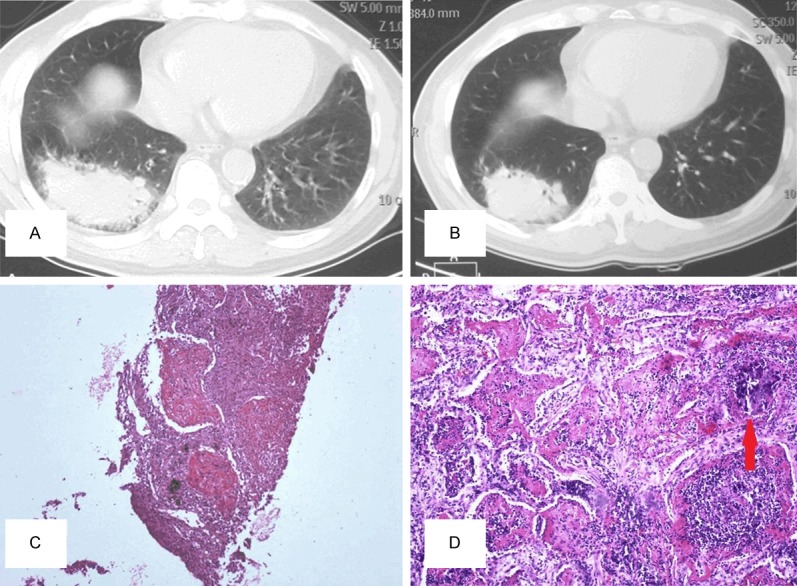
A: In case 2, CT-scan discovered an occupying mass under the pleura in the lower lobe of the right lung with irregular boundaries. B: 3 months after treated with antibiotic and steroids, CT-scan re-examination showed a slight shrink of the previous lesion size. C: Massive intra-alveolus fibrinous deposition with severe acute and chronic inflammatory cells infiltration (original magnification *100) was observed in the biopsy tissue. D: Lobectomy specimen examination discovered a poorly-differentiated adenocarcinoma, intra-alveolus fibrin balls irregularly mixed with cancer cells (original magnification *200, red arrowed pointing at poorly-differentiated adenocarcinoma).
3 months later, radiographic re-examination of the patient presented a slight shrink of the previous lesion size (Figure 2B). However, sputamentum exfoliative cytologic examination revealed “a small number of cells with nuclei atypia”. Therefore, a surgical resection of the right lower lobe of the lung was performed. Macroscopic examination discovered a 6 cm * 4.5 cm solid mass with irregular boundaries in the right lower lobe the lung. Pathology examinationidentified a poorly differentiated adenocarcinoma in the core of the mass. In addition, massive Masson bodies accompanied with eosinophilous intra-alveolus fibrin balls were also observed in the surrounding tissue of the cancer mass, occupying around 20% of the whole lesion. In some of the areas, intra-alveolus fibrin balls were seen irregularly mixed with cancer cells (Figure 2D). The patient underwent 1 course of chemotherapies, died of brain metastasis 10 months after surgery.
Discussion
AFOP has not yet been accepted as a distinct entity due to its close association with a spectrum of clinical conditions [1-6]. Most AFOPs present as diffuse or multifocal infiltrative lesions. Idiopathic AFOP with single occupying mass was also reported [11]. However, other occupying lesions, such as abscesses, tuberculosis and granulomas were observed containing typical histological pattern of AFOP [1]. It seems that AFOP occurred in these lesions acting as a nonspecific reaction adjacent to the major mass. This has proved diagnostic risks that the intrinsic lesion in the mass could be missed in small biopsies like in our cases. Therefore, Beasely emphasized that the diagnosismust be based on open-lung biopsy tissues rather than small biopsies [1]. However, large biopsy specimens obtained from open-lung biopsy are not as available as small biopsies, because open-lung biopsy is a much more costly and risky and traumatic examination method, and not frequently applied in clinical practice. PNLB is still primary approach for suspected infection and tumor, especially in patients with multiple or large lesions radiologically. It seems that misdiagnosis is hard to be avoided. As to clinical treatments, it was not found yet an ideal therapeutic strategy for AFOP, but there are reports of successful treatments with steroids and/or immunosuppressive agents [1,12,13] which may lead original infection aggravated.
In this manuscript, the patient of case 1 was discovered a subpleural lung consolidation opacities with typical AFOP histological pattern, agreed to the diagnosis of AFOP. But his rapid progression to diffuse military tuberculosis (within 1 month) indicated a tuberculosis lesion that possibly existed within the previous lesion, which was probably missed by the needle biopsy sampling. His symptoms and radiographic images were temporally alleviated following steroids therapies, maybe because the AFOP components in the lesion responded well to steroids. However, the inherent tuberculosis finally progressed to severe bilateral diffuse military tuberculosis by the effects of steroids.
In case 2, the occupying mass confirmed by CT-scan was initially treated as inflammatory mass. It is noticeable that massive eosinophilous fibrin-balls were seen accumulating in the alveolus of the surrounding tissues of the cancermass, occupying around 30% of the whole lesion which led to misdiagnosis in the first percutaneous needle lung biopsy. We may see that it is dangerous to make a pathology diagnosis as AFOP in occupying lesions, because in this case, the lesion size was slightly reduced after steroids and antibiotics therapies, providing an illusion that the mass was benign. Thus the optimal opportunity of curing cancer was delayed by misdiagnosis.
From the 2 cases above, we may see that it is not preferable to diagnose AFOP in lung consolidation and occupying lesions, even though the microscopic findings are very typical. As this histological presentation widely exists in other types of lesions, it could be a reactive change in the peripheral tissue reacting to the intrinsic lesion. Therefore, we concluded that (1) fully intercommunications with clinicians and adequate exclusions from other lung lesions are needed before a final diagnosis of idiopathic AFOP is made. (2) Patients with AFOP histological changes should be circumspectly treated with and closely surveyed. Another biopsy is required any time when the clinical conditions progressed or worsened. We suggest the image findings of AFOP should be described in the pathology report, and note that other types of lesions should be excluded before considering idiopathic AFOP.
Disclosure of conflict of interest
None.
References
- 1.Beasely MB. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126:1064–1070. doi: 10.5858/2002-126-1064-AFAOP. [DOI] [PubMed] [Google Scholar]
- 2.Prahalad S, Bohnsack JF, Maloney CG, Leslie KO. Fatal acute fibrinous and organizing pneumonia in a child with juvenile dermatomyositis. J Pediatr. 2005;146:289–292. doi: 10.1016/j.jpeds.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 3.Hariri LP, Unizony S, Stone J, Mino-Kenudson M, Sharma A, Matsubara O, Mark EJ. Acute fibrinous and organizing pneumonia in systemic lupus erythematosus: a case report and review of the literature. Pathol Int. 2010;60:755–759. doi: 10.1111/j.1440-1827.2010.02586.x. [DOI] [PubMed] [Google Scholar]
- 4.Cincotta DR, Sebire NJ, Lim E, Peters MJ. Fatal acute fibrinous and organizing pneumonia in an infant: The histopathologic variability of acute respiratory distress syndrome. Pediatr Crit Care Med. 2007;8:378–382. doi: 10.1097/01.PCC.0000269375.10806.60. [DOI] [PubMed] [Google Scholar]
- 5.Heo JY, Song JY, Noh JY, Yong HS, Cheong HJ, Kim WJ. Acute fibrinous and organizing pneumonia in a patient with HIV infection and Pneumocystis jiroveci pneumonia. Respirology. 2010;15:1259–1261. doi: 10.1111/j.1440-1843.2010.01845.x. [DOI] [PubMed] [Google Scholar]
- 6.Ribera A, Llatjós R, Casanova A, Santin M. Chlamydia pneumoniae infection associated to acute fibrinous and organizing pneumonia. Enferm Infecc Microbiol Clin. 2011;29:632–634. doi: 10.1016/j.eimc.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Hwang DM, Chamberlain DW, Poutanen SM, Low DE, Asa SL, Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol. 2005;18:1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshinouchi T, Ohtsuki Y, Kubo K, Shikata Y. Clinicopathological study on two types of cryptogenic organizing pneumonitis. Respir Med. 1995;89:271–278. doi: 10.1016/0954-6111(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 9.Hariri LP, Mino-Kenudson M, Shea B, Digumarthy S, Onozato M, Yagi Y, Fraire AE, Matsubara O, Mark EJ. Distinct histopathology of acute onset or abrupt exacerbation of hypersensitivity pneumonitis. Hum Pathol. 2012;43:660–668. doi: 10.1016/j.humpath.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Ding BS, Zhou YJ, Chen XY, Zhang J, Zhang PX, Sun ZY, Tan XY, Liu JN. Lung endothelium targeting for pulmonary embolism thrombolysis. Circulation. 2003;108:2892–2898. doi: 10.1161/01.CIR.0000103685.61137.3D. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi H, Sugimoto C, Kanoh S, Motoyoshi K, Aida S. Acute fibrinous and organizing pneumonia: initial presentation as a solitary nodule. J Thorac Imaging. 2005;20:291–293. doi: 10.1097/01.rti.0000168600.78213.85. [DOI] [PubMed] [Google Scholar]
- 12.Bhatti S, Hakeem A, Torrealba J, McMahon JP, Meyer KC. Severe acute fibrinous and organizing pneumonia (AFOP) causing ventilatory failure: successful treatment with mycophenolate mofetil and corticosteroids. Respir Med. 2009;103:1764–1767. doi: 10.1016/j.rmed.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Damas C, Morais A, Moura CS, Marques A. Acute fibrinous and organizing pneumonia. Rev Port Pneumol. 2006;12:615–620. [PubMed] [Google Scholar]


