Abstract
Acute respiratory distress syndrome (ARDS) is a serious medical condition occurring in patients with polytrauma, pulmonary or non-pulmonary sepsis, pneumonia and many other circumstances. It causes inflammation of the lung parenchyma leading to impaired gas exchange with a systemic release of inflammatory mediators, causing consequential lung tissue injury, hypoxemia and frequently multiple organ failure. The aim of current study was to describe expression of inflammatory markers (myeloperoxidase, CD163 and vascular endothelial growth factor) by the cells in acute phase of ARDS. The lung samples of a 20-year-old man who had suffered a serious motorbike accident were obtained for histological examination. He died on the seventh day as a consequence of respiratory failure. Our results imply that expression of CD163 was restricted to activated alveolar macrophages and monocytes. Immunopositivityof MPO was observed in neutrophil granulocytes within lung alveoli and lung blood vessels. Myeloperoxidase positivity was observed in alveolar macrophages, too. Vascular endothelial growth factor was expressed in cytoplasm of neutrophil granulocytes, monocytes, small-sized alveolar macrophages and type II pneumocytes localized mostly inside lung alveoli. On the contrary, no positivity was observed in lung endothelial cells of blood vessels.
Keywords: ARDS, myeloperoxidase, VEGF, CD163, immunohistochemistry
Introduction
Acute respiratory distress syndrome (ARDS) is a severe condition occurring in intensive care units. It represents clinical syndrome of non-cardiogenic pulmonary oedema associated with bilateral pulmonary infiltrates, stiff lungs and refractory hypoxemia. ARDS is an inflammatory disease initiated by a wide variety of systemic and/or pulmonary insults that lead to disruption of the alveolar-capillary unit and to a breakdown in the barrier and gas exchange functions of the lung [1]. Berlin definition in 2012 corrected criteria for determination of ARDS consisting of acute onset (within one week of known clinical insult), CT or RTG findings, non-cardiogenic pulmonary oedema and oxygenation changes. A draft definition proposed 3 mutually exclusive categories of ARDS based on degree of hypoxemia: mild, moderate and severe degree [2]. Histopathological features of the individual stages of ARDS are characteristic,but sometimes these stages cross each other. Most prominent and acute changes can be observed in exudative stage with formation of protein rich fluid in the interstitium and alveoli. In addition, hyaline membrane formation, necrosis of type I pneumocytes and destruction of alveolar basement membrane can be seen. Among several cells involved in this exudative stage play essential role lung neutrophils with their active substances. Their accumulation starts in pulmonary capillaries and during ARDS progress they migrate to the lung interstitium and alveolar lumen [3]. Many other cells such as macrophages, monocytes and vascular endothelium are involved in exudativestage of ARDS by secretion of inflammatory mediators [4,5]. The aim of our work was to describe and identify inflammatory cells using immunohistochemical detection of selected markers-myeloperoxidase (MPO), CD163 and vascular endothelial growth factor (VEGF).
Material and methods
Clinical features
A motorcycle rider (20-year-old male) was involved in a high-energy collision with a car, suffering traumatic amputation of the upper right arm in its middle third, compound fractures of the right thigh and right tibia with soft tissue devastation and problematic blood perfusion in the region. Patient was in hemorrhagic shock with 40% estimated blood loss (initial haemoglobin 59 g/l), 80/40 mmHg blood pressure, 120 beats per minute heart rate and mental confusion. Spiral head and torso CT scan revealed no other organ injury. After surgical soft tissue debridement and bone stabilization (external fixator, Kirschner-wire skeletal traction) the patient was transferred to the intensive care unit for further treatment. On the second day no signs of lung parenchyma infiltration were revealed by plain X-ray of the chest. There was an unsuccessful attempt to wean the patient from mechanical ventilation. Onthe third day after injury, diffuse crackles on chest auscultation and infiltration of the lung parenchyma at the right and left bases with right pleural effusion were revealed by X-ray. Positive end-expiratory pressure (PEEP) mode of mechanical ventilation was instigated. On the fourth day the patient became cyanotic with a drop in oxygen saturation to 72%. Emergency therapeutic fibreoptic bronchoscopy was carried out with suctioning of pink-tinged clear frothy fluid, and the right pleural space was drained with active suction. On the fifth day a CT scan of the lungs revealed signs of ARDS, atelectasis and minimal bilateral pleural effusions. Despite treatparenment with higher levels of PEEP with an increased fraction of inspired oxygen in the gas and repeated therapeutic fibreoptic bronchoscopies, the patient died from his injuries on day seven.
Immunohistochemical procedure
Necroptic lung specimens were harvested and immediately fixated in 4% paraformaldehyde and embedded in Paraplast wax, sectioned in 4-5 μm tissue slices. Histological sections were deparaffinised and rehydrated. Endogenous peroxidase activity was blocked with 3% H2O2 with methanol. Pre-treatment was performed in a microwave oven at 600 W for 15 min in 0.01 M citrate buffer at pH 6.0. A primary anti-MPO rabbit polyclonal antibody (Thermo Scientific, MA, USA), primary anti-VEGF rabbit polyclonal antibody (Thermo Scientific, MA, USA) and anti-CD163 rabbit clonal antibody (DB-Biotech, Košice, Slovakia) were used. Primary antibodies were labelled using a two-stage indirect immunoperoxidase technique. Primary antibodies were applied at the appropriate titre. Biotinylated secondary anti-mouse IgG (H+L)/anti-rabbit IgG (H+L) (Thermo Scientific, MA, USA), was used in labelling with R.T.U. Vectastain ABCReagent (Vector Laboratories) for detection of MPO-positive cells. Biotinylated secondary goat anti-mouse IgG/goat anti-rabbit IgG (Millipore Bioscience Research Reagents, MA, USA) antibody was used in labelling with IHC Select® Immunoperoxidase Secondary Detection System (Millipore Bioscience Research Reagents) for detection of the CD163-positive cell population. Positive cells were visualized with diaminobenzidine, DAB (Sigma-Aldrich, MO, USA) and counterstained with Mayer’s haematoxylin. Omitting the primary antibodies was considered as the negative control. The tissue sections were examined and photographed using an Olympus BX50 light microscope with an Olympus SP350 camera (Olympus, Japan) and were evaluated by two blinded and independent histologists.
Results
Alveolar macrophages demonstrated strong CD163-positivity of the cell membrane as well as distinct cytoplasmic positivity (Figure 1). In addition, much stronger CD163-immunopositivity was observed in small-sized cells morphologically similar to the monocytes or small macrophages, which were located especially inside interalveolar septa. Much higher CD163-positive cell concentration was found in alveolar space. Strong granular MPO expression wasevident within the cytoplasm of neutrophils which were present inside the lumen of dilated blood vessels as well as inside the fluid filling the alveoli (Figure 2). Minority of MPO-posi- tive cells was detected in the lung interstitialtissue. Strong expression of MPO as a homogenous cytoplasmic positivity was observed in interstitial small-sized macrophages as well. Large active macrophages positive for CD163 were MPO-negative. The VEGF immunoreactivity was observed in the neutrophil granulocytes and small-sized macrophages inside haemorrhagic lesions in lung parenchyma and inside dilated lung blood vessels (Figure 3). Distinct VEGF positivity was typical finding in cytoplasm of type II pneumocytes lining the alveolar surface and in the cytoplasm of detached cells within lung alveoli. Endothelial lining of pulmonary blood vessels showed no positivity for VEGF.
Figure 1.

Cytoplasmic expression of CD163 in monocyte-macrophage cell population (arrows). Strong CD163-immunopositivity of cell membrane surface is seen (arrowhead, left corner-detail).
Figure 2.

Cytoplasmic expression of MPO in neutrophil granulocytes within lung alveoli (asterisk). Strong MPO-immunoreactivity is seen in cytoplasm of small-sized macrophages within lung interstitium (arrowhead, left corner-detail).
Figure 3.
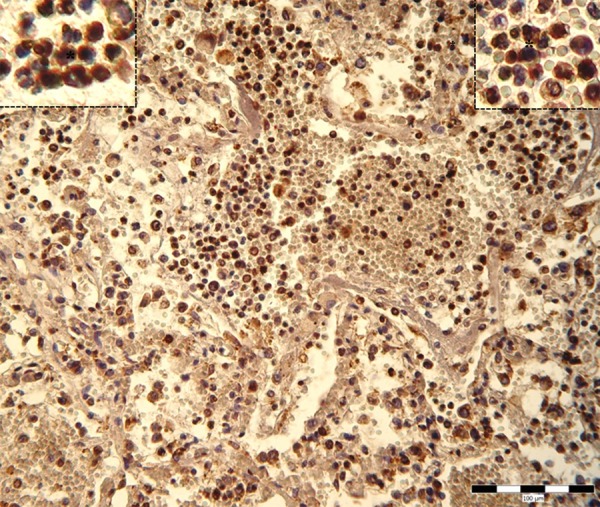
Cytoplasmic expression of VEGF in neutrophil granulocytes, small-sized macrophages and type II pneumocytes in lung lesions (asterisks).
Discussion
A main feature of ARDS acute phase is influx of protein rich fluid into the lung alveoli, which is caused by increased permeability of alveolar-capillary membrane. This condition is a result of endothelial injury accompanied by damage of alveolar epithelium. In our study the high number of neutrophils showed a typical feature for acute exudative phase of ARDS. Normally, the neutrophils cause no damage, but following activation they release oxygen radicals and hydrolytic enzymes that damage the endothelium of the lung capillaries. The development of diffuse alveolar impair is connected with pulmonary oedema and hypoxemia with consequent formation of hyaline membranes and type I pneumocyte necrosis. Detailed histopathological description of that was published previously [6].
Since neutrophils play critical and crucial role in ARDS development in acute phase, MPO expression as a part of neutrophil compartment is evident. Sugamata et al. studied MPO as a potent tissue damage factor and examinedits contribution in influenza pneumonia by using mice genetically lacking in MPO. The absence of MPO reduced inflammatory damage with suppression of leakage of total bronchoalveolar lavage fluid (BALF) proteins associatedwith alteration of claudins in the lung. They showed that in mice genetically absent in MPO, inflammatory response was reduced as well as decreased leakage of total BALF proteins [7].
Grattendick et al. pointed out on fact that neutrophil-derived MPO and enzymatically inactive MPO stimulate lung alveolar macrophages, resulting in an increased inflammatory and cytotoxic state, and thereby contributing to thegeneral lung inflammatory response [8]. Furthermore it was observed that some mediators like human recombinant complement factor 5a and human recombinant and granulocyte-macrophage colony-stimulating factor can inhibit rate of neutrophil apoptosis and that way increase life span of neutrophils in injured and inflamed tissue [9]. Our study showed definite granular MPO expression in the cytoplasm of neutrophils present inside the lumen of dilated blood vessels as well as inside the fluid filling the alveoli during acute phase of ARDS. During the resolution phase of an acute inflammatory response, infiltrated neutrophils undergo apoptosis and are subsequently cleared by residentmacrophages [10]. In our study, MPO expression was found as a homogenous cytoplasmic positivity in interstitial small-sized macrophages as well. Rousseau et al. noted in a study on ARDS that the alveolar cells in the patients had a phenotype similar to blood monocytes [11]. Based on studies in the mouse, the same group demonstrated that these cells are newly emigrated blood monocytes [12]. In the mouse blockade of monocyte immigration also blocked influx of neutrophils, indicating that the newly recruited monocytes are central to the inflammatory process [13]. MPO positivity found in the small macrophages in our case report needs further investigation and could be probablyconnected with their phagocytic activity. These cells located mostly in the interalveolar septa exhibit CD163-positivity as well. In human lung, CD163-positivity in both alveolar and interstitial mature macrophages was described [14]. The expression of CD163 can be regulated by a variety of factors which have been studied extensively in vitro. Consistent with the expression on mature macrophages in vivo, the in vitro differentiation of monocytesto macrophages strongly induces CD163 mRNA and protein expression. After in vitro treatment of monocytes with glucocorticoids the percentage of CD163 positive monocytes has been reported to rise from 10-30% to 90% [15]. When glucocorticoids were injected in vivo into human volunteers this also results in an increase of CD163 positive monocyte population of more than 80% within 6 h [16]. Recently was found, that interleukin-6 (IL-6) and tumor necrosis factor-α up-regulated CD163 expression in mononuclear cells of normal subjects [17].
Besides MPO, VEGF is another inflammatory mediator which is expressed in neutrophils. Taichman et al. showed that human neutrophil granulocytes are source of VEGF in inflamed tissue. He mentioned that although the amount of secreted VEGF is relatively small, high accumulation of cells may be sufficient to contribute VEGF to the local cytokine pool [18]. In our study VEGF immunoreactivity was observed in the neutrophil granulocytes, small-sized macrophages and type II pneumocytes. VEGF is a potent regulator of vascular permeability, inflammatory response, and cell survival in the lung. Mura et al concluded that VEGF in type II pneumocytes helps protect alveolar epithelial cells from caspase-dependent apoptosis [19]. However, VEGF produced from type II cells may contribute to increased vascular permeability during acute lung injury. In the lung, alveolar epithelial cells and microvascularendothelial cells are highly sensitive to hypoxia and together orchestrate a rapid and sustained adaptive response. Signorelli et al. confirmed inducing effect of hypoxia on VEGF and IL6 secretion by alveolar epithelial cells and microvascular endothelial cells. During ARDS progression hypoxia plays important role and could participate to strong VEGF production by type II pneumocytes found in our observing. Increased level of concurrent pro-inflammatory IL-6 production could be explanation for up-regulation CD163 expression in monocyte/macrophage cells. The absence of type I pneumocytes indicates their higher sensitivity to acute alveolar injury. Type II pneumocytes were more resistant and they persist in alveolar lining in terminal phase of disease, although some of them were detached and were found in alveolar space [20].
In conclusion, the inflammatory cell population taking part in exudative phase of ARDS is represented by neutrophils, which exhibit MPO and VEGF immunopositivity. From the group of monocytes/macrophages the small-sized cells had the same character together with CD163 positivity. From alveolar epithelial lining, the type II pneumocytes were present and they reveal strong VEGF immunoreactivity. Majority of immunoreactive cells were present inside alveolar air space spread in exudate.
Acknowledgements
This study was supported by the grant of European Regional Development Fund-Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123), CEMIO-ITMS-26220120058 and VEGA 1/0043/12. We gratefully acknowledge material and technical assistance o A. Hantke.
Disclosure of conflict of interest
None.
References
- 1.Crimi E, Slutsky AS. Inflammation and the acute respiratory distress syndrome. Best Pract Res Clin Anaesthesiol. 2004;18:477–492. doi: 10.1016/j.bpa.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 2.The Berlin Definition. Acute Respiratory Distress Syndrome. JAMA. 2012:307. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 3.Cranshawand JH, Griffiths JD. Inflammatory processes in the acute respiratory distress syndrome. Curr Anaesth Crit Care. 2003;14:66–73. [Google Scholar]
- 4.Schütte H, Lohmeyer J, Rosseau S, Ziegler S, Siebert C, Kielisch H, Pralle H, Grimminger F, Morr H, Seeger W. Bronchoalveolar and systemic cytokine profiles in patients with ARDS, severe pneumonia and cardiogenic pulmonary oedema. Eur Resp J. 1996;9:1858–1867. doi: 10.1183/09031936.96.09091858. [DOI] [PubMed] [Google Scholar]
- 5.Suter PM, Suter S, Girardin E, Roux-Lombard P, Grau GE, Dayer JM. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992;145:1016–1022. doi: 10.1164/ajrccm/145.5.1016. [DOI] [PubMed] [Google Scholar]
- 6.Tóth S, Pingorová S, Jonecová Z, Morochovic R, Pomfy M, Veselá J. Adult Respiratory Distress Syndrome and alveolar epithelium apoptosis: an histopathological and immunohistochemical study. Folia Histochem Cytobiol. 2009;3:431–4. doi: 10.2478/v10042-009-0046-7. [DOI] [PubMed] [Google Scholar]
- 7.Sugamata R, Dobashi H, Nagao T, et al. Contribution of neutrophil-derived myeloperoxidase in the early phase of fulminant acute respiratory distress syndrome induced by influenza virus infection. Microbiol Immunol. 2012;56:171–182. doi: 10.1111/j.1348-0421.2011.00424.x. [DOI] [PubMed] [Google Scholar]
- 8.Grattendick K, Stuart R, Roberts E, Lincoln J, Lefkowitz SS, Bollen A, Moguilevsky N, Friedman H, Lefkowitz DL. Alveolar Macrophage Activation by Myeloperoxidase: a Model for Exacerbation of Lung Inflammation. Am J Respir Cell Mol Biol. 2002;26:716–722. doi: 10.1165/ajrcmb.26.6.4723. [DOI] [PubMed] [Google Scholar]
- 9.Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- 10.Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol Res. 2009;43:25–61. doi: 10.1007/s12026-008-8049-6. [DOI] [PubMed] [Google Scholar]
- 11.Rosseau S, Hammerl P, Maus U, Walmrath HD, Schütte H, Grimminger F, Seeger W, Lohmeyer J. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2000;279:L25–35. doi: 10.1152/ajplung.2000.279.1.L25. [DOI] [PubMed] [Google Scholar]
- 12.Maus U, Herold S, Muth H, Maus R, Ermert L, Ermert M, Weissmann N, Rosseau S, Seeger W, Grimminger F, Lohmeyer J. Monocytes recruited into the alveolar air space of mice show a monocytic phenotype but upregulate CD14. Am J Physiol Lung Cell Mol Physiol. 2001;280:L58–68. doi: 10.1152/ajplung.2001.280.1.L58. [DOI] [PubMed] [Google Scholar]
- 13.Maus U, Herold S, Muth H, Maus R, Ermert L, Ermert M, Weissmann N, Rosseau S, Seeger W, Grimminger F, Lohmeyer J. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med. 2002;166:268–73. doi: 10.1164/rccm.2112012. [DOI] [PubMed] [Google Scholar]
- 14.Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology. 2005;210:153–60. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel I, Roth J, Sorg C. Identification of a novel surface molecule, RM3/1, that contributes to the adhesion of glucocorticoid-induced human monocytes to endothelial cells. Eur J Immunol. 1996;26:2758–2763. doi: 10.1002/eji.1830261131. [DOI] [PubMed] [Google Scholar]
- 16.Zwadlo-Klarwasser G, Bent S, Haubeck HD, Sorg C, Schmutzler W. Glucocorticoid-induced appearance of the macrophage subtype RM 3/1 in peripheral blood of man. Int Arch Allergy Appl Immunol. 1990;91:175–180. doi: 10.1159/000235111. [DOI] [PubMed] [Google Scholar]
- 17.Franzè E, Caruso R, Stolfi C, Sarra M, Cupi ML, Caprioli F, Monteleone I, Zorzi F, De Nitto D, Colantoni A, Biancone L, Pallone F, Monteleone G. Lesional accumulation of CD163-expressing cells in the gut of patients with inflammatory bowel disease. PLoS One. 2013;8:e69839. doi: 10.1371/journal.pone.0069839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taichman NS, Young S, Cruchley AT, Taylor P, Paleolog E. Human neutrophils secrete vascular endothelial growth factor. J Leukoc Biol. 1997;62:397–400. doi: 10.1002/jlb.62.3.397. [DOI] [PubMed] [Google Scholar]
- 19.Mura M, Binnie M, Han B, Li C, Andrade CF, Shiozaki A, Zhang Y, Ferrara N, Hwang D, Waddell TK, Keshavjee S, Liu M. Functions of type II pneumocyte-derived vascular endothelial growth factor in alveolar structure, acute inflammation, and vascular permeability. Am J Pathol. 2010;176:1725–34. doi: 10.2353/ajpath.2010.090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Signorelli S, Jennings P, Leonard MO, Pfaller W. Differential effects of hypoxic stress in alveolar epithelial cells and microvascular endothelial cells. Cell Physiol Biochem. 2010;25:135–44. doi: 10.1159/000272066. [DOI] [PubMed] [Google Scholar]


