Abstract
Genetic polymorphisms are important factors in effects and toxicity of chemotherapeutics. This study aimed to investigate whether there was a correlation between genotype or haplotype of inosine triphosph pyrophosphohydrolase(ITPA) and toxicities during maintenance therapy with mercaptopurine (6-MP) in Chinese patients with acute lymphoblastic leukemia (ALL). 95 ALL children who hospitalized between October 2004 and September 2007,were retrospectively analyzed. 6-MP toxicity was documented according to Common Toxicity Criteria, Version 2.0. ITPA sequencing was undertaken. Correlation between genotype/haplotype and 6-MP toxicity was analyzed. The results indicated that 50 cases (52.6%) had grade III-IV of bone marrow inhibition. These children had long-term disease-free survival (DFS), without hepatic and other organs’ dysfunction and secondary tumors. Three variations were observed in ITPA exon 2 (94 C → A), exon 3 (138 G → A), and exon 8 (561 G → A), the 94A carriers (CA and AA) had a lower risk of developing 6-MP toxicity when compared with carriers of the CC genotype (odds ratio [OR] 0.34, 95% confidence interval [CI] 0.12-0.98, P = 0.039). The risk of 6-MP intolerance was decreased in patients with 138 allele and 561 allele polymorphism, but with no significant difference. Patients carrying the haplotype 94A-138A-561A was tolerance compared to those with wild-type haplotype 94C-138G-561G (OR: 0.26, 95% CI 0.07-0.94 P = 0.043). In conclusion, the risk of 6-MP intolerance was decreased in patients with 138 allele and 561 allele polymorphism, but without significant difference. Patients carrying the haplotype 94A-138A-561A was tolerance compared to those with the wild-type haplotype 94C-138G-561G.
Keywords: ITPA, ALL children, mercaptopurine related toxicity
Introduction
Six-mercatopurine (6-MP) has been employed as a backbone agent in the whole course of chemotherapy for childhood acute lymphoblastic leukemia (ALL) [1,2]. Our previous studies have suggested that the tolerance to 6-MP varies very much depending on individuals, and that bone marrow inhibition and hepatic damage after standard doses of 6-MP in Chinese patients were more common than in Western patients, however the pathogenesis remains unclear [3]. Although genetic polymorphisms in the gene encoding human thiopurine methyltransferase (TPMT) are known to have a marked effect on mercaptopurine metabolism and toxicity, our previous study demonstrated that almost all of Chinese ALL patients with wild-type TPMT [3]. To explore the potential causes of individual differences in tolerance to mercaptopurine (6-MP) in Chinese ALL patients, we recently studied the effects of polymorphism in another gene encoding an enzyme involved in mercaptopurine metabolism, inosine triphospate pyrophosphatase (ITPA), which shows that genetic polymorphism of ITPA is a significant determinant of mercaptopurine metabolism in Chinese patients. In this study, we analyzed the Inosine Triphosphate Pyrophosphohydrolase (ITPA) polymorphic sequence variants in Chinese ALL children and possible association with severe intolerance to 6-MP.
Materials and methods
Criteria of patient entry
(i) All children with ALL hospitalized between October 1 in 2004 and September 30, 2007 entered this study; (ii) The ALL diagnosis standards, risk stratification and chemotherapy regimen were based on the BCH-ALL-2003 [4]; (iii) The 6-MP toxicity were documented according to Common Toxicity Criteria, Version 2.0, where all children were at the stage of in marrow remission and received >12-monthed maintenance chemotherapy; and (iv) All childrenwere evaluated for the CBC, bone marrow smear, immune function and abdomen B-Utrasonic scan every 3 months within the first year, and for marrow smear and the unrecovered parameters every 6 months within the second and third years after the whole chemotherapy was ended. (v) The ethical concern according to IRB approval was confirmed by their parents. We will do our best to make sure that the personal information in patient’s medical record will be kept private. Patient’s name and other personal information will not be used.
Protocol of 6-MP administration
For all children with entry, 6-MP (Shanghai Hualian Pharmaceutical Factory) was administered in the stage of maintenance chemotherapy with 6-MP (50 mg/m2.d, po, qn) and MTX (20 mg/m2/w, im) combined. All patients were followed up at the clinic until March 31, 2013.
Sequencing of ITPA gene fragment
Mononuclear cells were separated from 2 ml of heparin-anticoagulated bone marrow and then frozen at -70°C until use. DNA was extracted using a BloodGen Midi Kit (CWBIO) from peripheral blood, which was collected in remission states. ITPA fragment was amplified by PCR using the primers showed in Table 1. In brief, each 50-μl PCR consisted of ABI buffer II, MgCl2 (1.5 mM), dNTPs (0.2 mM/each), primer 10 pmol, cDNA 0.2 μg, Taq DNA polymerase 1 U. PCR amplification starts with an initial denaturation at 95°C for 5 min, continued with 30 cycles of 95°C for 15 s, 55-60°C for 15 s and 72°C for 30 s. Final extension was performed at 72°C for 5 min. The amplified segments were sent to sequencing directly using ABI 3730 sequencer. Sequence analysis and blast were completed using DNAstart software (accomplished by Joy orient translational medicine research center Co., Ltd).
Table 1.
Primes for ITPA exons and introns (2 and 3) amplification
| Primer | Sequences | length |
|---|---|---|
| ITPA-1-F | CCGGTTCTTGAAGATAGCGTCCCT | 518 |
| ITPA-1-R | CCCCGATTCTGCAGCCACTCCC | |
| ITPA-2-F | CGTAGAAGAGATAGAGAAGCAAGG | 1292 |
| ITPA-2-R | CTCCACTTACAGGTCGCATTC | |
| ITPA-3-F | CCTGGCTCTTGGAGGCATTCT | 256 |
| ITPA-3-R | GCTAGAACCTGGGACTCCTGATTACTA | |
| ITPA-4-F | TTGTATCGTCAGCCTGTGCCAGAG | 715 |
| ITPA-4-R | GCTTTGGGAATTGTCACCTTCAGTTTG | |
| ITPA-5-F | CCTTGTATTTTCAGGAACGCTGTCA | 786 |
| ITPA-5-R | GAAAGGCGAAACCACTGGCTACACA | |
| ITPA-6-F | AATGACTTTTGGGCACACTAGAGCA | 879 |
| ITPA-6-R | AACACTCCAGCTCACAGTGGCATC |
General clinical data
Among 95 children with ALL eligible for the study with gender male 55, female 40, and media age 68 months (range 18-188 months).
Statistical analysis
The allele frequencies of all polymorphisms were tested for Hardy-Weinberg equilibrium using the χ2 test. Toxicities were represented by the value 1 or 0, indicating whether an adverse event did or did not occur during the 6-mp therapy. Statistical associations between the susceptibility to toxicity and the ITPA polymorphisms were analyzed using logistic regression. Haplotype analysis and linkage disequilibrium analysis were assessed using the SNPStats web software.
Results
Toxic response to 6-MP
Fifty cases (52.6%) had III-IV grade of bone marrow inhibition, consisting of 39 cases (39/61 = 63.9%) with single marrow toxicity and 9 cases with marrow and hepatic toxicities. Twelve cases (19.7%) had single hepatic toxicity.Types of toxicity responses (severe intolerance to 6-MP) were not relevant to age, gender and WBC counts.
Follow-up and survival rate
All 95 children with ALL were followed up until March 31, 2013, with median 79 months (18-100.5 months). Expect for 8 cases of relapse, the remaining 87 children with ALL underwent chemotherapy discontinuation and survived until now. The period for immune function recovery was 6-12 months, for CBC recovery 2-4 months, for hepatic function recovery 3-6 months, and for fatty liver 0.5-1.5 years. Fifty cases (52.6%) had III-IV grade of bone marrow inhibition, and had been followed up for 6 years. The long-term disease-free survival (DFS) achieved 52.0% in these children, without hepatic and other organs’ dysfunction and secondary tumors (Figure 1).
Figure 1.
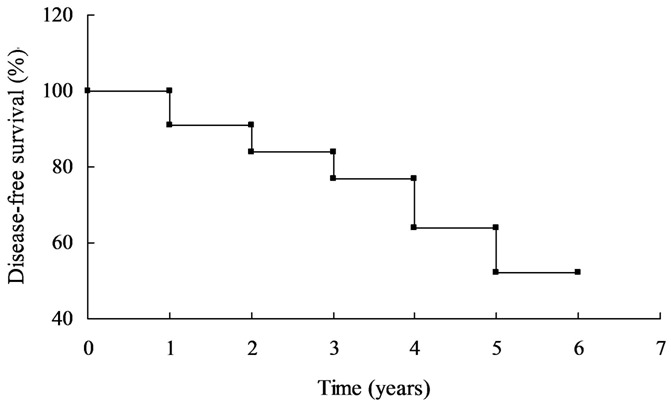
Long-term disease-free survival (DFS) of the ALL children.
Relationship between ITPA genotype and 6-mp tolerance
Table 2 shows the risk evaluation of 6-mp tolerance calculated by univariate analysis. Polymorphism was found to be related to 6-mp tolerance. Our data show that 6-mp intolerance was 0.4-fold decreased in the patients with the heterozygous (CA), when compared to patients with the wild-type genotype (CC). In addition, patients carrying the 94A allele (CA + AA) had a 0.34-fold decreased risk of 6-mp intolerance (P = 0.039). The risk of 6mp intolerance was decreased in patients with the 138 allele and 561 allele polymorphism, but with no significant difference.
Table 2.
Logistic regression analysis of association between ITPA genotype and 6-mp tolerance
| Polymorphism (n) | Tolerance | Intolerance | OR | P value |
|---|---|---|---|---|
| 94C>A | ||||
| CC | 32 (71.1%) | 44 (88%) | reference | |
| CA | 11 (24.4%) | 6 (12%) | 0.40 (0.13-1.18) | 0.11 |
| AA | 2 (4.4%) | 0 (0%) | 0.0 (0.0-NA) | 0.081 |
| CA + AA | 13 (28.9%) | 6 (12%) | 0.34 (0.12-0.98) | 0.039 |
| 138G>A | ||||
| A/A | 18 (40%) | 11 (22%) | reference | |
| G/A | 20 (44.4%) | 25 (50%) | 2.05 (0.79-5.31) | 0.08 |
| G/G | 7 (15.6%) | 14 (28%) | 3.27 (1.01-10.62) | 0.11 |
| GA + AA | 38 (84.4%) | 36 (72%) | 2.11 (0.76-5.83) | 0.14 |
| 561G>A | ||||
| G/G | 10 (22.2%) | 13 (26%) | reference | |
| G/A | 22 (48.9%) | 28 (56%) | 0.98 (0.36-2.65) | 0.49 |
| A/A | 13 (28.9%) | 9 (18%) | 0.53 (0.16-1.74) | 0.21 |
| GA + AA | 35 (77.8%) | 37 (74%) | 0.81 (0.32-2.09) | 0.67 |
Relationship between 6-mp tolerance and haplotype
In this study, we analyzed the haplotype frequency, and all of the heplotype frequency was listed in Table 3.
Table 3.
Haplotype frequencies estimation (n = 95)
| SNP1 | SNP2 | SNP3 | Total | Cumulative frequency |
|---|---|---|---|---|
| C | G | G | 0.3982 | 0.3982 |
| C | A | A | 0.3311 | 0.7293 |
| A | A | A | 0.104 | 0.8333 |
| C | A | G | 0.1005 | 0.9338 |
| C | G | A | 0.0597 | 0.9934 |
| A | A | G | 0.0066 | 1 |
| A | G | A | 0 | 1 |
| A | G | G | 0 | 1 |
Table 4 showed the association between 6mp tolerance and the possession of each haplotype, which was analyzed using SNPStats. Patients carrying the haplotype 94A-138A-561A was tolerance compared to patients with the wild-type haplotype 94C-138G-561G (OR: 0.26, 95% CI 0.07-0.94 P = 0.043).
Table 4.
Haplotype association with response (n = 95, crude analysis)
| SNP1 | SNP2 | SNP3 | Freq | OR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| 1 | C | G | G | 0.3983 | 1 | --- |
| 2 | C | A | A | 0.3319 | 0.73 (0.36-1.50) | 0.4 |
| 3 | A | A | A | 0.1032 | 0.26 (0.07-0.94) | 0.043 |
| 4 | C | A | G | 0.0996 | 0.51 (0.17-1.57) | 0.24 |
| 5 | C | G | A | 0.0596 | 1.27 (0.37-4.34) | 0.7 |
| rare | A | G | G | 0.0073 | 0.44 (0.00-222.91) | 0.8 |
Global haplotype association p-value: 0.17.
Discussion
Although the long-term DFS of childhood ALL has reached up to 80% following employment of the effective combined chemotherapy [5-7], the toxicity of chemotherapeutic agents also impact the prognosis. Our study has suggested that 6-MP tolerance varies very much between individuals with ALL. Approximately 50% of ALL children are intolerant to standard doses of 6-MP and manifest 1/4 grade of toxicity, and correspondingly the chemotherapy must be discontinued, otherwise, repetitively modified to avoid occurrence of more severe toxicity. In this study, 95 children with ALL For 50 6-MP-intolerant children with ALL, 6-MP toxicity assumed different characteristics. Bone marrowtoxicity occurred in more than 3/4 of patients, companied with toxicity in a minority of patients. Thus, CBC and liver function testing should not be employed as objective parameters for modifying 6-MP doses. The mechanism remainsto be explored in the future.
Neutropenia and consequent respiratory infection are the highlighting complications in the course of 6-MP-based maintenance chemotherapy [8,9], whereas severe hemorrhage and intermediate to severe anemia were rare. Recurrent or continuous liver dysfunction may be treated with liver protective agents [10-12]. Except for 8 cases relapsed, the remaining ALL children did not receive chemotherapy and survived until now, with CBC, liver function and immune function recovered, without severe 6-MP toxicity and secondary tumor.
So far, these children have been followed up for 6 years, with estimated DFS up to 52.0%. Certainly, the follow-up should be continued to evaluate long-term effect of severe 6-MP intolerance.
In conclusion, the risk of 6-MP intolerance was decreased in patients with the 138 allele and 561 allele polymorphism, but with no significant difference. Patients carrying the haplotype 94A-138A-561A was tolerance compared to patients with the wild-type haplotype 94C-138G-561G.
Acknowledgements
This study was granted by Special Funds of the National Natural Science Foundation of China (Grant No. 81141038) and thank Dr. Weiyue Gu from Joy orient translational medicine research center for the sequences analysis and blast.
Disclosure of conflict of interest
None.
References
- 1.Leei CK, Lin WL, Han CP. Monoclonal antibody Cam 5.2 targeted mainly CK8, but not CK18--Comment on: “Chromophobe renal cell carcinoma with liposarcomatous dedifferentiation--report of a unique case. Int J Clin Exp Pathol. 2010 May 5; 3(5): 534-40.”. Int J Clin Exp Pathol. 2010;3:742. [PMC free article] [PubMed] [Google Scholar]
- 2.Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, Wartman LD, Lamprecht TL, Liu F, Xia J, Kandoth C, Fulton RS, McLellan MD, Dooling DJ, Wallis JW, Chen K, Harris CC, Schmidt HK, Kalicki-Veizer JM, Lu C, Zhang Q, Lin L, O’Laughlin MD, McMichael JF, Delehaunty KD, Fulton LA, Magrini VJ, McGrath SD, Demeter RT, Vickery TL, Hundal J, Cook LL, Swift GW, Reed JP, Alldredge PA, Wylie TN, Walker JR, Watson MA, Heath SE, Shannon WD, Varghese N, Nagarajan R, Payton JE, Baty JD, Kulkarni S, Klco JM, Tomasson MH, Westervelt P, Walter MJ, Graubert TA, DiPersio JF, Ding L, Mardis ER, Wilson RK. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150:264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma XL, Wang B, Guo HY, Zhang YH, Zhu GH, Duan YL. Tolerability of 6-mercaptopurine in children with acute lymphoblastic leukemia. Zhonghua Er Ke Za Zhi. 2010;48:289–292. [PubMed] [Google Scholar]
- 4.Common Toxicity Criteria, Version 2.0, Common Toxicity Criteria Manual. 1999. Jun 1, Cancer Therapy Evaluation Program. [Google Scholar]
- 5.Sliwa T, Awsa S, Vesely M, Rokitte D, Grossschmidt P, Jilch R, Ulrich W, Geissler K. Hyperexpression of NOTCH-1 is found in immature acute myeloid leukemia. Int J Clin Exp Pathol. 2014;7:882–889. [PMC free article] [PubMed] [Google Scholar]
- 6.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 7.Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011;89:387–391. doi: 10.1038/clpt.2010.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terada T. Acute onset pyothorax-associated lymphoma (PAL) with inflammatory features. Int J Clin Exp Pathol. 2012;5:163–166. [PMC free article] [PubMed] [Google Scholar]
- 9.Higgs JE, Payne K, Roberts C, Newman WG. Are patients with intermediate TPMT and ITPA activity at increased risk of myelosuppression when taking thiopurine medications? Pharmacogenomics. 2010;11:177–188. doi: 10.2217/pgs.09.155. [DOI] [PubMed] [Google Scholar]
- 10.Stoccob G, Kristine RC, William EE. Genetic polymorphism of inosinetriphosphate -pyrophosphatase influences mercaptopurine metabolism and toxicity during treatment of acute lymphoblastic leukemia individualized for thiopurine-S-methyl-transferase status. Expert Opin Drug Saf. 2010;9:23–37. doi: 10.1517/14740330903426151. [DOI] [PubMed] [Google Scholar]
- 11.Orsolic N, Benkovic V, Lisicic D, Dikic D, Erhardt J, Knezevic AH. Protective effects of propolis and related polyphenolic/flavonoid compounds against toxicity induced by irinotecan. Med Oncol. 2010;27:1346–1358. doi: 10.1007/s12032-009-9387-5. [DOI] [PubMed] [Google Scholar]
- 12.Levran O, Awolesi O, Linzy S, Adelsom M, Kreek MJ. Haplotype block structure of the genomic region of the mu opioid receptor gene. J Hum Genet. 2011;56:147–155. doi: 10.1038/jhg.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]


