Abstract
Chemical profiling is always the first task in the standardization and modernization of Traditional Chinese Medicine. HPLC and LC-MS were employed to find out the common chromatographic peaks in various batches of Tianshu Capsule (TSC) and the contribution of the characteristic peaks from individual herbs to the whole chromatographic profile of TSC sample. A total of 38 constituents were identified in TSC sample based on the comparison of retention time and UV spectra with authentic compounds as well as by summarized MS fragmentation rules and matching of empirical molecular formula with those of published components. This is the first systematic report on the chemical profiling of the commercial TSC product, which provides the sufficiently chemical evidence for the global quality evaluation of TSC products.
1. Introduction
Traditional Chinese Medicine (TCM) is getting more attention all over the world due to its exact clinical practice, especially prescription application, which comprehensively highlights the quintessence of the theory of traditional Chinese medical science. Da Chuan Xiong Fang (DCXF), a well-known and extensively used TCM decoction for the treatment of migraine, first appeared in Xuan Ming Lun Fang, a famous formula book written by Wansu Liu who lived in Jin Dynasty (1115–1234). It is composed of two herbs, namely, Chuanxiong (Chuanxiong rhizoma) and Tianma (Gastrodiae rhizoma), with a crude weight ratio of 4 : 1. Eight dosage forms of DCXF such as capsule, tablet, dripping-pill, honeyed pill, oral liquid, and granule, have been authorized to Chinese market. Tianshu Capsule (TSC), as a representative of DCXF preparations, is widely used in clinics for treating the blood stasis type of headache and migraine [1, 2].
Phytochemical and pharmacological investigations showed that phenols, organic acids, phthalides, and nitrogen-containing compounds were the major active ingredients of DCXF [3]. Several qualitative analyses have been reported concerning main types of constituents in DCXF [4–7]. One study described the identification of 17 different constituents in the 50% EtOH extract of DCXF by LC-Q-TOF/MS, containing gastrodin, parishin C, ferulic acid, guanosine, adenosine, palmitic acid, and 11 phthalide compounds [4]. Another similar study identified 3 compounds of Chuanxiong (ferulic acid, senkyunolide I, and senkyunolide H) and 8 constituents of Tianma (gastrodin, s-(4-hydroxybenzyl)-glutathione, parishin, parishin B, parishin C, p-hydroxybenzaldehyde, etc.) by using the HPLC-DAD-MSn coupling technique, respectively [5]. Continuous reports from the second research group confirmed that 10 different compounds, including 6 original substances of Chuanxiong and 4 original ones of Tianma, were detected in the rat plasma after the gavage of DCXF active components [6, 7]. However, all these investigations mentioned above were carried out based on the samples of the 50% EtOH extract of the mixture of both herbs (4 : 1) or active components of single crude herb. No systematic reports could be available involving the chemical profiling of the commercial finished products derived from DCXF. TSC was produced from both crude herbs by employing the various pharmaceutical engineering technologies and the complex manufacturing processes such as extraction, concentration, and preparation. The accumulating studies showed that decocting could induce chemical changes of medicinal herbs or combinatorial formula [8]. It is well-known that ferulic acid and some of the phthalides such as Z-ligustilide and dimeric phthalide are unstable at high temperature [9, 10]. Therefore, during the preparation of TSC, these thermolabile components may undertake chemical transformation, consequently leading to the difference of chemical compositions of finished products with DCXF decoction. Understanding the chemical profiles of TSC samples would be helpful in selecting suitable chemical markers for the quality control and pharmacokinetic study. In this work, a combination of HPLC, LC-DAD-MSn, and LC-DAD-ESI-IT-TOF/MS analyses was employed to find out and identify the common components in various batches of commercial TSC samples. The contribution of the characteristic peaks from individual herb to the whole chemical profiling of TSC was also discussed. A total of 38 constituents were identified or tentatively characterized, among which the water-soluble compounds with higher polarity from Gastrodiae rhizoma are detected in TSC samples for the first time.
2. Materials and Methods
2.1. Materials and Reagents
Five batches of Tianshu Capsules and related crude herbal materials (Chuanxiong rhizoma and Gastrodiae rhizoma) were provided by Kanion Pharmaceutical (Lianyungang, China). The reference substances of gastrodin (Lot. 110807-200205), 5-hydroxymethyl-2-furfural (5-HMF, Lot. 111626-201007), ferulic acid (Lot. 0773-9607), and Z-ligustilide (Lot. 111737-201102) were purchased from the National Institutes for Food and Drug Control (Beijing, China). Parishin B (174972-79-3), parishin C (174972-80-6), and parishin (62499-28-9) were from the collection of Dr. Li Wang, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (Dalian, China). The purity for each of these compounds was over 98% by HPLC assay and their structures were shown in Figure 1. HPLC grade methanol (Fisher, Fair Lawn, NJ, USA) and ultrapure water were used for HPLC analyses. All other chemical reagents were of analytical grade from Beijing Chemical Corporation (Beijing, China).
Figure 1.
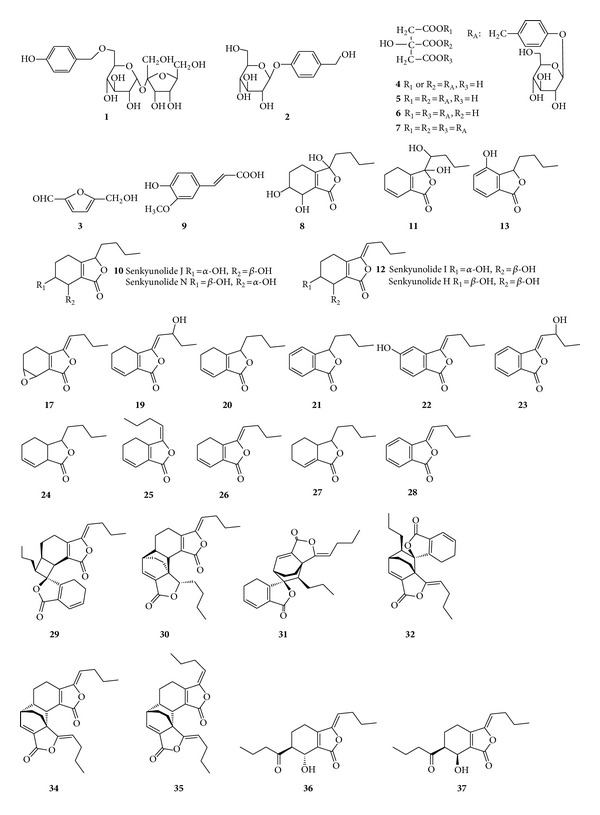
The structures of the identified compounds in TSC sample.
2.2. Sample Preparation
2.2.1. TSC Sample
1.0 g of pulverized contents of TSC samples was extracted with aqueous methanol (MeOH-H2O, 1 : 1, 25 mL) by ultrasonication (250 w, 40 kHz) for 30 min at room temperature, and the extract was then centrifuged for 10 min at 14800 rpm. A volume of 10 μL of the supernatant was used for HPLC and LC-DAD-MSn analysis.
2.2.2. Extraction of Chuanxiong Rhizoma, Gastrodiae Rhizoma, and DCXF
The ethanolic and aqueous extracts of individual herb (Chuanxiong rhizoma and Gastrodiae rhizoma) and the mixture of both herbs (w/w, 4 : 1, DCXF) were prepared according to the manufacturing processes of TSC (Figure S1 in the Supplementary Material available online at http://dx.doi.org/10.1155/2014/580745) described in the current Chinese pharmacopoeia. The ethanolic and aqueous extracts were diluted with aqueous methanol (MeOH-H2O, 1 : 1) to the concentration of 0.05 g·mL−1 and then centrifuged for 10 min at 14800 rpm. Each of the supernatants was used for HPLC and LC-MS analyses.
2.2.3. Reference Solution
Stock solutions with a concentration of about 0.010 mg·mL−1 were prepared by dissolving an accurately weighed amount of each reference substance in aqueous methanol (MeOH-H2O, 1 : 1). The mixture of 7 reference solutions was prepared from the stock solutions.
2.3. Qualitative HPLC Analyses of 5 Batches of TSC Samples
The analyses were performed on a Shimadzu HPLC system (Shimadzu, Japan) equipped with a LC-20AT binary pump, a DGU-20A5 degasser, a SIL-20AC autosampler, a CTO-20AC column oven, and a SPD-M20A photodiode array detector. The samples were separated on a Phenomenex Luna C18 column (5 μm, 4.6 × 250 mm). The mobile phase consisted of methanol (A) and water containing 0.1% formic acid (B) using a gradient program as follows: 0 min, 15% A; 5 min, 15% A; 55 min, 95% A; 60 min, 95% A. The flow rate was 1.0 mL·min−1 and the column temperature was set at 30°C. The PDA detector recorded UV spectra in the range from 190 nm to 400 nm and HPLC chromatogram was monitored at 276 nm.
2.4. Comparison of Typical TSC Sample and Its Related Crude Herbal Materials as Well as DCXF by HPLC and LC-MS
TSC sample and its related crude herbal material, Chuanxiong rhizoma and Gastrodiae rhizoma as well as DXCF, were analyzed under the same chromatographic conditions by HPLC and LC-MS to find the contribution of individual herb to the whole chemical profile of TSC sample. The HPLC system was the same as those in Section 2.3. LC-MS analyses were performed using an Agilent 6130 Quadrupole LC-MS (Agilent, Waldbronn, Germany) connected to an Agilent 1200 HPLC system (Agilent, Waldbronn, Germany). The parameters for MS analysis in the positive and negative ion mode were as follows: nebulizer, 35 psi; ionization voltage, 3500 V; dry temperature, 350°C; flow rate of carrier gas, 9.0 L·min−1. Full-scan mass spectra were acquired in the range of 100–800 m/z.
2.5. LC-DAD-ESI-IT-MSn Analysis of Typical TSC Sample
To comprehensively identify the chemical constituents in TSC sample by the fragmentation rules, a LC-DAD-ESI-IT-MSn experiment was performed using an Agilent 6320 ion-trap spectrometer (Agilent, Waldbronn, Germany) connected to an Agilent 1200 HPLC system (Agilent, Waldbronn, Germany). The HPLC conditions were the same as those described in Section 2.3. The LC effluent was introduced into an electrospray ionization source after a postcolumn split ratio of 2 : 1. The parameters for MS analysis in the positive ion mode were as follows: nebulizer, 45 psi; ionization voltage, 4000 V; dry temperature, 350°C; flow rate of carrier gas, 12.0 L·min−1. Full-scan mass spectra were acquired in the range of 100–800 m/z. The optimized parameters for MS/MS analysis were as follows: collision energy, 1.5 V; nitrogen was used as the collision gas. MSn spectra of pure substances were obtained using the same parameters as mentioned above.
2.6. LC-DAD-ESI-IT-TOF/MS of Analysis of Typical TSC Sample
To confirm the elemental composition of precursor ions and their fragments with high-accurate mass, a LC-ESI-IT-TOF/MS experiment was performed on a Shimadzu LC-MS-IT-TOF instrument equipped with a Shimadzu UFLCXR HPLC system (Shimadzu, Kyoto, Japan). The HPLC system consisted of a CBM-20A controller, two LC-20AD binary pumps, an SPD-M20A diode array detector, an SIL-20AC autosampler, a CTO-20A column oven, and a DGU-20A3 degasser. The HPLC conditions were the same as those for HPLC-DAD-ESI-MSn analysis. The LC effluent was directed into the ESI source as a rough split ratio of 3 : 1. The optimized MS conditions were as follows: positive and negative ion mode; electrospray voltage, +4.5 kV/−3.5 kV; detector voltage, 1.65 kV; curved desolvation line (CDL) temperature, 200°C; heat block temperature, 200°C; nebulizing gas (N2), 1.5 L·min−1; drying gas (N2), 10 L·min−1; scan range, m/z 100–1100 for MS1, 100–800 for MS2, and 100–500 for MS3. The ultrahigh purity argon was used as the collision gas for collision-induced dissociation (CID) experiments, and the collision energy was set at 50% for MS2 and MS3; ion accumulated time was 30 ms. The MSn data were collected in an automatic mode and the software could automatically select precursor ions for MSn analysis according to criteria settings. Accurate mass determination was corrected using the external standard method. The data acquisition and analysis were performed by LC-MS Solution Version 3.6 software (Shimadzu, Kyoto, Japan).
3. Results and Discussion
3.1. Qualitative Analyses of TSC and Its Related Crude Herbal Materials by HPLC and LC-MS
Under the HPLC conditions as described in the current Chinese Pharmacopoeia [1], 5 batches of TSC samples, together with the reference compounds, were examined and their HPLC chromatograms were shown in Figure S2. High similarity in the number, type, and amount of chemical constituents was observed in the HPLC profiles of different batches of TSC samples. General chromatographic profile was obtained by Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine software and characteristic peaks were found in the HPLC profile of each individual sample.
In order to identify the origin of these characteristic peaks from individual herbs, a comparative study was carried out by using various extracts of herbs and TSC samples. Accordingly, the possible individual contribution from the corresponding herbs to the general chromatographic profile was found. Compared with the HPLC profiles of the ethanolic and aqueous extracts of Chuanxiong rhizoma and Gastrodiae rhizome (Figure S3), 29 of 38 peaks occurring in HPLC profile of TSC sample were contributed by Chuanxiong rhizoma and other 9 peaks came from Gastrodiae rhizome (Figures S4 ~ S10). In addition to the comparison of retention time and on-line UV spectra with those of reference compounds gastrodin, 5-HMF, parishin B, parishin C, parishin, ferulic acid, and Z-ligustilide, the precursor ions obtained by the positive and negative LC-MS (Figure 2) such as [M+H]+, [M+NH4]+, [M+Na]+, and [M-H]− further confirmed the contribution of the characteristic peaks from individual herbs to the chromatographic profile of TSC sample (Table 1).
Figure 2.
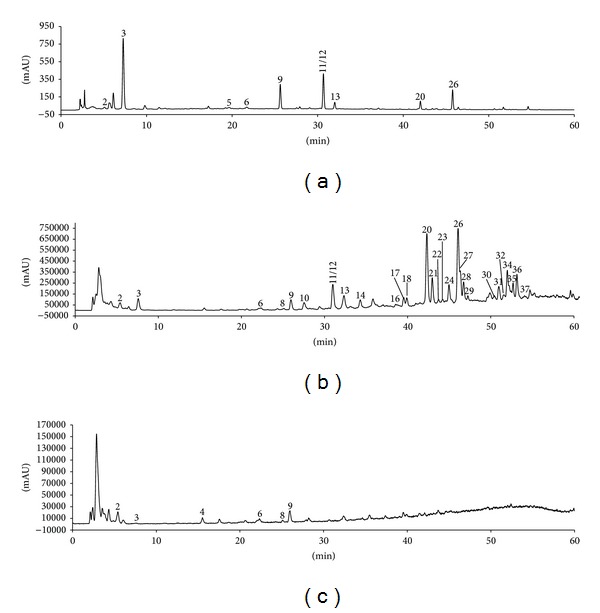
HPLC and TIC of typical TSC sample obtained using an Agilent 6130 Quadrupole LC-MS connected to an Agilent 1200 HPLC system. (a) HPLC (276 nm), (b) (+) TIC, and (c) (−) TIC.
Table 1.
Retention time (t R), UV, and LC-MS data of the identified compounds in Tianshu Capsule and related crude materials.
| Number | t R/min | UV λ max/nm | MW | [M − H]− | [M + H]+ | [M + NH4]+ | [M + Na]+ | [2M + Na]+ | [M + HCOO]− | Tianma | Chuanxiong | Identified compounds |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 5.403 | 221, 276 | 286 | 304 | 331 | + | − | Gastrodin | ||||
| 3 | 7.736 | 230, 283 | 126 | 127 | 144 | 149 | + | − | 5-HMF | |||
| 4 | 15.579 | 225 | 460 | 459 | 478 | 483 | + | − | Parishin E/G | |||
| 5 | 20.691 | 221, 272 | 728 | 727 | 746 | 751 | + | − | Parishin B | |||
| 6 | 22.220 | 221, 274 | 728 | 727 | 746 | 751 | + | − | Parishin C | |||
| 8 | 25.080 | 218 | 242 | 241 | 243 | 265 | 507 | − | + | 3-Butyl-3,6,7-trihydroxy-4,5,6,7 -tetrahydrophthalide |
||
| 9 | 25.567 | 220, 236, 323 | 194 | 193 | 195 | 217 | + | + | ferulic acid | |||
| 10 | 27.529 | 220, 330 | 226 | 227 | 249 | 475 | − | + | Senkyunolide J/N | |||
| 11 | 30.667 | 280 | 224 | 225 | 247 | 471 | − | + | 4,5-Dihydro-3,1′-dihydroxy-3 -butylphthalide |
|||
| 13 | 32.436 | 275 | 206 | 205 | 207 | − | + | 4-hydroxyl-3-butylphthalide | ||||
| 14 | 34.299 | 325 | 396 | 397 | + | + | unidentified | |||||
| 16 | 38.635 | 220, 270 | 222 | 221 | 223 | 245 | − | + | Senkyunolide D or 4,7-dihydroxy-3-butylidenephthalide |
|||
| 17 | 39.502 | 230, 310 | 206 | 205 | 207 | 229 | 435 | − | + | Z-6,7-Epoxyligustilide | ||
| 18 | 39.898 | 280 | 208 | 207 | 209 | 231 | 439 | − | + | Senkyunolide K/G | ||
| 19 | 41.465 | 220 | 206 | 205 | 207 | 229 | 435 | − | + | Senkyunolide F | ||
| 20 | 42.332 | 220, 240, 325 | 192 | 193 | 215 | 407 | + | + | Senkyunolide A | |||
| 21 | 42.956 | 226, 280 | 190 | 191 | 208 | 213 | 403 | − | + | Butylphthalide | ||
| 22 | 43.717 | 218, 270 | 204 | 203 | 205 | 227 | 431 | 249 | − | + | Senkyunolide C | |
| 23 | 44.147 | 210, 285, 330 | 204 | 203 | 205 | 227 | 431 | 249 | − | + | Senkyunolide E | |
| 24 | 44.949 | 220, 270, 330 | 194 | 195 | 217 | 411 | − | + | Cnidilide | |||
| 26 | 45.831 | 205, 280, 328 | 190 | 191 | 213 | 403 | − | + | Ligustilide | |||
| 27 | 46.394 | 220 | 194 | 195 | 217 | 411 | − | + | Neocnidilide | |||
| 28 | 46.653 | 210, 275, 330 | 188 | 189 | 206 | 211 | 399 | − | + | 3-Butylidenephthalide | ||
| 29 | 47.231 | 225, 280, 310 | 380 | 381 | 398 | 403 | − | + | Riligustilide | |||
| 30 | 50.042 | 276 | 382 | 383 | 399 | 405 | − | + | Senkyunolide P | |||
| 31 | 50.476 | 220, 280 | 380 | 381 | 398 | 403 | − | + | 3′,6,8′,3a-Biligustilide | |||
| 32 | 50.970 | 220, 280 | 380 | 381 | 398 | 403 | − | + | Tokinolide B | |||
| 33 | 51.065 | 230, 279 | 382 | 383 | 400 | 405 | − | + | Unidentified | |||
| 34 | 51.962 | 220, 275 | 380 | 381 | 403 | − | + | Levistolide A | ||||
| 35 | 52.267 | 220, 285 | 380 | 381 | − | + | 3′Z-3,3′a,8,6′a-Biligustilide | |||||
| 36 | 52.693 | 220, 280 | 278 | 279 | 301 | 579 | − | + | Senkyunolide M | |||
| 37 | 53.134 | 220, 280 | 278 | 279 | 301 | 579 | − | + | Senkyunolide Q | |||
| 38 | 53.852 | 225, 278 | 382 | 383 | 400 | 405 | 383 | − | + | Unidentified |
3.2. Identification of Chemical Constituents in TSC by LC-DAD-EST-IT-MSn and LC-DAD-ESI-IT-TOF/MS
The combination of LC-DAD-ESI-IT-MSn and LC-DAD-ESI-IT-TOF/MS experiments was employed for the identification of chemical constituents in TSC sample, and, as a result, a total of 38 compounds was identified or tentatively characterized. The structures of the identified compounds are shown in Figure 1 and their chromatographic and mass spectrometric data are shown in Tables 2 and 3. The total ion chromatograms (TICs) of TSC sample are shown in Figures S11 and S12, respectively. Among the identified constituents, 7 compounds (2, 3, 5, 6, 7, 9, and 26) were unambiguously identified as gastrodin, 5-HMF, parishin B, parishin C, parishin, ferulic acid, and Z-ligustilide based on the direct comparison of their retention times, UV spectra, and mass spectra with those of the authentic compounds. Furthermore, 31 compounds were tentatively characterized according to their UV spectra, empirical molecular formula, and mass fragmentation pathways as well as their eluted sequence from ODS column reported in the literature [11–14] and acquired in the present experiments.
Table 2.
Retention time (t R), UV, and MSn data obtained by LC-DAD-ESI-IT-MSn of the identified compounds in Tianshu Capsule.
| Number | t R/min | Identified compounds | UV λ max/nm | [M + Na]+ | [M + H]+ | [2M + Na]+ | Main product ions | [M − H]− | [M + HCOO]− | Main product ions |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.3 | Gastrodin glucose (448) | 221, 276 | 471 | 447 | 493 | 323[M – H-C7H8O2]−, 285[M – H-glc]−, 179 |
|||
| 2 | 6.3 | Gastrodin (286) | 220, 269 | 309 | 185[M + Na-C7H8O2]+ | 285 | 331 | 161[M – H-C7H8O2]−, 123[M – H-glc]− |
||
| 3 | 8.1 | 5-HMF (126) | 230, 283 | 127 | 109[M + H-H2O]+ | 125 | ||||
| 5 | 20.3 | Parishin B (728) | 221, 272 | 751 | 483[M + Na-268]+, 215[483-268]+ | 727 | 459, 441, 423, 397, 379, 217 | |||
| 6 | 21.8 | Parishin C (728) | 221, 274 | 751 | 483[M + Na-268]+, 215[483-268]+ | 727 | 459, 441, 423, 397, 379, 217 | |||
| 7 | 23.4 | Parishin (996) | 221, 271 | 995 | 727[M – H-268]− | |||||
| 9 | 26.0 | Ferulic acid (194) | 220, 236, 323 | 195 | 177[M + H-H2O]+, 145[177-CH3OH]+ |
|||||
| 10 | 27.4 | Senkyunolide J/N (226) | 220, 330 | 249 | 475 | 209[M + H-H2O]+, 191[M + H-2H2O]+, 163[M + H-2H2O-28]+ |
||||
| 11 | 30.4 | 4,5-Dihydro-3,1′-dihydroxy -3-butylphthalide (224) |
280 | 247 | 207[M + H-H2O]+, 189[M + H-2H2O]+, 165, 121 | |||||
| 12 | 31.6 | Senkyunolide I/H (224) | 276 | 247 | 207[M + H-H2O]+, 189[M + H-2H2O]+, 161[189-H2O]+, 133[189-56]+ |
|||||
| 15 | 37.0 | Unidentified (316) | 230, 276, 280 | 339 | 317 | 299[M + H-H2O]+, 281[M + H-2H2O]+, 271[M + H-H2O-28]+, 243 |
315 | |||
| 17 | 37.8 | Z-6,7-Epoxyligustilide (206) | 230 | 229 | 413 | 189[M + Na-H2O]+, 171[189-H2O]+ | ||||
| 18 | 38.1 | Senkyunolide K/G (208) | 233, 280 | 231 | 439 | 191[M + H-H2O]+, 173[191 − H2O]+, 149[191-42]+, 135[191-56]+ |
||||
| 20 | 40.2 | Senkyunolide A (192) | 280 | 215 | 193 | 407 | 175[M + H-H2O]+, 147, 119, 105 | |||
| 21 | 40.7 | Butylphthalide (190) | 232, 275 | 213 | 191 | 403 | 173[M + H-H2O]+, 145, 117 | |||
| 24 | 42.6 | Cnidilide (194) | 238, 279, 327 | 217 | ||||||
| 25 | 43.3 | E-Ligustilide (190) | 281, 327 | 213 | 191 | 403 | 173[M + H-H2O]+, 130 | |||
| 26 | 43.5 | Z-Ligustilide (190) | 237, 260, 312 | 213 | 191 | 403 | 173[M + H-H2O]+, 145[173-28]+ 130, 117, 105 |
|||
| 28 | 44.0 | 3-Butylidenephthalide (188) | 230, 277, 326 | 211 | 189 | 171[M + H-H2O]+, 153[171-H2O]+ | ||||
| 29 | 47.4 | Riligustilide (380) | 275 | 403 | 381 | 213[2M + Na-190]+, 191, 173 | ||||
| 30 | 47.5 | Senkyunolide P (382) | 278 | 405 | 383 | 192[M + H-190]+ | ||||
| 31 | 47.8 | 3′,6,8′,3a-Biligustilide (380) | 278, 363 | 403 | 381 | 213[2M + Na-190]+, 191[M + H-190]+, 145, 117 |
||||
| 32 | 48.0 | Tokinolide B (380) | 278 | 403 | 381 | 213[2M + Na-190]+, 191[M + H-190]+ | ||||
| 33 | 48.2 | Unidentified (382) | 230, 279 | 405 | 383 | 193[M + H-190]+ | ||||
| 34 | 48.5 | Levistolide A (380) | 276 | 403 | 381 | 213[2M + Na-190]+, 191[M + H-190]+, 145, 117 |
||||
| 35 | 48.8 | Senkyunolide O (380) | 278 | 403 | 381 | 173[191-H2O]+, 191[M + H-190]+, 145, 117 |
||||
| 36 | 49.0 | Senkyunolide M (278) | 279 | 301 | 279 | 245[M + Na-56]+, 189, 171 | ||||
| 37 | 49.4 | Senkyunolide Q (278) | 278 | 301 | 279 | 245[M + Na-56]+, 189, 171 | ||||
| 38 | 49.9 | Unidentified (382) | 225, 278 | 405 | 383 |
Table 3.
Retention time (t R) and MS data obtained by LC-DAD-ESI-IT-TOF/MS of the identified compounds in the sample of Tianshu Capsule.
| Number | t R/min | Identified compounds | Formula | Mea. mass/m/z | Calc. mass/m/z | Error/ppm | Other precursor ions | Main product ions |
|---|---|---|---|---|---|---|---|---|
| 3 | 7.6 | 5-HMF (126) | C6H6O3 | 127.0384[M + H]+ | 127.0390[M + H]+ | −4.72 | 149.0240[M + Na]+ | |
| 5 | 19.8 | Parishin B (728) | C32H40O19 | 727.2123[M − H]− | 727.2091[M − H]− | 4.40 | ||
| 6 | 21.5 | Parishin C (728) | C32H40O19 | 727.2132[M − H]− | 727.2091[M − H]− | 5.64 | ||
| 8 | 24.5 | 3-Butyl-3,6,7-trihydroxy-4,5,6,7-tetrahydrophthalide (242) | C12H18O5 | 241.1090[M − H]− | 241.1081[M − H]− | 3.72 | 223.0885[M – H-H2O]−, 197.1142, 179.1107, 141.0930, 123.0854 |
|
| 243.1220[M + H]+ | 243.1227[M + H]+ | 2.88 | 265.1060[M + Na]+ | |||||
| 9 | 25.3 | Ferulic acid (194) | C10H10O4 | 195.0649[M + H]+ | 195.0652[M + H]+ | −1.54 | 217.0458[M + Na]+ | |
| 10 | 27.0 | Senkyunolide J/N (226) | C12H18O4 | 227.1259[M + H]+ | 227.1278[M + H]+ | −3.37 | 249.1082[M + Na]+ | 209.1177[M + H-H2O]+, 191.1026[M + H-2H2O]+ |
| 11 | 30.3 | 4,5-Dihydro-3,1′-dihydroxy-3-butylphthalide (224) | C12H16O4 | 225.1105[M + H]+ | 225.1121[M + H]+ | −7.11 | ||
| 12 | 31.6 | Senkyunolide I/H (224) | C12H16O4 | 225.1105[M + H]+ | 225.1121[M + H]+ | −7.11 | ||
| 15 | 37.8 | Unidentified (316) | C18H20O5 | 317.1380[M + H]+ | 317.1384[M + H]+ | −1.26 | 339.1210[M + Na]+ | 299.1378[M + H-H2O]+, 271.1343[M + H-H2O-28]+ |
| 16 | 37.8 | 4,7-Dihydroxy-3-butylidenephthalide or senkyunolide D (222) | C12H14O4 | 223.0986[M + H]+ | 223.0965[M + H]+ | 9.41 | 245.0774[M + Na]+ | |
| 17 | 38.7 | Z-6,7-Epoxyligustilide (206) | C12H14O3 | 207.1008[M + H]+ | 207.1016[M + H]+ | 3.66 | 229.0824[M + Na]+ | 189.0856[M + H-H2O]+, 133.0318 |
| 18 | 39.1 | Senkyunolide K/G (208) | C12H16O3 | 209.1167[M + H]+ | 209.1172[M + H]+ | −2.39 | 231.0974[M + Na]+ | 191.1091[M + H-H2O]+, 119.0819 |
| 19 | 40.6 | Senkyunolide F (206) | C12H14O3 | 207.1009[M + H]+ | 207.1016[M + H]+ | 3.38 | 229.0815[M + Na]+ | 189.0911[M + H-H2O]+, 161.0959 |
| 205.0874[M − H]− | 205.0870[M − H]− | 1.95 | 411.1821[2M − H]− | 161.0976[M – H-44]−, 106.0421 | ||||
| 20 | 41.4 | Senkyunolide A (192) | C12H16O2 | 193.1219[M + H]+ | 193.1223[M + H]+ | −2.07 | 215.1027[M + Na]+, 407.2196[2M + Na]+ |
175.1119[M + H-H2O]+, 147.1155, 105.0718 |
| 21 | 42.1 | Butylphthalide (190) | C12H14O2 | 191.1061[M + H]+ | 191.1067[M + H]+ | −3.14 | 213.0872[M + Na]+, 403.1888[2M + Na]+ |
173.0989[M + H-H2O]+, 145.1049[M + H-H2O-28]+ |
| 22 | 42.9 | Senkyunolide C (204) | C12H12O3 | 205.0847[M + H]+ | 205.0859[M + H]+ | −5.85 | 227.0685[M + Na]+, 431.1431[2M + Na]+ |
187.0763[M + H-H2O]+, 169.0757[M + H-2H2O]+, 141.0699[169-28]+ |
| 23 | 43.4 | Senkyunolide E (204) | C12H12O3 | 205.0843[M + H]+ | 205.0859[M + H]+ | −7.80 | 227.0663[M + Na]+ | |
| 24 | 44.1 | Cnidilide (194) | C12H18O2 | 195.1372[M + H]+ | 195.1380[M + H]+ | −4.10 | 217.1185[M + Na]+ | 177.1323[M + H-H2O]+, 149.1340[M + H-H2O-28]+ |
| 25 | 44.1 | E-Ligustilide (190) | C12H14O2 | 191.1061[M + H]+ | 191.1067[M + H]+ | −3.14 | 213.0871[M + Na]+ | |
| 26 | 45.2 | Z-Ligustilide (190) | C12H14O2 | 191.1056[M + H]+ | 191.1067[M + H]+ | −5.76 | 213.0859[M + Na]+, 403.1901[2M + Na]+ |
173.0984[M + H-H2O]+, 145.0999[M + H-H2O-28]+, 117.0690 |
| 27 | 45.5 | Neocnidilide (194) | C12H18O2 | 195.1372[M + H]+ | 195.1380[M + H]+ | −4.10 | 217.1179[M + Na]+ | 177.1323[M + H-H2O]+, 149.1306[M + H-H2O-28]+, 121.0998 |
| 28 | 45.8 | 3-Butylidenephthalide (188) | C12H12O2 | 189.0907[M + H]+ | 189.0910[M + H]+ | −1.59 | 211.0720[M + Na]+ | 171.0817[M + H-H2O]+, 153.0800[M + H-H2O-H2O]+, 143.0882[M + H-H2O-28]+, 129.0724 |
| 29 | 46.2 | Riligustilide (380) | C24H28O4 | 381.2058[M + H]+ | 381.2060[M + H]+ | −0.52 | 403.1872[M + Na]+, 191.1060, 213.0875 |
|
| 30 | 48.9 | Senkyunolide P (382) | 383.2220[M + H]+ | 383.2217[M + H]+ | 405.2031[M + Na]+ | 193.1212[M/2 + H]+ | ||
| 31 | 49.2 | 3′,6,8′,3a-Biligustilide (380) | C24H28O4 | 381.2058[M + H]+ | 381.2060[M + H]+ | −0.52 | 403.1885[M + Na]+, 191.1091 |
|
| 32 | 49.8 | Tokinolide B (380) | C24H28O4 | 381.2037[M + H]+ | 381.2060[M + H]+ | −6.03 | 403.1878[M + Na]+, 191.1062 |
|
| 33 | 49.9 | Unidentified (382) | 383.2220[M + H]+ | 383.2217[M + H]+ | 405.2040[M + Na]+ | 193.1214[M/2 + H]+ | ||
| 34 | 50.3 | Levistolide A (380) | C24H28O4 | 381.2064[M + H]+ | 381.2060[M + H]+ | 1.05 | 403.1887[M + Na]+, 191.1054 |
|
| 35 | 50.8 | Senkyunolide O (380) | C24H28O4 | 381.2061[M + H]+ | 381.2060[M + H]+ | 0.26 | 403.1879[M + Na]+, 191.1057 |
191.1051[M/2 + H]+ |
| 36 | 51.4 | Senkyunolide M (278) | 279.1582[M + H]+ | 301.1402[M + Na]+, 579.2952[2M + Na]+ |
||||
| 37 | 52.0 | Senkyunolide Q (278) | 279.1596[M + H]+ | 301.1381[M + Na]+, 579.2952[2M + Na]+ |
||||
| 38 | 52.7 | Unidentified (382) | 383.2217[M + H]+ | 383.2217[M + H]+ | 0.00 | 405.2008[M + Na]+ | 365.2115, 347.1905, 193.1201[M/2 + H]+ |
3.2.1. Fragmentation Characteristic of Reference Compounds
In the present HPLC and MS conditions, characteristic MS adduct ions were observed for phenolic glycosides, organic acid, and phthalide derivatives. Phenolic glycosides and organic acids could be well-detected in positive and negative ionization mode and adduct ions such as [M+H]+, [M+NH4]+, [M+Na]+, and [2M+Na]+ or [M-H]− and [M+HCOO]− were found, whereas phthalides were only detected in positive mode and mainly showed the abundant [M+H]+, [M+Na]+, and [2M+Na]+ ions. The fragmentation characteristic of reference compounds was similar as those described in the literature [11, 14, 15]. For example, gastrodin, the glucoside of p-hydroxybenzyl alcohol, mainly showed characteristic product ions at m/z 123 and 161 corresponding to the elimination of a molecule of glucose and a molecule of p-hydroxybenzyl alcohol from m/z 285 [M-H]−, respectively. Parishin B and C, the gastrodin derivatives with citric acid, mainly indicated the fragmentation of the ester glucoside bond and the neutral loss of gastrodin residue (268 Da) corresponding to the ion at m/z 459. The ions at m/z 441, 423, 397, and 379 were also observed, which were related to elimination of H2O and CO2 from the tertiary alcoholic hydroxyl group and the free carboxylic groups produced by breaking of the ester glucoside bond. Phthalides mainly displayed two pathways: side-chain cleavage with loss of alkenes and ring-opening followed by elimination of H2O and CO. These series of characteristic ion rules would be beneficial to elucidate the chemical constituents in TSC sample.
3.2.2. Identification of Phenolics Derivatives in TSC Sample
Compounds 1 and 2 gave the same on-line UV spectrum which is in accordance with that of gastrodin. The structure of 2 was identified as gastrodin based on the comparison of retention time, UV spectrum, and characteristic fragment ions with those of authentic compound as well as accurate molecular weight. The molecular weight of 1 was deduced as 448 from the sodium adduct ion at m/z 471 [M+Na]+ detected in positive mode and deprotonated molecular ion at m/z 447 [M-H]− in negative mode, respectively. A prominent neutral loss of 162 Da, corresponding to the loss of hexose, and disaccharide residue ions at m/z 323 were observed in MS2 of the [M-H]− ion at m/z 447. The loss of 124 Da was assigned to p-hydroxybenzyl alcohol just like the characteristic loss of gastrodin. Therefore, compound 1 was identified as elatoside, namely, 6′-(p-hydroxybenzyl methyl)-gastrabiose, which was previously isolated from the rhizome of G. elata [16].
Compounds 4, 5, 6, and 7 gave the adduct ions [M+Na]+ and [M+NH4]+ in positive LC-MS and [M-H]− in negative LC-MS experiments as well as UV spectra, which were similar to those of gastrodin derivatives. Compounds 5 and 6 had the same [M-H]− ions at m/z 727, which produced the prominent fragment ion at m/z 459 in the MS2, due to loss of gastrodin residue (268 Da) as well as other ions at m/z 441, 423, 397, and 379. Combining with retention time and addition of reference substances, 5 and 6 were identified as parishin B and C. Similarly, compound 4 was assigned to parishin E or its positional isomer parishin G with identical molecular mass of 460 [17]. Compound 7 had the molecular mass of 996 and exhibited consecutive loss of gastrodin residue (268 Da), and, therefore, it was identified as parishin, a conjugate of one citric acid and three gastrodins.
3.2.3. Identification of Phthalide Derivatives in TSC Sample
The molecular formula of compound 8, detected from individual herb Chuanxiong rhizoma, was calculated as C12H18O5 by [M+H]+ ion at m/z 243.1220 and [M-H]− ion at m/z 241.1090 in its LC-IT-TOF/MS experiment. It had the same molecular weight of 242 as those of known compounds, senkyunolide L and 3-butyl-3,6,7-trihydroxy-4,5,6,7-tetrahydrophthalide, present in Chuanxiong rhizoma; however, compound 8 could not be senkyunolide L (C12H15ClO3) because both had the different element composition [18]. The fragmentation ion of 8 at m/z 197.1142, which was derived from a retro-Diels-Alder cleavage of the [M-H-H2O]− ion at m/z 223.0885, suggested that the structure of 8 was proposed as 3-butyl-3,6,7-trihydroxy-4,5,6,7-tetrahydrophthalide.
Compound 10 displayed [M+H]+ ion at m/z 227.1259, suggesting the molecular formula of C12H18O4. The fragment ions at m/z 209 and 191 in the MS2, indicating consecutive loss of H2O, supported that 10 was a dihydroxylated derivative of ligustilide. UV and MS data of 10 were in accordance with those of senkyunolide J and senkyunolide N, but the stereochemistry information of two hydroxy groups could not be provided. Thus, 10 was tentatively assigned as one of senkyunolide J and senkyunolide N. Compounds 11 and 12 gave the same [M-H]− ions at m/z 224 and their molecular formula was established as C12H18O4 according to the [M+H]+ ion at m/z 225.1105. However, both constituents had different fragmentation rules. The MS2 of 11 displayed the fragment ions at m/z 207, 189 and the characteristic ion at m/z 165, which were similar to those of 4,5-dihydro-3,1′-dihydroxy-3-butylphthalide [15], whereas 12 showed elimination of two H2O followed by side-chain cleavage. Therefore, 11 was deduced as 4,5-dihydro-3,1′-dihydroxy-3-butylphthalide and 12 could be one of senkyunolide I and senkyunolide H [14, 15].
Compounds 13, 17, and 19 have the same molecular formula of C12H14O3 and were tentatively characterized as 4-hydroxy-3-butylphthalide, Z-6,7-epoxyligustilide, and senkyunolide F. Compared with monohydroxy phthalide derivatives 13 and 19, the instable structure of 17 resulted in the ion at m/z 189 as base peak observed in MS1, suggesting loss of H2O from the [M-H]− ion at m/z 207. Senkyunolide A 20 and Z-ligustilide 26 as main constituents were identified and butylphthalide 21, senkyunolide C 22, senkyunolide E 23, E-ligustilide 24, and 3-butylidenephthalide 28 were characterized as minor constituents. For the compounds 20, 21, 24, and 26, the neutral loss of CO (28 Da) and side-chain cleavage (56 Da) from [M+H-H2O]+ were the common fragmentation rules.
Compound 16 (C12H14O3) was tentatively proposed to 4,7-dihydroxy-3-butylidenephthalide or senkyunolide D. Compound 18 was assigned as senkyunolide K or senkyunolide G. These compounds and their isomers could not be differentiated by available MS data.
Five dimeric ligustilides 29, 31, 32, 34, and 35, which produced the protonated ion [M+H]+ at m/z 381, were detected in extracted ion chromatograms. They showed the base peak at m/z 403 [M+Na]+ in MS1 and the fragment ion at m/z 191 [M+H-190]+ as base peak in MS2 by the loss of a RDA fragment (145 Da) followed by the loss of H2O and HCOOH (46 Da). Comprehensively considering the information described in the literature [14], they were tentatively identified as riligustilide, 3′,6,8′,3a-biligustilide, tokinolide B, levistolide A, and senkyunolide O, respectively. Three compounds, indicating the protonated ion [M+H]+ at m/z 383, were detected in extracted ion chromatograms; however, only senkyunolide P was previously reported in Chuanxiong Rhizoma. Thus, compound 30 was tentatively characterized as senkyunolide P and the other compounds (33 and 38) were still indefinite based on the available information.
In the modernization of TCM, chemical profiling is always the first task. It is of importance for development of the suitable quality standard and control strategy, study of pharmacokinetics, and interpretation of therapeutic character of TCM [19, 20]. As a marketed product in China, the quality control system of TSC is still to be improved and its pharmacokinetics and action mechanism are not completely clear. Usually, gastrodin, ferulic acid, and 6,7-dihydroxyligustilide are selected as marker compounds for the quality control or the pharmacokinetic study of Tianshu Capsule [1, 21, 22]. The present investigation provides more chemical information for the selection of the marker compounds and the improvement of quality control. It also tells the pharmacokinetic scientists and ethnopharmacologists which compounds in TSC samples are worth further evaluation. In addition to gastrodin and ferulic acid, more efforts should be made to the group of phthalide derivatives such as major constituents, senkyunolide I/H 12, 4-hydroxy-3-butylphthalide 13, senkyunolide A 20, and Z-ligustilide 26 as well as their dimers. Additionally, it is worth noting that 5-HMF 3, a potent toxic compound, was found as main peak in the chemical profile of TSC samples. This compound originated from the crude material Gastrodiae rhizoma and its content in TSC samples was up to 2.67 mg·g−1 [23]. If it is necessary to develop its limit standard in TSC product or not, more study are expected. Also, ligustrazine is not detected in TSC sample, even in the extracted ion chromatogram, which could result from its very low amount in crude herbal material (about 0.01~0.02%), although, actually, it is often considered as one of active components in Chuanxiong rhizoma.
4. Conclusions
In this study, HPLC analysis was employed to find out the common chromatographic peak in various batches of TSC samples. The contribution of the characteristic peaks from individual herbs to the whole chromatographic profile was discussed based on comparative HPLC and LC-MS analyses. A total of 38 constituents were identified based on the comparison of retention time and UV spectra with authentic compounds as well as by summarized MS fragmentation rules and matching empirical molecular formula with those of published components. The present investigation provided the good basis for monitoring the manufacturing processes and improving quality control of TSC products.
Supplementary Material
Figure S1 The pharmaceutical manufacture process of TSC described in current Chinese Pharmacopoeia
Figure S2. HPLC of 5 batches of TSC samples at 276 nm
Figure S3. HPLC of reference solution (RS) and TSC sample. A. RS at 276 nm, B. RS at 221 nm, C. TSC sample at 276 nm, D. TSC sample at 221 nm.
Figure S4. HPLC of ethanolic (e) or aqueous (a) extracts of Da Chuanxiong Fang (D.), chuanxiong rhizoma (C.) and gastrodiae rhizoma (G.) at 276 nm.
Figure S5. HPLC and TIC of ethanolic extract of Da Chuanxiong Fang. A. HPLC (276 nm), B. (+) TIC, C. (-) TIC.
Figure S6. HPLC and TIC of aqueous extract of Da Chuanxiong Fang. A. HPLC (276 nm), B. (+) TIC, C. (-) TIC.
Acknowledgments
This research work was supported by China Postdoctoral Science Foundation (2012M510731) and State Key Laboratory of New-tech for Chinese Medicine Pharmaceutical Process (SKL2010M0204). Meanwhile great thanks are due to Dr. Feng Ji from Shimadzu (China) Co., Ltd., Beijing Office, for measuring the LC-DAD-ESI-IT-TOF/MS experiments.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia. Vol. 1. Beijing, China: China Medical Science Press; 2010. [Google Scholar]
- 2.Xia W, Zhu MJ, Zhang Z, et al. Effect of Tianshu capsule in treatment of migraine: a meta-analysis of randomized control trials. Journal of Traditional Chinese Medicine. 2013;33(1):9–14. doi: 10.1016/s0254-6272(13)60093-x. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Zhang JM, Hong YL, Feng Y, Chen MW, Wang YT. Phytochemical and pharmacological review of Da Chuanxiong formula: a famous herb pair composed of Chuanxiong Rhizoma and Gastrodiae Rhizoma for headache. Evidence-Based Complementary and Alternative Medicine. 2013;2013:16 pages. doi: 10.1155/2013/425369.425369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ni SM, Qian DW, Shang EX, Duan JA, Guo JM, Tang YP. Analysis of Chemical composition of Da chuanxiong Fang by ultra performance liquid chromatography-electrospray ionization quadrupole time of flight/mass spectrometry. Chinese Journal of Experimental Traditional Medical Formulae. 2010;16(1):40–45. [Google Scholar]
- 5.Shen L, Lin X, Liang S, et al. Characterization on central ingredients of active components in dachuanxiong fang based on HPLC-DAD-MSn. Chinese Journal of Experimental Traditional Medical Formulae. 2012;18(7):128–134. [Google Scholar]
- 6.Wei YF, Lin X, Zhang N, Feng Y. UPLC-MS analysis of constituents of Dachuanxiong Fang active parts absorbed into blood. Zhongguo Zhongyao Zazhi. 2011;36(9):1245–1248. [PubMed] [Google Scholar]
- 7.Wei Y, Zhang N, Lin X, Feng Y. Release characteristics in vitro and pharmacokinetics of Da Chuanxiong Fang multiunit drug delivery system in rats. Acta Pharmaceutica Sinica. 2011;46(9):1150–1155. [PubMed] [Google Scholar]
- 8.Zhang XL, Liu LF, Zhu LY, et al. A high performance liquid chromatography fingerprinting and ultra high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry chemical profiling approach to rapidly find characteristic chemical markers for quality evaluation of dispensing granules, a case study on Chuanxiong Rhizoma. Journal of Pharmaceutical and Biomedical Analysis. 2014;88:391–400. doi: 10.1016/j.jpba.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Zhang XZ, Xiao HB, Xu Q, Li XL, Wang JN, Liang XM. Characterization of phthalides in Ligusticum chuanxiong by liquid chromatographic-atmospheric pressure chemical ionization-mass spectrometry. Journal of Chromatographic Science. 2003;41(8):428–433. doi: 10.1093/chromsci/41.8.428. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Yan R, Tam Y, Lin G. Post-harvest alteration of the main chemical ingredients in Ligusticum chuanxiong HORT. (Rhizoma Chuanxiong) Chemical and Pharmaceutical Bulletin. 2007;55(1):140–144. doi: 10.1248/cpb.55.140. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Xiao HB, Liang XM, Wei L. Identification of phenolics and nucleoside derivatives in Gastrodia elata by HPLC-UV-MS. Journal of Separation Science. 2007;30(10):1488–1495. doi: 10.1002/jssc.200600469. [DOI] [PubMed] [Google Scholar]
- 12.Yi T, Leung KS, Lu GH, Zhang H. Comparative analysis of Ligusticum chuanxiong and related umbelliferous medicinal plants by high performance liquid chromatography-electrospray ionization mass spectrometry. Planta Medica. 2007;73(4):392–398. doi: 10.1055/s-2007-967139. [DOI] [PubMed] [Google Scholar]
- 13.Yan R, Li SL, Chung HS, Tam Y, Lin G. Simultaneous quantification of 12 bioactive components of Ligusticum chuanxiong Hort. by high-performance liquid chromatography. Journal of Pharmaceutical and Biomedical Analysis. 2005;37(1):87–95. doi: 10.1016/j.jpba.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 14.Yi T, Leung KS, Lu GH, Chan K, Zhang H. Simultaneous qualitative and quantitative analyses of the major constituents in the rhizome of Ligusticum chuanxiong using HPLC-DAD-MS. Chemical and Pharmaceutical Bulletin. 2006;54(2):255–259. doi: 10.1248/cpb.54.255. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q, Yang XW. GC-MS analysis of essential oil from rhizomes of Ligusticum chuanxiong cultivated in GAP Base for Chinese Medicinal Materials of China. China Journal of Chinese Materia Medica. 2008;33(3):276–280. [PubMed] [Google Scholar]
- 16.Ning L. Studies on the constituents of Gastrodia elata and three medicinal plants belongs to Curculigo [Doctoral thesis] Kunming, China: Kunming Institute of Botany, Chinese Academy of Sciences; 2004. [Google Scholar]
- 17.Wang L, Xiao H, Yang L, Wang ZT. Two new phenolic glycosides from the rhizome of Gastrodia elata . Journal of Asian Natural Products Research. 2012;14(5):457–462. doi: 10.1080/10286020.2012.669755. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Mitsuhashi H. Studies on the constituents of umbelliferea plants X VII structure of three new ligustilide derivatives from Ligusticum wallichii . Chemical & Pharmaceutical Bulletin. 1987;35(12):4789–4792. [Google Scholar]
- 19.Shi ZQ, Song DF, Li RQ, et al. Identification of effective combinatorial markers for quality standardization of herbal medicines. Journal of Chromatography A. 2014;1345:78–85. doi: 10.1016/j.chroma.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Hu Z, Yang J, Cheng C, et al. Combinatorial metabolism notably affects human systemic exposure to ginsenosides from orally administered extract of Panax notoginseng roots (Sanqi) Drug Metabolism and Disposition. 2013;41(7):1457–1469. doi: 10.1124/dmd.113.051391. [DOI] [PubMed] [Google Scholar]
- 21.Zhang HF, Chen XH, Huo YS, Bi KS. RP-HPLC simultaneous determination of the contents of gastrodin , ferulic acid and 6,7-dihydroxyligustilide in Tianshu capsules under a changed UV wavelengths. Journal of Shenyang Pharmaceutical University. 2012;29(6):443–447. [Google Scholar]
- 22.Zhang H, Sun L, Chen X, Huo Y, Yan B, Bi K. Simultaneous quantification of 6,7-di-hydroxyligustilide and gastrodin in rat plasma by LC-MS: application to pharmacokinetic study of Tianshu capsule. Latin American Journal of Pharmacy. 2012;31(1):112–119. [Google Scholar]
- 23.Liang JJ, Gao HM, Chen LM, et al. Simultaneous determination of three constituents in Tiansu Capsule by HPLC. Chinese Journal of Experimental Traditional Medical Formulae. 2013;19(17):81–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The pharmaceutical manufacture process of TSC described in current Chinese Pharmacopoeia
Figure S2. HPLC of 5 batches of TSC samples at 276 nm
Figure S3. HPLC of reference solution (RS) and TSC sample. A. RS at 276 nm, B. RS at 221 nm, C. TSC sample at 276 nm, D. TSC sample at 221 nm.
Figure S4. HPLC of ethanolic (e) or aqueous (a) extracts of Da Chuanxiong Fang (D.), chuanxiong rhizoma (C.) and gastrodiae rhizoma (G.) at 276 nm.
Figure S5. HPLC and TIC of ethanolic extract of Da Chuanxiong Fang. A. HPLC (276 nm), B. (+) TIC, C. (-) TIC.
Figure S6. HPLC and TIC of aqueous extract of Da Chuanxiong Fang. A. HPLC (276 nm), B. (+) TIC, C. (-) TIC.


