Abstract
We evaluated the utility of follow-up interferon-gamma release assays (IGRAs) for the diagnosis of reactivation of latent tuberculosis infection (LTBI) or new tuberculosis in ankylosing spondylitis (AS) patients receiving anti-tumor necrosis factor alpha (anti-TNFα). The study participants (n=127) had a negative IGRA screening before receiving anti-TNFα and were evaluated by follow-up IGRA. We retrospectively examined data of the subjects according to age, gender, tuberculosis prophylaxis, concomitant medications, IGRA conversion and anti-TNFα, including type and treatment duration. The median duration of anti-TNFα was 21.5 months, and the median age was 35.3 yr. Of the 127 patients, IGRA conversion was found in 10 patients (7.9%). There was no significant variation between IGRA conversion rate and any risk factors except for age. IGRA conversion rate was not significantly different between AS and rheumatoid arthritis (P=0.12). IGRA conversion was observed in AS patients receiving anti-TNFα in Korea. A follow-up IGRA test can be helpful for identifying LTBI or new tuberculosis in AS patients receiving anti-TNFα.
Graphical Abstract
Keywords: Ankylosing Spondylitis, Interferon-Gamma Release Assay, Anti-Tumor Necrosis Factor, Latent Tuberculosis Infection
INTRODUCTION
Anti-tumor necrosis factor alpha (TNFα) is often used in patients with rheumatic disease who do not respond to conventional treatments, such as non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroid, or disease modifying anti-rheumatic drugs (DMARDs) (1, 2). Dramatic improvements have been shown in clinical symptoms and signs in patients with ankylosing spondylitis (AS) receiving anti-TNFα. However, anti-TNFα has a number of rare but serious adverse effects, including infection, tuberculosis (TB), increased risk of certain malignancies, congestive heart failure and drug-induced lupus (3, 4). Among these, latent tuberculosis infection (LTBI) or new TB are of the most particular concern in Korea. The degree of concern for LTBI differs by country and its frequency of TB. Hence, the standard for TB surveillance may be different before and after treatment. Physicians should be aware of TB, especially in patients receiving anti-TNFα or other biologic agents because the incidence rate of TB in Korea is considerably high (5). In addition, AS patients have higher risk of TB than the general population (6).
In the 2012 update of the American College of Rheumatology (ACR) recommendation, annual LTBI testing is suggested in rheumatoid arthritis (RA) patients taking anti-TNFα who live in situations where TB exposure is likely (1). Therefore, follow-up LTBI testing also seems to be important in AS patients receiving anti-TNFα. Because of the increasing use of anti-TNFα in AS patients, a well-established guideline concerning follow-up LTBI testing needs to be generated. Park et al. (7) reported that the tuberculin skin test (TST) conversion rate is significantly higher in the AS than in the RA patients. However, the TST may not be the appropriate value of measurement to employ because prior Bacillus Calmette-Guerin (BCG) vaccination can produce false-positive results. Recently, Kim et al. (8) showed that AS patients have an independent association with discordant IGRA-negative/TST-positive results. In contrast to the TST, because IGRA is unaffected by BCG vaccination status. In addition, TST testing has a boosting effect, which could lead to conversion in repeated testing, whereas IGRA does not exhibit such testing error. Hence, IGRA may serve as an appropriate guideline measurement (9, 10). However, follow-up IGRA testing in AS patients receiving anti-TNFα has rarely been studied. Moreover, there are no country-specific guidelines for IGRA testing.
In the present study, we assessed the pattern of serial IGRA results in AS patients receiving anti-TNFα in Korea with intermediate TB burden, and analyzed the characteristics of AS and RA patients.
MATERIALS AND METHODS
Study population
The study participants were enrolled from September 2008 to August 2012, among AS patients who were registered with the outpatient clinic of Hanyang University Hospital for Rheumatic Diseases in Seoul. A total of 127 patients with AS started to receive anti-TNFα (etanercept, adalimumab, and infliximab) during this period, and had negative initial IGRA result and follow-up testing. These patients were evaluated by follow-up IGRAs until November 2013. The patients were of Korean nationality, and met the 1984 New York criteria for AS (11). We reviewed data from the subjects according to age, gender, TB prophylaxis, concomitant treatment (methotrexate, steroid), IGRA conversion and anti-TNFα, including type, and treatment duration. No patient had a prior history of active TB.
To compare the characteristics of AS and RA patients, we also reviewed 26 RA patients in the same study design. All the RA patients met the American College of Rheumatology 1987 classification criteria for RA (12).
The interferon-gamma release assay
From September 2008, the QuantiFERON-TB Gold In-Tube test (QFT-GIT) as IGRA had applied to the LTBI screening. Therefore the QFT-GIT was performed in all patients before initiating anti-TNFα and for follow-up testing, according to the manufacturer's instructions (Cellistics Limited, Carnegie, Victoria, Australia). Interferon-gamma concentration≥0.35 IU/mL, and ≥25% of the negative control were interpreted as positive. Conversion of QFT-GIT was defined as negative QFT-GIT at baseline and positive QFT-GIT at follow-up. We excluded the indeterminate QFT-GIT results from our study.
Statistical analysis
We analyzed patient characteristics and IGRA conversion rates using multivariable logistic regression analysis adjusted for age, sex, LTBI prophylaxis, concomitant use of methotrexate and steroid, and treatment duration of anti-TNFα. We compared the characteristics of AS and RA patients using the Wilcoxon rank sum test for quantitative variables and the chi-square test for qualitative variables. Results are presented as means±SDs unless specified otherwise. When P values were less than 0.05, results were considered statistically significant. All statistical analyses were performed using SPSS (version 18.0, Inc., Chicago, IL, USA).
Ethics statement
This study was approved by institutional review board of Hanyang University Hospital (IRB No. 2012-08-001). Informed consent was not required because data were collected retrospectively from medical records.
RESULTS
Patient characteristics and IGRA conversion result
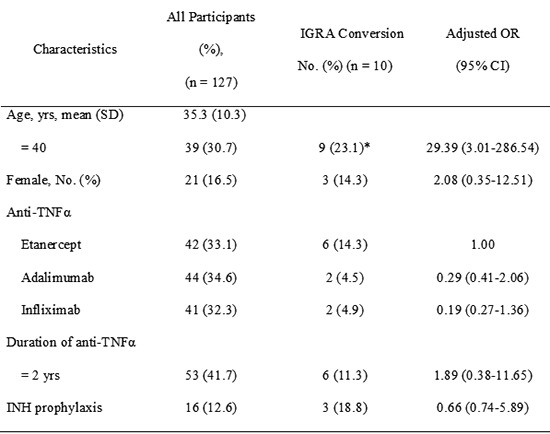
The 127 AS patients were enrolled in the study. The mean age was 35.3±10.3 yr. There were 106 (83.5%) male and 21 (16.5%) female patients. The median duration of anti-TNFα was 22.6±11.4 months. Forty two patients received etanercept, 44 received adalimumab, and 41 received infliximab (Table 1).
Table 1.
Patients characteristics and interferon-gamma release assay (IGRA) conversion rate
TNF, tumor necrosis factor; SD, standard deviation; OR, odds ratio; CI, confidence interval. *P<0.05.
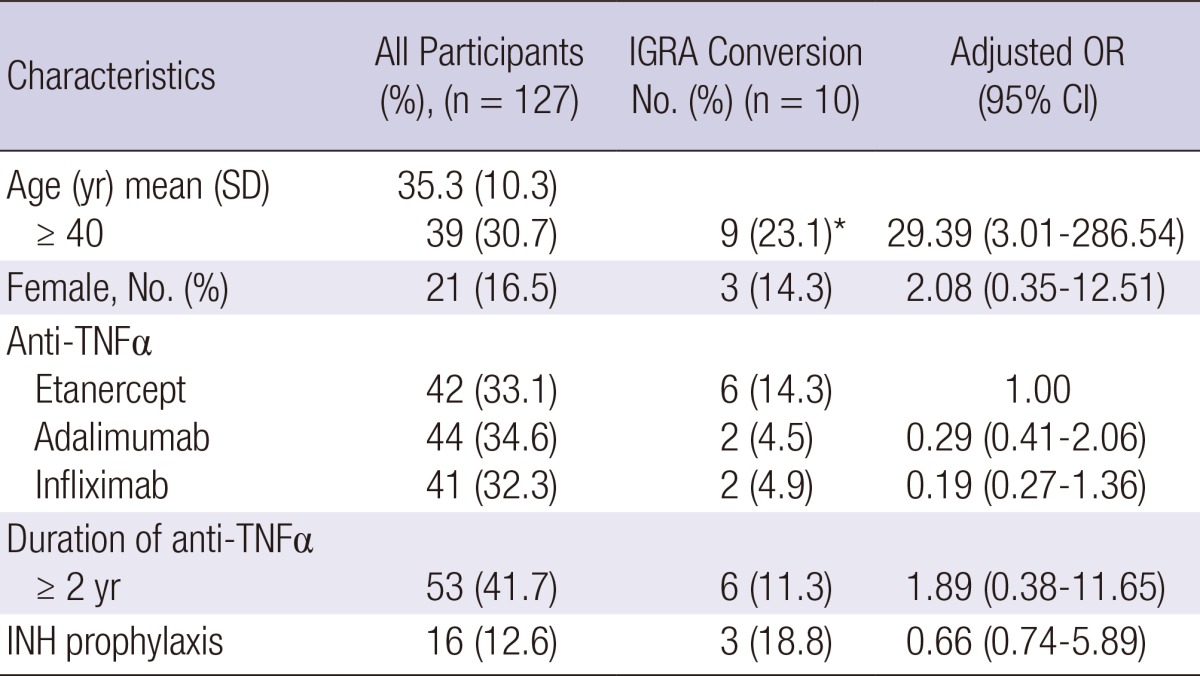
Among the 127 AS patients, IGRA conversion was found in 10 patients (7.9%). There was no significant variation between IGRA conversion rate and any risk factors except for age. Type of anti-TNFα was not associated with IGRA conversion rate. The IGRA conversion rate was higher in older patients (≥40 yr) (9/39, 23.1%) than in younger patients (<40 yr) (1/88, 1.1%) (P<0.05). Table 2 shows the characteristics of AS patients with IGRA conversion. In spite of isoniazid (INH) prophylaxis, three patients among 16 AS patients with INH prophylaxis had positive IGRA conversion result. However, no patient among the 127 AS patients has developed TB.
Table 2.
Clinical characteristics of IGRA conversion in 10 patients with AS undergoing anti-TNFα
IGRA, interferon-gamma release assay; AS, ankylosing spondylitis; TNF, tumor necrosis factor; INHP, isoniazid prophylaxis.
Comparison results of IGRA between AS and RA
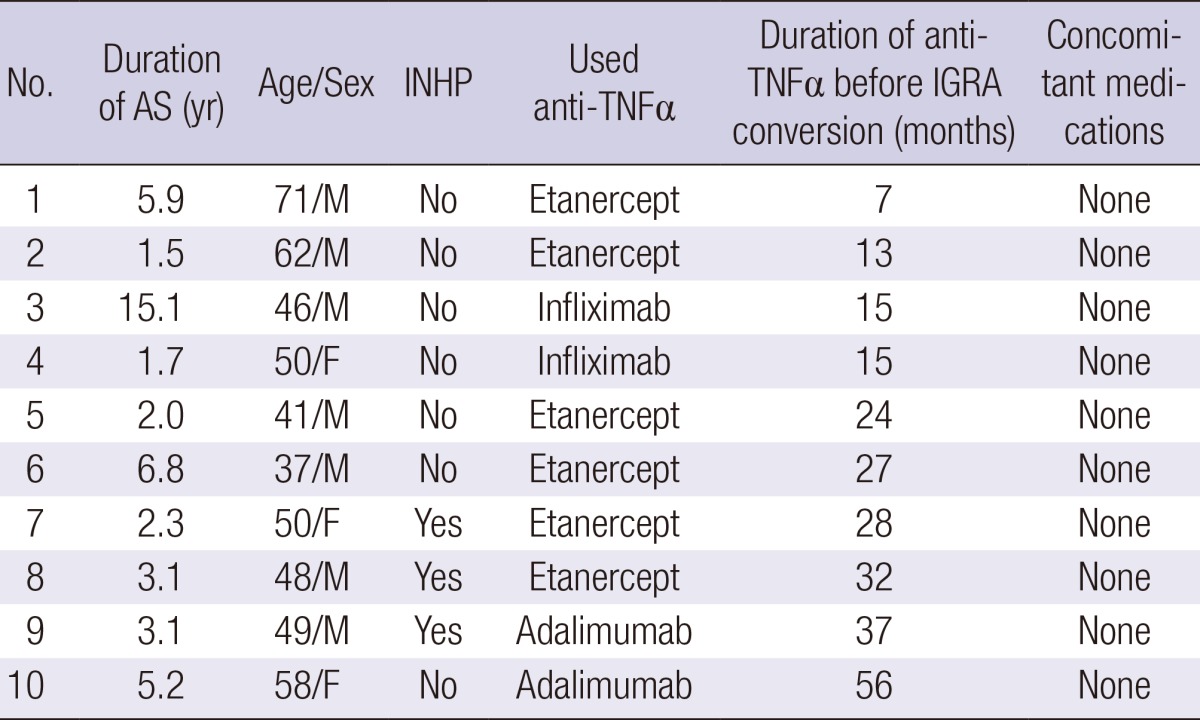
As shown in Table 3, the mean age of RA patients was 54.3±16.4 yr. RA patients were older than AS patients. While 83.5% of AS patients were male, only 7.7% of RA patients were male. The median duration of anti-TNFα showed no difference between AS and RA. Type of anti-TNFα was not different between RA and AS. RA patients used methotrexate and steroids more often than AS patients. Among the 26 RA patients, positive conversion was found in 5 patients (19.2%). The IGRA conversion rate was not significantly different between AS and RA patients (P=0.12).
Table 3.
Comparison of characteristics of AS and RA patients
AS, ankylosing spondylitis; RA, rheumatoid arthritis; SD, standard deviation; TNF, tumor necrosis factor; INH, insoniazid; IGRA, interferon-gamma release assay.
DISCUSSION
AS patients undergoing anti-TNFα in Korea have an ongoing risk of TB because they have a higher risk of reactivation of LTBI or contracting new TB than patients in low TB-burden areas do. This is because Korea is an intermediate TB burden area, posing a greater risk for TB exposure (6), which in turn leads to an increased risk of new TB infection. As a result, the number of infected patients who cannot be diagnosed through IGRA may become higher than that of developed countries. Chen et al. (13) reported that development of active tuberculosis presented persistently high interferon gamma level or IGRA conversion in RA patients receiving anti-TNFα. Therefore, interferon gamma (IFNγ) level and serial IGRA testing may be useful for the control of TB in patients receiving anti-TNFα (1). We assessed the utility of a follow-up IGRA in AS patients who were receiving anti-TNFα. Age was the only risk factor that seemed to influence the IGRA conversion rate. Therefore, a follow-up IGRA test may be more useful for elderly AS patients receiving anti-TNFα.
There is no gold standard for the diagnosis of LTBI. A TST, QFT-GIT, or T-SPOT TB (Oxford Immunotec, Abingdon, UK) are frequently used for LTBI screening (14, 15). The TST represents delayed type hypersensitivity reaction in the skin to intradermal injection of PPD. QFT-GIT detects secretory INFγ in response to TB-specific antigens (EAST-6, CFP-10, and TB7.7). T-SPOT TB uses ESAT-6 and CFP-10 as TB-specific antigens. Previous study showed the TST conversion rate was significantly higher in the AS (50%) than in the RA (17.5%) patients (7). Kim et al. (16) reported that eight patients among 26 AS patients treated with immunosuppressive agents or anti-TNFα had positive IGRA conversion (8/26, 30.8%). However, in our study, IGRA conversion rate in AS patients undergoing anti-TNFα were 7.9%, and IGRA conversion rate had no statistical difference between AS and RA.
In Korea, the BCG vaccination is given at birth. Until 1996, Korean children underwent a second TST at the age of 12 or 13 yr. Any child who had negative TST results received a second BCG vaccination. AS patients are relatively younger than those with other rheumatic diseases. Thus, relatively young AS patients might be more affected than RA patients by previous BCG vaccination. Recently, Kim et al. (8) showed that AS patients had association of discordant QFT-GIT-negative/TST-positive. With respect to AS, IGRA has several advantages for monitoring of LTBI. IGRA is not affected by BCG (9, 10). Second, patients do not need to visit the clinic once more. AS patients find it difficult to make a return visit for TST interpretation 72 hr later. There is also subjective variability in the interpretation of TST results (17).
Our study has some limitations. We usually start LTBI treatment before anti-TNFα following a positive LTBI screening result (1, 18). However, there is no strong evidence showing that LTBI treatment is needed in AS patients receiving anti-TNFα if they have an IGRA conversion result. We retrospectively analyzed the IGRA conversion rate. The duration of follow-up testing was quite long (22.6±11.4 months), therefore we do not know exactly when IGRA conversion occurred. Although the duration of anti-TNFα was not associated with IGRA conversion rates in our study, in a recent article, LTBI reactivation probably occurred during the first 6 months after starting anti-TNFα (13). However, in this study 9 of 10 patients with IGRA conversion had follow-up testing after 1 yr. If newly activated TB was detected in a follow-up IGRA, it would be necessary to prescribe the anti-TB medication (quadruple therapy) instead of INH prophylaxis. On the other hand, if the IGRA conversion presented a false-positive result, then INH prophylaxis would be socioeconomically impractical and medically unethical, and could lead to the risk of INH resistance. IGRA also exhibits variability of test results. Therefore, it is not guaranteed that a patient will continue to have positive results once a positive diagnosis is made, and not all of the patients with positive results will be diagnosed with TB. In countries with low rates of TB incidence, PPD conversion sometimes occurs, whereas IGRA conversion rarely does (19). In Korea, since the IGRA conversion rate is high, we should implement differentiated treatment method rather than following the examples of developed countries.
In conclusion, IGRA conversion was observed in AS patients receiving anti-TNFα in Korea. A follow-up IGRA test could be helpful in identifying LTBI or new TB in AS patients receiving anti-TNFα. The interpretation of IGRA conversion and how best to proceed in such cases require further clarification.
Footnotes
This work was supported by the research fund of Hanyang University (HY-2013-G).
The authors have no conflicts of interest to disclose.
References
- 1.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, Moreland LW, O'Dell J, Winthrop KL, Beukelman T, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kievit W, Fransen J, Adang EM, den Broeder AA, Bernelot Moens HJ, Visser H, van de Laar MA, van Riel PL. Long-term effectiveness and safety of TNF-blocking agents in daily clinical practice: results from the Dutch Rheumatoid Arthritis Monitoring register. Rheumatology (Oxford) 2011;50:196–203. doi: 10.1093/rheumatology/keq325. [DOI] [PubMed] [Google Scholar]
- 3.Jain A, Singh JA. Harms of TNF inhibitors in rheumatic diseases: a focused review of the literature. Immunotherapy. 2013;5:265–299. doi: 10.2217/imt.13.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasparyan AY, Ayvazyan L, Cocco G, Kitas GD. Adverse cardiovascular effects of antirheumatic drugs: implications for clinical practice and research. Curr Pharm Des. 2012;18:1543–1555. doi: 10.2174/138161212799504759. [DOI] [PubMed] [Google Scholar]
- 5.Lew WJ, Lee EG, Bai JY, Kim HJ, Bai GH, Ahn DI, Lee JK, Kim SJ. An Internet-based surveillance system for tuberculosis in Korea. Int J Tuberc Lung Dis. 2006;10:1241–1247. [PubMed] [Google Scholar]
- 6.Kim EM, Uhm WS, Bae SC, Yoo DH, Kim TH. Incidence of tuberculosis among korean patients with ankylosing spondylitis who are taking tumor necrosis factor blockers. J Rheumatol. 2011;38:2218–2223. doi: 10.3899/jrheum.110373. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, Seo GY, Lee JS, Kim TH, Yoo DH. Positive conversion of tuberculin skin test and performance of interferon release assay to detect hidden tuberculosis infection during anti-tumor necrosis factor agent trial. J Rheumatol. 2009;36:2158–2163. doi: 10.3899/jrheum.090150. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Cho SK, Han M, Choi CB, Kim TH, Jun JB, Bae SC, Yoo DH, Sung YK. Factors influencing discrepancies between the QuantiFERON-TB gold in tube test and the tuberculin skin test in Korean patients with rheumatic diseases. Semin Arthritis Rheum. 2013;42:424–432. doi: 10.1016/j.semarthrit.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Matulis G, Juni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a Mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann Rheum Dis. 2008;67:84–90. doi: 10.1136/ard.2007.070789. [DOI] [PubMed] [Google Scholar]
- 10.Chang B, Park HY, Jeon K, Ahn JK, Cha HS, Koh EM, Kang ES, Koh WJ. Interferon-γ release assay in the diagnosis of latent tuberculosis infection in arthritis patients treated with tumor necrosis factor antagonists in Korea. Clin Rheumatol. 2011;30:1535–1541. doi: 10.1007/s10067-011-1771-9. [DOI] [PubMed] [Google Scholar]
- 11.Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 12.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 13.Chen DY, Shen GH, Chen YM, Chen HH, Hsieh CW, Lan JL. Biphasic emergence of active tuberculosis in rheumatoid arthritis patients receiving TNFα inhibitors: the utility of IFNγ assay. Ann Rheum Dis. 2012;71:231–237. doi: 10.1136/annrheumdis-2011-200489. [DOI] [PubMed] [Google Scholar]
- 14.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4:761–776. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 15.Lee JY, Choi HJ, Park IN, Hong SB, Oh YM, Lim CM, Lee SD, Koh Y, Kim WS, Kim DS, et al. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur Respir J. 2006;28:24–30. doi: 10.1183/09031936.06.00016906. [DOI] [PubMed] [Google Scholar]
- 16.Kim KH, Lee SW, Chung WT, Kim BG, Woo KS, Han JY, Kim JM. Serial interferon-gamma release assays for the diagnosis of latent tuberculosis infection in patients treated with immunosuppressive agents. Korean J Lab Med. 2011;31:271–278. doi: 10.3343/kjlm.2011.31.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K IGRA Expert Committee; Centers for Disease Control and Prevention (CDC) Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep. 2010;59:1–25. [PubMed] [Google Scholar]
- 19.Papay P, Primas C, Eser A, Novacek G, Winkler S, Frantal S, Angelberger S, Mikulits A, Dejaco C, Kazemi-Shirazi L, et al. Retesting for latent tuberculosis in patients with inflammatory bowel disease treated with TNF-α inhibitors. Aliment Pharmacol Ther. 2012;36:858–865. doi: 10.1111/apt.12037. [DOI] [PubMed] [Google Scholar]


