Abstract
The purpose of this study was to evaluate treatment patterns, outcome and prognosticators for patients with leptomeningeal metastases from solid tumor. Medical records of 80 patients from January 1, 2004 to May 31, 2011 were retrospectively reviewed. Most frequent site of origin was the lung (59%) followed by the breast (25%). Most patients were treated with intrathecal chemotherapy (90%) and/or whole brain radiotherapy (67.5%). Systemic therapy was offered to 27 patients (33.8%). Percentage of patients treated with single, dual, and triple modality were 32.5%, 43.8%, and 23.8%, respectively. Median survival was 2.7 months and 1 yr survival rate was 11.3%. Multivariate analysis showed that negative cerebrospinal fluid cytology, fewer chemotherapy regimen prior to leptomeningeal metastases, whole brain radiotherapy, systemic therapy, and combined modality treatment (median survival; single 1.4 vs. dual 2.8 vs. triple 8.3 months, P<0.001) had statistical significance on survival. Subgroup analysis of non-small cell lung cancer (NSCLC) patients showed that targeted therapy had significant independent impact on survival (median survival; 10.5 vs. 3.0 months, P=0.008). Unlike previous reports, survival of patients with NSCLC primary was comparable to breast primary. Furthermore, combined modality treatment for all patients and additionally targeted therapy for NSCLC patients should be considered in the treatment of leptomeningeal metastases from solid tumor.
Graphical Abstract
Keywords: Leptomeningeal Metastases, Prognostic Factor, Solid Tumor
INTRODUCTION
Leptomeningeal metastasis (LM) is dissemination of malignant tumor cells into the subarachnoid space. Typically, LM presents with advanced stage, but it may be the first manifestation of cancer for 6%-21% of the patients (1). LM occurs 5%-8% among all cancer patients. According to autopsy data, the incidence is estimated at 19%, and 40% of them had negative cerebrospinal fluid (CSF) cytology prior to death (2). Recently, the incidence of LM is increasing. Despite lengthened survival of cancer patients with improvement in chemotherapeutic agents, intracranial area still remains sanctuary due to blood brain barrier (BBB). Moreover, advanced diagnostic capability for LM contributed to the increase in the detection rate (3, 4).
It is well-known that LM carries dismal prognosis. Even though patients surviving longer than 1 or 2 yr are occasionally observed, most patients succumbed to disease only 3 to 4 months after the diagnosis (5). Many studies have proposed various prognostic factors to predict the response to therapy or patients' survival. However, there is no consensual prognostic marker. Recently, for the treatment of LM, novel chemotherapeutic agents, targeted therapy and immunotherapy were added to more traditional treatments including intrathecal chemotherapy (IT-CTx), radiotherapy and systemic chemotherapy (6, 7, 8). However, there is no gold standard treatment and very few, if any, prospective randomized trial. Multimodality treatment is recommended only for patients with breast primary among various solid tumors according to recent National Comprehensive Cancer Network (NCCN) guideline (9). Therefore, the purpose of this study was to evaluate treatment patterns, outcome and prognosticators in patients with LM from solid tumors.
MATERIALS AND METHODS
Among patients treated at Seoul National University Hospital from January 2004 to March 2011, 82 patients met following eligibility criteria; 1) histological verification of primary solid tumor and 2) presence of malignant cell in cytologic evaluation of CSF, or demonstration of findings consistent with LM on brain or spine magnetic resonance imaging (MRI). Retrospective review of patient medical records was conducted to gather demographics, treatment patterns, and clinical outcomes. Two patients were excluded from further analysis due to the following reasons after review of medical records. One patient was diagnosed with sepsis simultaneously and expired prior to treatment of LM due to sepsis. The other patient was lost to follow up immediately after the diagnosis of LM.
Clinical endpoint and statistical analysis
Endpoint was determined as date of death or last available follow up. Date of diagnosis of primary tumor and LM were defined as the date of histological verification and reported date of CSF cytology or image study, respectively. Overall survival (OS) was calculated from date of diagnosis of LM to the endpoint. Estimated OS was obtained by using the Kaplan-Meier method. Possible prognostic factors including demographics, clinical manifestations, laboratory results, image findings and treatment patterns were analyzed to evaluate the effects on survival. Univariate analyses were done using the Log-rank test and multivariate analyses were performed through the Cox proportional hazards regression analyses with a backward stepwise selection. P values less than 0.05 were considered statistically significant. All statistical analyses were performed by IBM SPSS statistics version 19 (SPSS Inc., an IBM Company, Chicago, IL, USA).
Ethics statement
This study was approved by the institutional review board of Seoul National University Hospital (IRB No. H-1108-036-372). Informed consent for study enrollment was waived by the IRB.
RESULTS
Clinical profiles
Median age at diagnosis was 54 yr (range: 27-78 yr). Forty-nine patients (61.3%) were female and 31 (38.8%) were male. About one-third of the patients (36.3%) had an ECOG performance status class of 0 or 1 at the time of diagnosis of LM. The most frequent site of primary tumor was the lung (58.8%) followed by the breast (25.0%) and the stomach (16.3%). Most patients (93.8%) had distant metastasis prior to the diagnosis of LM. Forty-nine patients (61.3%) were treated with multiple chemotherapy regimens before diagnosis of LM (Table 1).
Table 1.
Patient characteristics
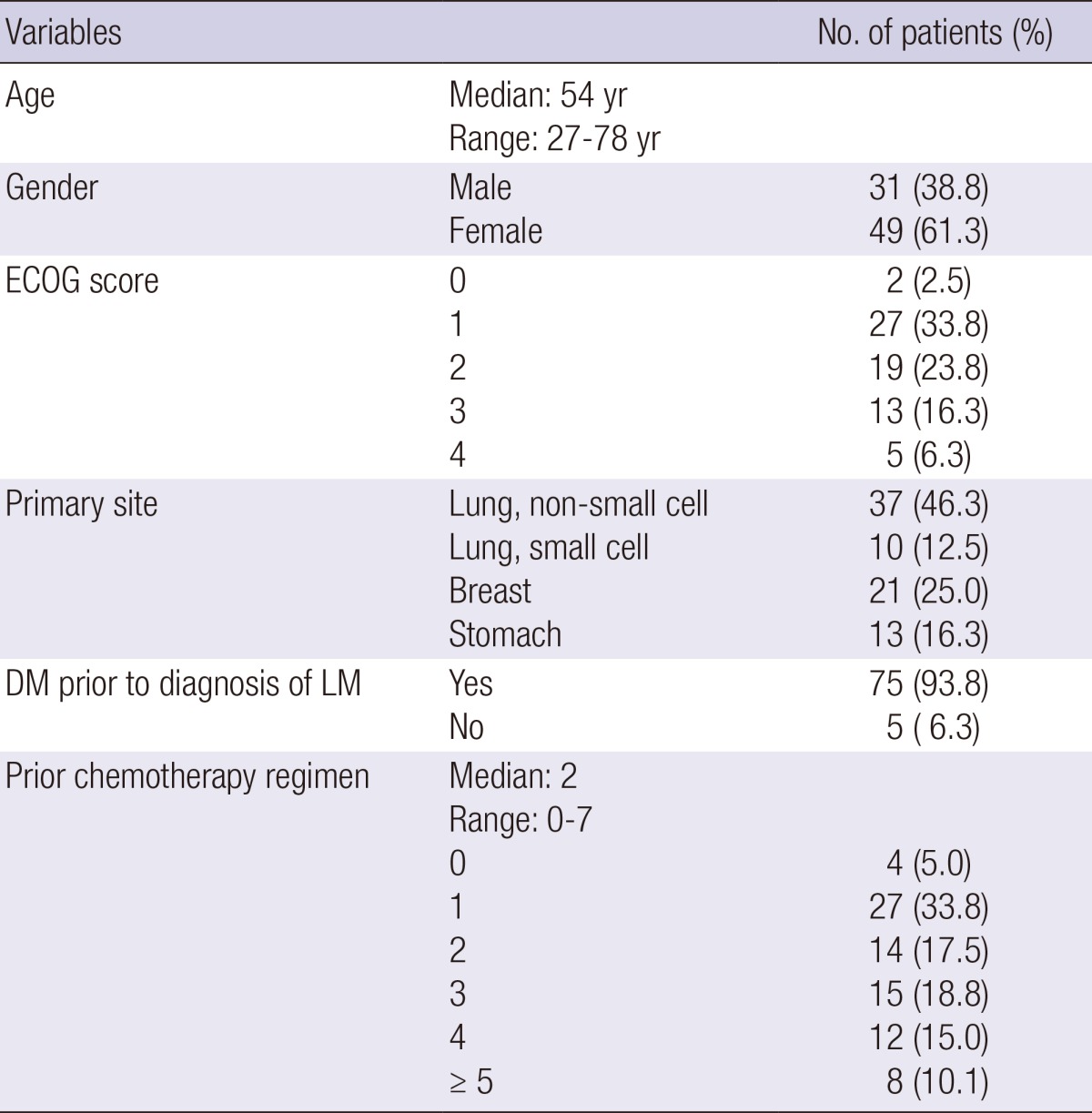
ECOG, Eastern Cooperative Oncology Group; DM, distant metastasis; LM, leptomeningeal metastasis.
Presenting symptoms of LM were categorized into three subgroups; symptoms suggestive of the involvement of the cerebrum, spinal cord or cranial nerve. Among cerebral involvement symptoms (62 patients, 77.5%), headache was most common with 43 patients (53.8%) followed by nausea or vomiting in 28 patients (35.0%). Among 23 patients (28.8%) presented with spinal involvement symptoms, weakness of extremities was most common manifestation with 13 patients (16.3%) and bowel/bladder dysfunction was second most common symptom with 9 patients (11.3%). Fifteen patients (18.8%) had symptoms of the cranial nerve involvement such as visual disturbance (6 patients, 7.5%) or diplopia (5 patients, 6.3%).
Diagnostic CSF tapping was performed in 77 patients. Among them, malignant cells in CSF cytology were found in 55 patients (68.8%). Increased opening pressure was observed in 17 patients (21.3%). CSF leukocytosis (WBC≥4) was found in 62 patients (77.5%). Number of patients with elevated protein (>50 mg/dL) and decreased glucose (<60 mg/dL) in CSF were 51 (63.8%) and 45 (56.3%), respectively. Brain MRI was obtained from all patients suspected of LM. Linear, nodular or diffuse leptomeningeal enhancement after administration of gadolinium was considered as positive finding for LM and these findings were shown in 70 patients (87.5%). Radiologic finding consistent with LM was found in 21 cases (26.3%) among 35 patients who underwent spine MR for suspected spinal involvement.
Treatment patterns
IT-CTx was given to 72 patients (90%). IT-CTx was mainly given through a lumbar puncture (95%). Methotrexate (15 mg) was most commonly administered agent with 57 patients. Triple regimen of methotrexate (15 mg), hydrocortisone (15 mg/m2) and cytarabine (30 mg/m2) was injected in 15 patients. Median number of cycle for IT-CTx was 6. IT-CTx was performed twice per week initially. After two consecutive negative conversions of CSF cytologies, IT-CTx was repeated weekly. For the maintenance IT-CTx, frequency of treatment was tapered to bi-weekly twice followed by tri-weekly twice and then patients were off the treatment.
Radiotherapy for LM was whole brain radiotherapy (WBRT) and/or focal spinal radiotherapy. WBRT was offered to 54 patients. The scheme of 30 Gy/10 fractions was most commonly applied (70.9%), and additional boost to focal lesion was given in 9 patients (range, 8-24 Gy). Spinal radiotherapy was delivered to 14 patients (17.5%). Most common field included lumbar spine (7 patients) followed by sacrum (5 patients). Most frequently applied RT schedule was also 30 Gy over 10 fractions.
Systemic therapy (Sys-Tx) was offered to 27 patients and various regimens were used according to primary tumor type. Sys-Tx included both cytotoxic chemotherapy (19 patients, taxane, platinum, etc.) and/or targeted therapy (14 patients, epidermal growth factor receptor - tyrosine kinase inhibitor (EGFR-TKI) or dual tyrosine kinase inhibitor).
Patients were grouped into three according to the number of treatment modalities. Number of patients treated with single treatment modality was 26 (32.5%). IT-CTx was most frequently chosen single modality with 19 patients (23.8%). Thirty-five patients (43.8%) were treated with dual modalities. Dominant combination was IT-CTx and WBRT with 29 patients (36.3%). Number of patients treated with triple modalities was 19 (23.8%) (Table 2).
Table 2.
Treatment modality for leptomeningeal metastases
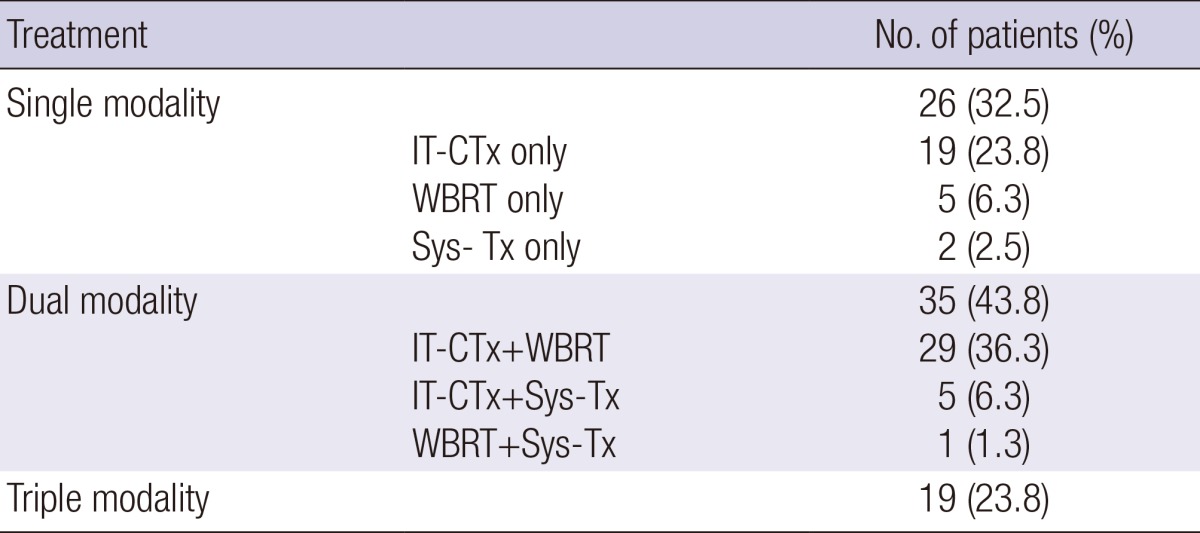
IT-CTx, intrathecal chemotherapy; Sys-Tx, Systemic therapy; WBRT, whole brain radiotherapy.
Survival & prognostic factors
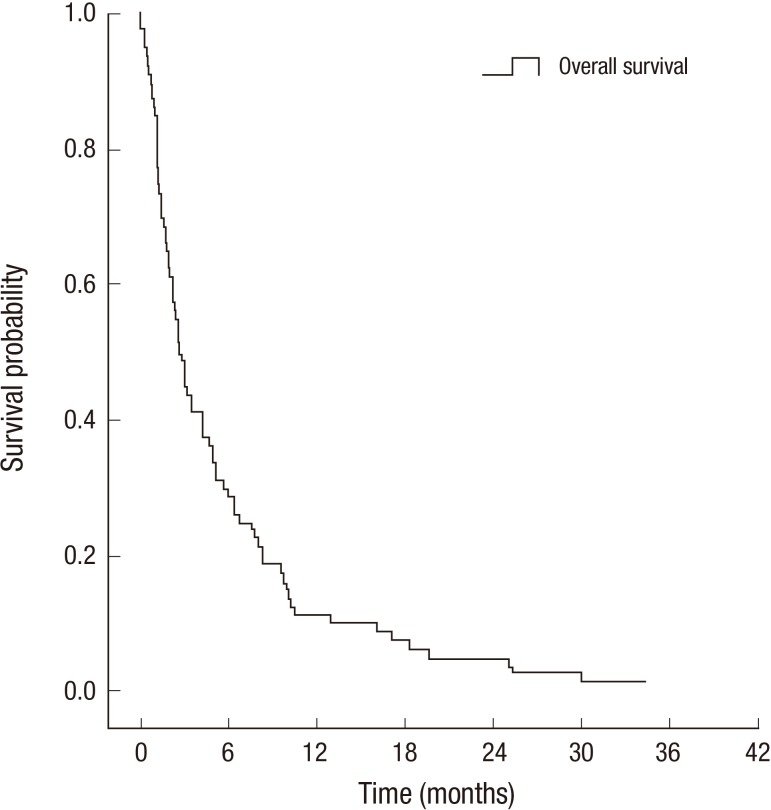
Median interval from diagnosis of primary tumor to LM was 15.7 months. Median follow-up duration after the diagnosis of LM was 2.8 months. Median survival (MS) was 2.7 months and the 1-yr survival rate was 11.3% (Fig. 1). Only one patient was alive at the time of analysis.
Fig. 1.
Overall survival rate.
Results of statistical analyses for OS are summarized in Table 3. Patients who were 65 yr old or younger had longer survival (P=0.003). Survival outcomes were not affected by gender, performance status or manifesting symptoms. There was significant difference according to primary site (P=0.003); LM from non-small cell lung cancer (NSCLC) showed most favorable outcomes with MS of 4.3 months whereas LM from small cell lung cancer (SCLC) had worst outcome with that of 1.4 months. MS of patients with breast cancer and stomach cancer were 2.7 and 1.6 months, respectively. Patients treated with multiple chemotherapy regimen prior to the LM diagnosis had trend toward decreased survival (P=0.074). Among laboratory results, patients with positive CSF cytology had inferior survival (P=0.005), whereas patients with CSF leukocytosis survived longer (P=0.004). There were no relationships between increased opening pressure, CSF protein or glucose level and survival outcome. Identification of LM on neuraxis image study did not affect outcomes, as well. In respect to treatment modality, Sys-Tx (P<0.001) significantly improved survival outcomes. IT-CTx (P=0.058) and WBRT (P=0.065) also showed marginally statistically significant impact on survival. Patients treated with combined modality had statistically significant survival prolongation (P<0.001).
Table 3.
Prognostic factors for survival
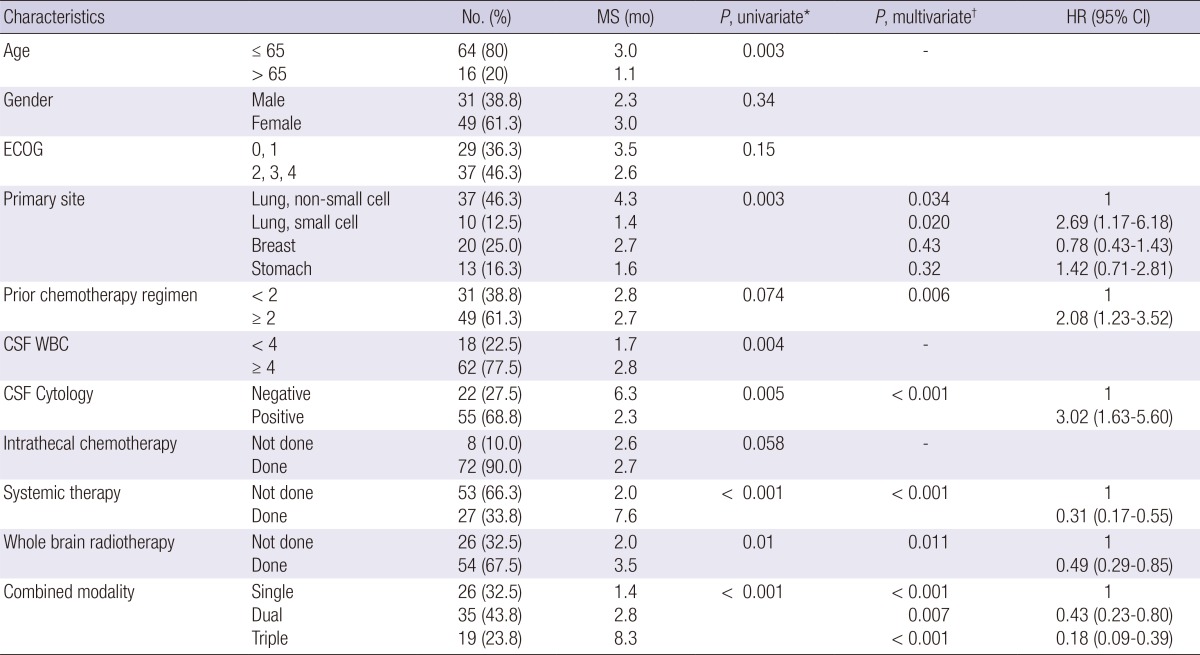
*Log-rank test; †Cox proportional hazards regression analyses with a backward stepwise selection. ECOG, Eastern Cooperative Oncology Group; CSF, cerebrospinal fluid; MS, median survival; HR, hazard ratio; CI, confidence interval.
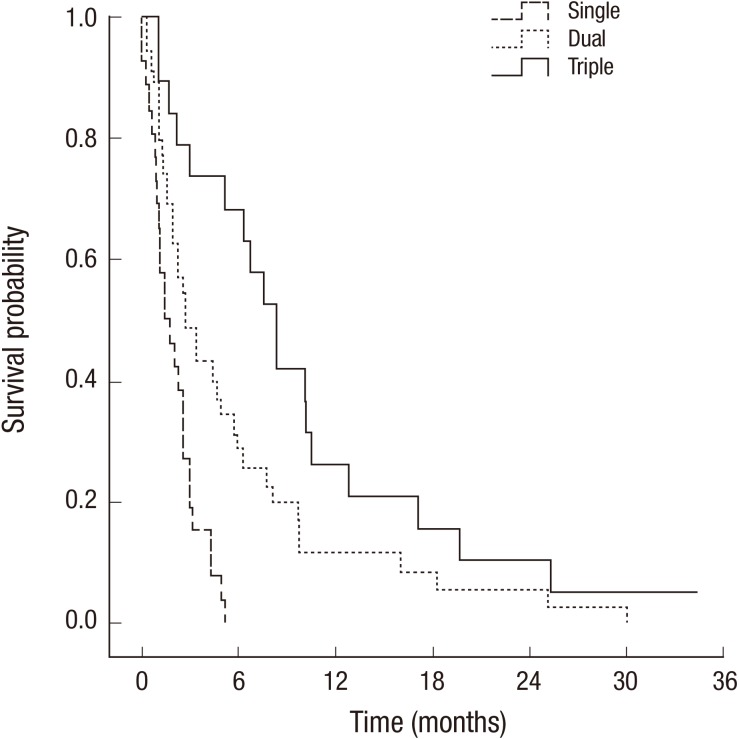
In multivariate analysis, survival outcomes were unfavorable in patients treated with multiple chemotherapy regimen before LM (P=0.006). CSF cytology-positive group had shortened survival (P<0.001). Statistically significant survival improvement was shown in patients treated with Sys-Tx group (P<0.001) and WBRT (P=0.011). Combined modality treatment (CMT) showed superior survival outcomes (P<0.001, Fig. 2), whereas age, CSF leukocytosis, and IT-CTx lost their statistical significance, when adjusted for other factors.
Fig. 2.
Overall survival rate according to combined treatment modality.
Subgroup analysis for NSCLC patients
Subgroup analysis was performed for NSCLC patients (n=37). Factors with improved survival in univariate analysis were; absence of cranial nerve symptoms (P=0.036), fewer chemotherapy regimen prior to LM (P=0.024), CSF leukocytosis (P=0.013), negative CSF cytology (P=0.038), IT-CTx (P=0.007), Sys-Tx (P<0.001), combined modality treatment (P=0.001) (Table 4).
Table 4.
Univariate analysis for NSCLC patients
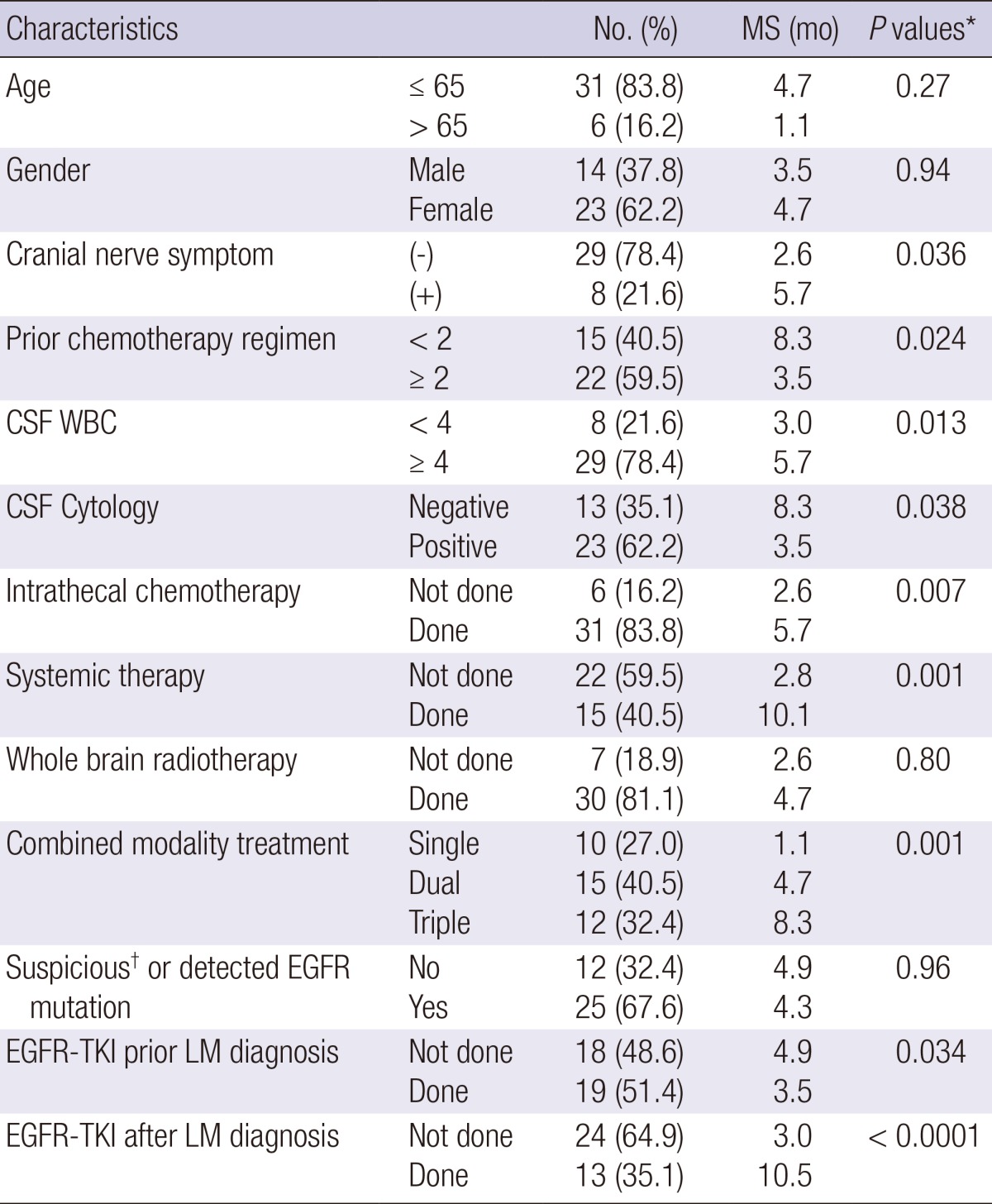
*Log-rank test; †highly suspicious of EGFR mutation, clinically based on adenocarcinoma histology and never-smoking history. NSCLC, non-small cell lung cancer; CSF, cerebrospinal fluid; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; LM, leptomeningeal metastasis; MS, median survival.
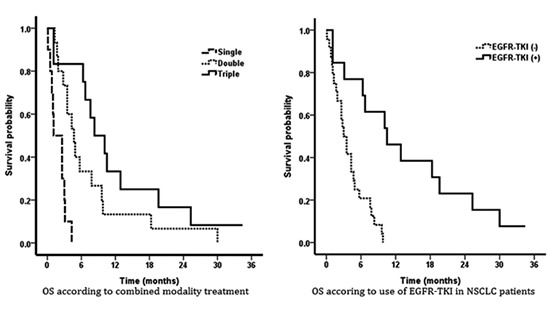
Unlike other solid tumors, target agents were commonly used in NSCLC patients. Further analysis was performed on EGFR-TKI and its associated factors. Evaluation of EGFR mutation was performed in 19 patients and 12 patients (32.4%) had gene mutation. Among patients without EGFR mutation analysis, 13 patients were suspected to have high probability of mutation based on two favorable clinical parameters, adenocarcinoma histology and never-smoking history. EGFR-TKI was prescribed to these patients based on this speculation. Detected or suspicious EGFR mutation had no significant impact on survival in univariate analysis (P=0.96). During the entire course of treatment for NSCLC and LM, 28 patients (75.7%) were exposed to EGFR-TKI and EGFR-TKI exposure was related with marginal prolongation of survival (P=0.056). Nineteen (51.4%) and 13 patients (35.1%) received the EGFR-TKI before and after LM diagnosis, respectively. Patients exposed to EGFR-TKI prior to LM diagnosis had a relatively short survival (P=0.034), whereas, EGFR-TKI after LM diagnosis led to statistically significant improvement in survival (P<0.001). Multivariate analysis revealed that positive CSF cytology, treatment of combined modality rather than single modality, and EGFR-TKI after LM diagnosis were statistically significant prognostic factors (Table 5, Fig. 3).
Table 5.
Multivariate analysis for NSCLC patients
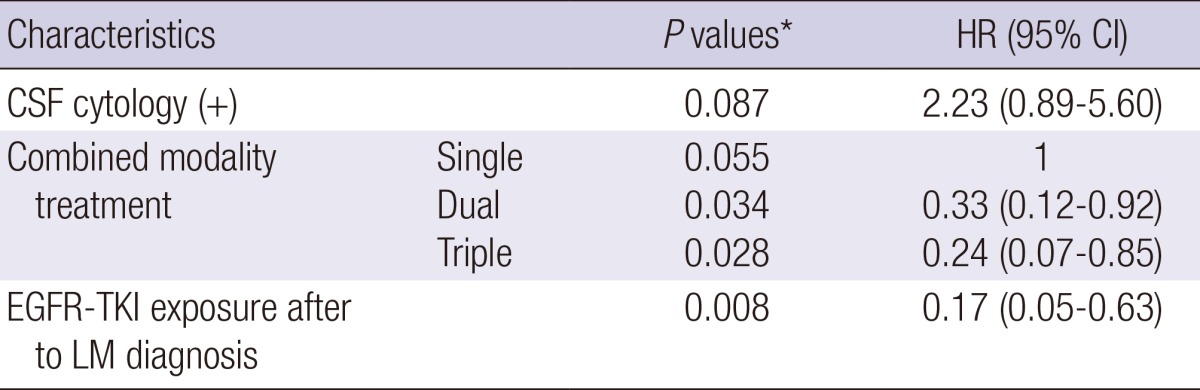
*Cox proportional hazards regression analyses with a backward stepwise selection. NSCLC, non-small cell lung cancer; CSF, cerebrospinal fluid; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; LM, leptomeningeal metastasis, HR, hazard ratio; CI, confidence interval.
Fig. 3.
Overall survival according to combined modality treatment (A) and use of EGFR-TKI after diagnosis of leptomeningeal metastasis (B) in NSCLC patients.
DISCUSSION
Patients with LM, which is the third most common metastatic site of the central nervous system (7), have a poor prognosis with reported MS of 4-6 weeks for untreated patients (1, 7, 10, 11). The proper treatment for LM could give not only palliation of symptoms but also prolongation of survival. But treatment of LM is complicated as standard of care is not established and the role of each treatment modality is not well defined (10). Prognostic factors also have been reported in many literatures, but it is still controversial.
In the current study including 80 patients with LM from various solid tumors, MS was 2.7 months and 1-yr survival rate was 11.3%, which is similar to previous reports (1, 3, 4, 8, 11, 12). Patients with NSCLC showed the most favorable outcome with a MS of 4.3 months followed by patients with breast cancer in presenting cohort. This is in contrast to previous reports where breast cancer is considered most favorable primary tumor (3, 13, 14). Reported MS for breast primary ranges from 3 to 7.5 months (11, 13, 15), whereas that for NSCLC primary is from 1.5 to 5 months (10). Shorter survival of patients with breast primary in the current study compared to other series may be related with inclusion of patients with more aggressive tumor biology. The influence of molecular subtype in patients with LM from breast has been reported by Gauthier et al. (13, 16). In the current cohort, proportion of patients without hormonal receptor was relatively higher than other studies (13, 16). MS of hormonal receptor positive group (7 patients, 35%) was 5.1 months, whereas that for hormonal receptor negative group (12 patients, 60%) was only 1.7 months. Though statistical significance was not found, this may be mere reflection of insufficient number of analyzed patients.
Survival outcomes were significantly affected by positive CSF cytology, exposure to multiple chemotherapy regimens for the primary tumor treatment, administration of Sys-Tx, WBRT and CMT for LM, after adjusting for other possibly related clinical factors. There were no significant effect on survival by performance status, age, presence of symptoms suggesting specific structure involvement, extent of the central nervous system (CNS) infiltration observed on imaging study, and CSF laboratory findings except for cytology. In the previous studies, performance status was considered as significant prognostic factor (10, 13, 16). But it is not conclusive because some studies failed to show the significance in multivariate analysis, as in the current study (17). As most studies were conducted retrospectively, performance status was estimated by researcher based on medical records in contrast to other variables, such as CSF or image findings and therefore is subject to bias. Boogerd et al. (15) reported that age older than 55 yr is one of negative prognostic factors, but study by Balm et al. (18) and current study showed that age is not a predictive factor. According to manifesting symptoms, Boogerd et al. (15) and Balm et al. (18) demonstrated that patients with symptoms related to cranial nerve or cerebral involvement had worse prognosis, respectively. However the relationship between symptoms and outcomes was conflicted with each other studies (15, 16, 19). Although the neuraxis imaging study is one of most important diagnostic tools for LM, it is unclear whether extent of CNS infiltration on imaging study affects the survival. Other studies as well as current study, showed that presence of parenchymal CNS metastases or greater imaging evidence of LM is not indicative of inferior survival (15, 16, 20).
The meaning of CSF study also has controversies. Boogerd et al. (15) reported that patients with CSF glucose of 43 mg/dL or less had significantly short survival in multivariate analysis. They explained that CSF glucose level was decreased because of consumption by circulating tumor cells and impairment of glucose transfer caused by meningeal involvement. They also described that normal CSF protein means the absence of wide spread leakage through involved meninges and found that patients with normal protein level had longer MS. But studies by Park et al. (10) and Gauthier et al. (16) failed to show its significance. In contrast to the current study, malignant tumor cell in CSF at diagnosis was not correlated with survival in the study by Harstad et al. (20). These results might reflect the quantitative uncertainty of CSF study. According to Murray et al., even if there is no impairment of CSF flow, level of glucose, protein and malignant cells in CSF specimen might vary at different levels of the neuraxis (21).
Of our cohort, patients treated with two or more regimen of chemotherapy prior to LM showed poor outcome. Gauthier et al. (16) also showed that overall survival was independently influenced by the number of prior chemotherapy which reflects chemo-sensitivity of remaining cancer cells.
In the current study, trend toward improved survival for patients undergoing IT-CTx in univariate analysis was lost after multivariate analysis, whereas positive impact of WBRT, Sys-Tx and CMT was sustained. In the past, IT-CTx was considered as the main treatment modality in LM, whereas the role of Sys-Tx was questioned due to belief that intravenously injected chemotherapeutic agents was unavailing because of BBB (13). Thus, older studies mainly focused on validating the role of IT-CTx (18, 19, 20, 22, 23). Recently, however, the role of IT-CTx has been questioned. In the study by Boogerd et al. (15), survival outcomes of non-IT-CTx treated group were identical to those of IT-CTx group. Park et al. (10) and Bokstein et al. (24) also showed no significant difference of MS when two groups treated with or without IT-CTx was compared.
Nowadays, the role of Sys-Tx is more emphasized. In patients with LM, disturbed BBB enables Sys-Tx agents to penetrate into the CSF space (13). In concordance with results from current study, other studies also confirmed survival improvement by Sys-Tx in patients with LM from solid tumor (10, 13, 16). In the study by Glantz et al. (25), high dose Sys-Tx showed significant improvement of survival over IT-CTx (MS, 13.8 mo vs. 2.3 mo). Particularly, in several studies, LM from breast primary seemed to respond to intravenously injected CTx (13, 16). Thus, NCCN guideline recommends Sys-Tx to responsive cancer such as breast cancer or lymphoma (9).
However, with introduction of EGFR TKIs in NSCLC, there were many attempts to use it for LM from NSCLC. Most studies showed the favorable outcomes. Park et al. (10) and Yi et al. (26) demonstrated that Sys-Tx such as EGFR TKIs could lengthen survival in patients with LM from NSCLC as well. Our results of NSCLC subgroup analysis also showed that EGFR TKI for LM significantly reduced risk of death (HR 0.15, MS 10.1 mo) and the prior exposure to EGFR-TKI did not compromised the survival. In the current study, gefitinib was mainly used before LM diagnosis, whereas most patients were treated with erlotinib after LM diagnosis. Many previous studies reported that gefitinib does not seem to penetrate BBB. Thus, tumor cells within neuraxis were naive to gefitinib and were not resistant to the EGFR-TKI. In addition, erlotinib seems to penetrate the BBB more readily, making it more effective for control of the LM (26, 27). As for the EGFR mutation, though it was not directly related to the prognosis in the current study, any conclusion should not be drawn, considering small sample size.
On the other hand, radiotherapy for LM has traditionally been limited to bulky disease or site of CSF flow obstruction (9, 11). The purpose of these focal RT was to provide symptom palliation. WBRT has been used for LM in limited institutions. Rudnicka et al. (13) reported that WBRT prolonged survival in univariate analysis. Though this was not valid in multivariate analysis, they contended that use of WBRT has a positive impact on the quality of life and thus should be included in multimodality treatment for LM. Study of Park et al. (10) demonstrated the impact of WBRT on survival in multivariate analysis (P=0.032, Adjusted HR 2.76 [1.09-7.0]). However, recently published largest series in this regard by Patrick et al. (28) showed that survival was not improved by WBRT in patients with primary lung cancer (P=0.84). In current series, though WBRT was found to be statistically significant prognosticator in entire cohort, statistical significance was lost in the NSCLC subgroup. This may reflect a need for disease specific treatment strategy. Unlike NSCLC series, Kim et al. (29) reported survival benefit from addition of chemotherapy to WBRT in patients with LM from breast cancer (P<0.001). They also showed that chemotherapy after WBRT was the most powerful prognostic factors for survival in the multivariate analysis, stressing the importance of combined modality management.
Aforementioned improved treatment outcomes from Sys-Tx and RT suggest the need of CMT for LM (13, 23). Even though performance status did not affect survival in this study, CMT might not be feasible to compromised patients. In the current study, there was slight difference in the proportion of patients with poor performance status (ECOG 2 or higher) according to treatment modality (60% in single, 62% in double, and 41% in triple, respectively), though it was not statistically significant (P=0.353). Thus, other factors to select patients more suited to undergo CMT for LM should be studied in the future to improve the survival of these patients with dismal prognosis.
In the present study, estimated survival curve is relatively reliable because all patients included were not censored. However, as it was retrospective study, it is not free from inherent limitations including treatment selection bias. There is also limitation stemming from limited number of patients and heterogeneity of primary sites. It should also be noted that in the current study, role of radiotherapy in conjunction with EGFR-TKI could not be sought, as there were no patients exposed to both modalities.
Unlike previous studies, survival was not affected by patient characteristics such as age, performance status and symptoms at the time of diagnosis of LM in the current study. However, as these results were drawn from relatively small heterogeneous cohort, it should not be conclusive. Survival in patients with primary NSCLC was comparable to that in patients with primary breast cancer. Furthermore, survival improvement was significant with combined modality treatment including Sys-Tx and WBRT over single modality treatment. Thus, multimodality treatment should be sought for patients with feasible performance to tolerate treatment and those with not only breast primary but also NSCLC primary tumors. In addition, use of EGFR-TKI after LM diagnosis should be considered for NSCLC patients. Further study is warranted to define the role of EGFR-TKI in conjunction with radiotherapy.
Footnotes
Authors have no conflict of interest to disclose.
References
- 1.Bruno MK, Raizer J. Leptomeningeal metastases from solid tumors (Meningeal carcinomatosis) Cancer Treat Res. 2005;125:31–52. doi: 10.1007/0-387-24199-x_3. [DOI] [PubMed] [Google Scholar]
- 2.Glass JP, Melamed M, Chernik NL, Posner JB. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology. 1979;29:1369–1375. doi: 10.1212/wnl.29.10.1369. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain MC. Neoplastic meningitis. Oncologist. 2008;13:967–977. doi: 10.1634/theoncologist.2008-0138. [DOI] [PubMed] [Google Scholar]
- 4.Park JO, Shin HJ, Kim HJ, Lee SW, Jeung HC, Kim SM, Yoo NC, Chung HC, Kim JH, Kim BS, et al. Leptomeningeal carcinomatosis in solid tumors; clinical manifestation and treatment. J Korean Cancer Assoc. 2001;33:34–40. [Google Scholar]
- 5.Jaeckle KA. Neoplastic meningitis from systemic malignancies: diagnosis, prognosis and treatment. Semin Oncol. 2006;33:312–323. doi: 10.1053/j.seminoncol.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Herrlinger U, Weller M, Schabet M. New aspects of immunotherapy of leptomeningeal metastasis. J Neurooncol. 1998;38:233–239. doi: 10.1023/a:1005948722912. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain MC. Leptomeningeal metastasis. Semin Neurol. 2010;30:236–244. doi: 10.1055/s-0030-1255220. [DOI] [PubMed] [Google Scholar]
- 8.Groves MD. New strategies in the management of leptomeningeal metastases. Arch Neurol. 2010;67:305–312. doi: 10.1001/archneurol.2010.18. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Central Nervous System Cancers Version 2. 2013. [accessed on 4 April 2013]. Available at http://www.nccn.org/professionals/physician_gls/pdf/cns.pdf.
- 10.Park JH, Kim YJ, Lee JO, Lee KW, Kim JH, Bang SM, Chung JH, Kim JS, Lee JS. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer. 2012;76:387–392. doi: 10.1016/j.lungcan.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain MC. Leptomeningeal metastasis. Curr Opin Neurol. 2009;22:665–674. doi: 10.1097/WCO.0b013e3283322a92. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain MC. Leptomeningeal metastases: a review of evaluation and treatment. J Neurooncol. 1998;37:271–284. doi: 10.1023/a:1005976926058. [DOI] [PubMed] [Google Scholar]
- 13.Rudnicka H, Niwińska A, Murawska M. Breast cancer leptomeningeal metastasis--the role of multimodality treatment. J Neurooncol. 2007;84:57–62. doi: 10.1007/s11060-007-9340-4. [DOI] [PubMed] [Google Scholar]
- 14.Hildebrand J. Prophylaxis and treatment of leptomeningeal carcinomatosis in solid tumors of adulthood. J Neurooncol. 1998;38:193–198. doi: 10.1023/a:1005976003347. [DOI] [PubMed] [Google Scholar]
- 15.Boogerd W, Hart AA, van der Sande JJ, Engelsman E. Meningeal carcinomatosis in breast cancer. Prognostic factors and influence of treatment. Cancer. 1991;67:1685–1695. doi: 10.1002/1097-0142(19910315)67:6<1685::aid-cncr2820670635>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier H, Guilhaume MN, Bidard FC, Pierga JY, Girre V, Cottu PH, Laurence V, Livartowski A, Mignot L, Diéras V. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol. 2010;21:2183–2187. doi: 10.1093/annonc/mdq232. [DOI] [PubMed] [Google Scholar]
- 17.Lee JL, Kang YK, Kim TW, Chang HM, Lee GW, Ryu MH, Kim E, Oh SJ, Lee JH, Kim SB, et al. Leptomeningeal carcinomatosis in gastric cancer. J Neurooncol. 2004;66:167–174. doi: 10.1023/b:neon.0000013462.43156.f4. [DOI] [PubMed] [Google Scholar]
- 18.Balm M, Hammack J. Leptomeningeal carcinomatosis: presenting features and prognostic factors. Arch Neurol. 1996;53:626–632. doi: 10.1001/archneur.1996.00550070064013. [DOI] [PubMed] [Google Scholar]
- 19.Bruna J, González L, Miró J, Velasco R, Gil M, Tortosa A Neuro-Oncology Unit of the Institute of Biomedical Investigation of Bellvitge. Leptomeningeal carcinomatosis: prognostic implications of clinical and cerebrospinal fluid features. Cancer. 2009;115:381–389. doi: 10.1002/cncr.24041. [DOI] [PubMed] [Google Scholar]
- 20.Harstad L, Hess KR, Groves MD. Prognostic factors and outcomes in patients with leptomeningeal melanomatosis. Neuro Oncol. 2008;10:1010–1018. doi: 10.1215/15228517-2008-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray JJ, Greco FA, Wolff SN, Hainsworth JD. Neoplastic meningitis. Marked variations of cerebrospinal fluid composition in the absence of extradural block. Am J Med. 1983;75:289–294. doi: 10.1016/0002-9343(83)91207-x. [DOI] [PubMed] [Google Scholar]
- 22.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49:759–772. doi: 10.1002/1097-0142(19820215)49:4<759::aid-cncr2820490427>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, Swinnen LJ, Maria B, LaFollette S, Schumann GB, Cole BF, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5:3394–3402. [PubMed] [Google Scholar]
- 24.Bokstein F, Lossos A, Siegal T. Leptomeningeal metastases from solid tumors: a comparison of two prospective series treated with and without intra-cerebrospinal fluid chemotherapy. Cancer. 1998;82:1756–1763. [PubMed] [Google Scholar]
- 25.Glantz MJ, Cole BF, Recht L, Akerley W, Mills P, Saris S, Hochberg F, Calabresi P, Egorin MJ. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol. 1998;16:1561–1567. doi: 10.1200/JCO.1998.16.4.1561. [DOI] [PubMed] [Google Scholar]
- 26.Yi HG, Kim HJ, Kim YJ, Han SW, Oh DY, Lee SH, Kim DW, Im SA, Kim TY, Kim CS, et al. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for leptomeningeal metastasis from non-small cell lung cancer patients with sensitive EGFR mutation or other predictive factors of good response for EGFR TKI. Lung Cancer. 2009;65:80–84. doi: 10.1016/j.lungcan.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Lee E, Keam B, Kim DW, Kim TM, Lee SH, Chung DH, Heo DS. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol. 2013;8:1069–1074. doi: 10.1097/JTO.0b013e318294c8e8. [DOI] [PubMed] [Google Scholar]
- 28.Morris PG, Reiner AS, Szenberg OR, Clarke JL, Panageas KS, Perez HR, Kris MG, Chan TA, DeAngelis LM, Omuro AM. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7:382–385. doi: 10.1097/JTO.0b013e3182398e4f. [DOI] [PubMed] [Google Scholar]
- 29.Kim HJ, Im SA, Keam B, Kim YJ, Han SW, Kim TM, Oh DY, Kim JH, Lee SH, Chie EK, et al. Clinical outcome of central nervous system metastases from breast cancer: differences in survival depending on systemic treatment. J Neurooncol. 2012;106:303–313. doi: 10.1007/s11060-011-0664-8. [DOI] [PubMed] [Google Scholar]