Abstract
Pulmonary surfactant (PS) therapy was proven to be highly successful for the treatment of respiratory distress syndrome in premature infants. As a results, early prophylactic (EP) PS therapy has been introduced recently in Europe, the US and Korea. However, no multi-center study was compared EP and late selective (LS) PS therapies in Korea. We performed a retrospective multi-center study to compare the outcomes of EP and LS PS therapies in very preterm infants. We analyzed clinical morbidity and mortality for 1,291 infants in 2010 (LS group) and 1,249 infants in 2011 (EP group); the infants were born <30 weeks of gestation and had birth weight ≤1,250 g, and were chosen from 53 neonatal intensive care units in Korea. Compared to the LS group (22.5%), the overall mortality was better in the EP group (19.9%) and there was no increased need for retreatment.There were additional benefits in the EP group such as fewer associated complications. To the best of knowledge, our study is the first nationwide Korean study to compare the outcomes of EP and LS therapies, and it provides evidences that EP PS therapy is important in very preterm infants to improve for survival and reduce morbidities.
Graphical Abstract
Keywords: Surfactant; Effectiveness; Treatment; Treatment Outcome; Infant; Preterm; Respiratory Distress Syndrome, Newborn
INTRODUCTION
Respiratory distress syndrome (RDS) is defined as respiratory failure due to lack of pulmonary surfactant (PS). It is the most common pulmonary disease and is a major cause of death in premature infants. In 1980, an artificial PS preparation was successfully developed by Fujiwara et al. (1) by mixing bovine PS extracts with artificial phospholipids; clinical trial in 10 premature infants showed dramatic improvement in clinical symptoms and prognosis. From the 1990s onwards, several artificial PSs have been produced commercially around the world as standard therapy for RDS. This has markedly improved the survival of preterm, low birth weight, and very low birth weight infants, and has resulted in improvements in neonatal and infant mortality (2).
In Korea, Surfacten® (Mitsubishi Pharma Corporation, Tokyo, Japan) has been used since 1991, Exosurf® (Wellcome Foundation Ltd, London, UK) from 1991 to 1997, Curosurf® (ChiesiFarmaceutici, Parma, Italy) since 2003, Newfactan® (Yuhan Pharm Corporation, Seoul, Korea) since 1996, and Infasurf® (Samaritan Pharmaceuticals, Las Vegas, NV, USA) since 2007. There have been five national reports (3, 4, 5, 6, 7) in Korea on the outcomes of PS therapy in all newborn infants, showing a marked improvement in mortality ranging from 40.0% in 1990, to 30.0% in 1996, 18.7% in 2002, 14.3% in 2007 and 10.1% in 2010. The PS therapy in these reports was late selective (LS), not early prophylaxis (EP).
PS therapy can be divided into EP and LS (8, 9) according to the timing of therapy. EP therapy corresponds to both application of PS after diagnosis of RDS for early rescue (1-2 hr after birth) and prophylaxis within 15 min of birth before a diagnosis of RDS in infants born at <30-32 weeks of gestation. LS therapy corresponds to application of PS after diagnosis of RDS for late rescue after birth (>2 hr after birth). EP PS therapy and rapid extubation (InSuRE) immediately after birth, which comprises the early administration of PS replacement followed by immediate extubation and stabilization on continuous positive airway pressure (CPAP), has had a favorable outcome (10, 11). Several meta-analyses report that EP therapy has a better outcome than LS therapy (2, 9, 12, 13). Among them, Soll and Morley reported that EP resulted in an odds ratio of 0.62 for overall mortality (14).
PS therapy for RDS in Korea is controlled by the national medical insurance policy of the Korean Ministry of Health and Welfare (KMHW), which has limited its usage strictly to cases of oxygen need or mechanical ventilation and positive signs on chest radiograph since 1991. Repeated dosing was approved in 2002, and thereafter, EP PS replacement therapy for RDS was approved in 2011. We therefore undertook a comparison the efficacy of EP therapy (<2 hr after birth) in 2011 with that of LS therapy (≥2 hr after birth) in 2010 in Korea, analyzing mortality and complications among very preterm infants born at <30 weeks of gestation or with birth weight ≤1,250 g.
MATERIALS AND METHODS
We analyzed 2,540 cases of PS use in infants from 53 neonatal intensive care units (NICUs) in Korea during two study periods. The infants were born at <30 weeks of gestation or had birth weight ≤1,250 g. We divided the patients into two groups based on the study period and type of PS therapy: the LS group (1,291 cases in January to December 2010) and the EP group (1,249 cases in January to December 2011).
From January to December 2010, the indications in the national medical insurance policy of the KMHW for PS therapy for RDS in the LS group were as follows: 1) clinical evidence of respiratory difficulties; 2) radiologic evidences of RDS (diffuse granular opacities or air-bronchogram in the both lung fields; 3) need for mechanical ventilation (FiO2>0.4) to sustain optimal blood O2 saturation (50-80 mmHg). From January to December 2011, the national health insurance policy was changed as follows (EP group): administration of PSs to infants born at <30 weeks of gestation or with birth weight ≤1,250 g within 2 hr of delivery in the delivery room or neonatal intensive care unit (NICU) without radiological evidence of RDS.
We collected the following data for each group from each NICU; birth weight, gestational age, time of PS therapy after birth, PS dose, response after PS use, length of endotracheal intubation, length of mechanical ventilation, days of oxygen supply, and admission days in the NICU. Response after PS administration was graded as follows: 1) good response: persistent increase of arterial-to alveolar oxygen pressure ratio (a/APO2) >0.2, decrease of mean airway pressure (MAP) >2 cmH2O at least 120 hr after PS use; 2) relapse: good response during the first 24 hr, then decrease of a/APO2 to 50% of the baseline level, increase in MAP to 50% of baseline level; 3) poor: no increase in a/APO2 >0.2 after 6 hr of PS use.
We also compared the followings, such as sepsis, disseminated intravascular coagulation (DIC), patent ductus arteriosus (PDA), pneumothorax or pneumomediastinum, intraventricular hemorrhage (IVH) ≥grade 2, pulmonary interstitial emphysema (PIE), bronchopulmonary dysplasia (BPD), persistent pulmonary hypertension (PPHN), retinopathy of prematurity (ROP), necrotizing enterocolitis (NEC), pulmonary hemorrhage, pneumonia, and cholestasis. PDA was diagnosed by echocardiography, and only symptomatic cases were identified. IVH was defined as a diagnosis of higher than grade II on cranial ultra-sonography by classification of Papile et al. (15). BPD was defined by Jobe and Bancalari criteria (16) as a need for supplemental oxygen at 36 weeks of gestation. PPHN was defined as right-to-left or bidirectional shunting across a patent foramen ovale and a ductus arteriosus and evidence of increased pulmonary pressure without other cardiac anomalies visualized by real-time echocardiography. NEC was defined as a diagnosis higher than stage II in the modified Bell's staging criteria (17), or necessitating surgery. Cholestasis was defined as an elevation of serum direct bilirubin levels to >2.0 mg/dL. Sepsis was defined by clinical findings, and the presence of bacteria or fungus in blood culture. Pneumothorax, pneumopericardium and PIE were diagnosed using chest radiography.
The data were collected results of two years (2010 and 2011) in hospital NICUs, so we could only analyze aggregated data by hospital units. Summary measures (means, proportions) of study variables in each year were estimated with weighting by the number of cases in each hospital and compared before and after introduction of the indications of the national medical insurance policy of the KMHW for PS therapy for RDS in 2011. Weighted paired t-test and multilevel regression anaysis were performed assuming that the effect of each hopsital was random. P values<0.05 were considered significant. Statistical analysis was performed by a statistician without knowledge of patients group or outcomes by using Stata 12.
Ethics statement
This study was approved by the institutional review board of Kyung Hee University Hospital at Gangdong (IRB No. 2014-02-001).
RESULTS
We analyzed 1,291 cases in 2010 (LS group) and 1,249 cases in 2011 (EP group) from 53 NICUs. Table 1 shows the clinical demographic characteristics and method of PS use in enrolled cases. The mean birth weight of the infants in the EP group (959 g) was significantly smaller than that of the infants in the LS group (1,005 g) (P=0.040), although the gestational age of the two groups was similar. The time interval from birth to treatment was significantly different between the two groups, as expected (2.9 hr in LS group vs. 0.6 hr in EP group, P=0.002). Surfacten® was most popular preparation used in both groups, and the use of Curosurf® increased from the 2010 to the 2011 study period. A single dose of PS was frequently used in both groups. The use of multiple doses of PS was not significantly different between the two groups, even though the use of PS in the EP group was earlier compared to that in the LS group.
Table 1.
Demographic characteristics and pulmonary surfactant use in enrolled cases for late selective and early prophylactic groups in Korea
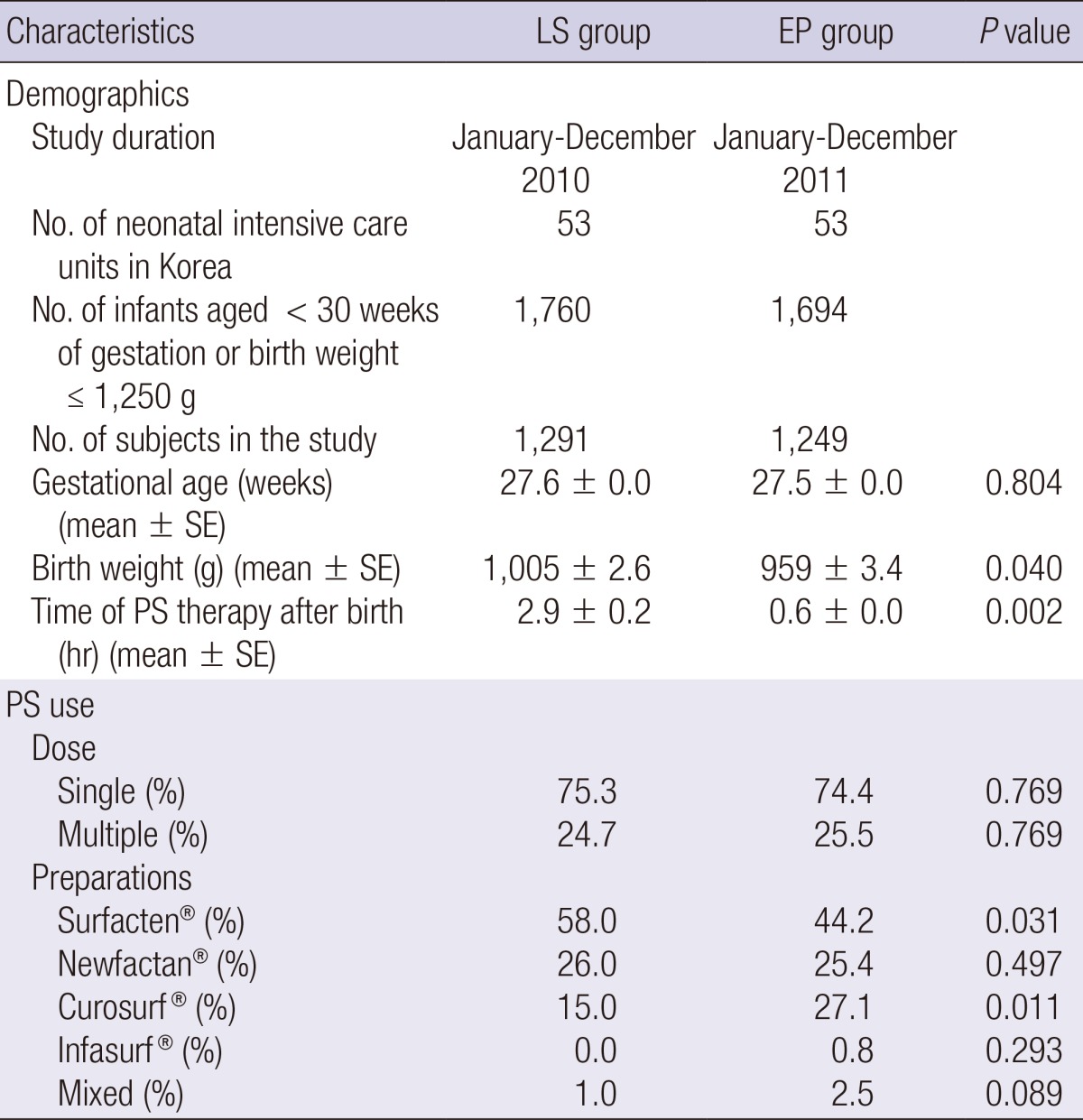
EP, early prophylactic use; LS, late selective use; PS, pulmonary surfactant.
Table 2 shows the clinical outcome after PS therapy and the morbidity of enrolled cases. There was no difference in the relapse rate after PS between the two groups, although the EP group had less frequency of good responses than those in the LS group due to more frequent use of PS in the delivery room. The length of endotracheal intubation was shorter in the EP group than in the LS group, even though no significant difference in the time of mechanical ventilation (including CPAP) between the two groups. Duration of oxygen supply was longer in the EP group than in the LS group, probably due to the smaller birth weight of infants in the EP group. There was no significant difference in NICU admission days between the two groups. Regarding morbidities in the two groups, there was a significant reduction in the incidence of PDA, BPD, PPHN, pneumonia, and cholestasis in the EP group than in the LS group.
Table 2.
Comparison of clinical outcomes after pulmonary surfactant therapy and morbidity in late selective and early prophylactic groups for pulmonary surfactant use in Korea
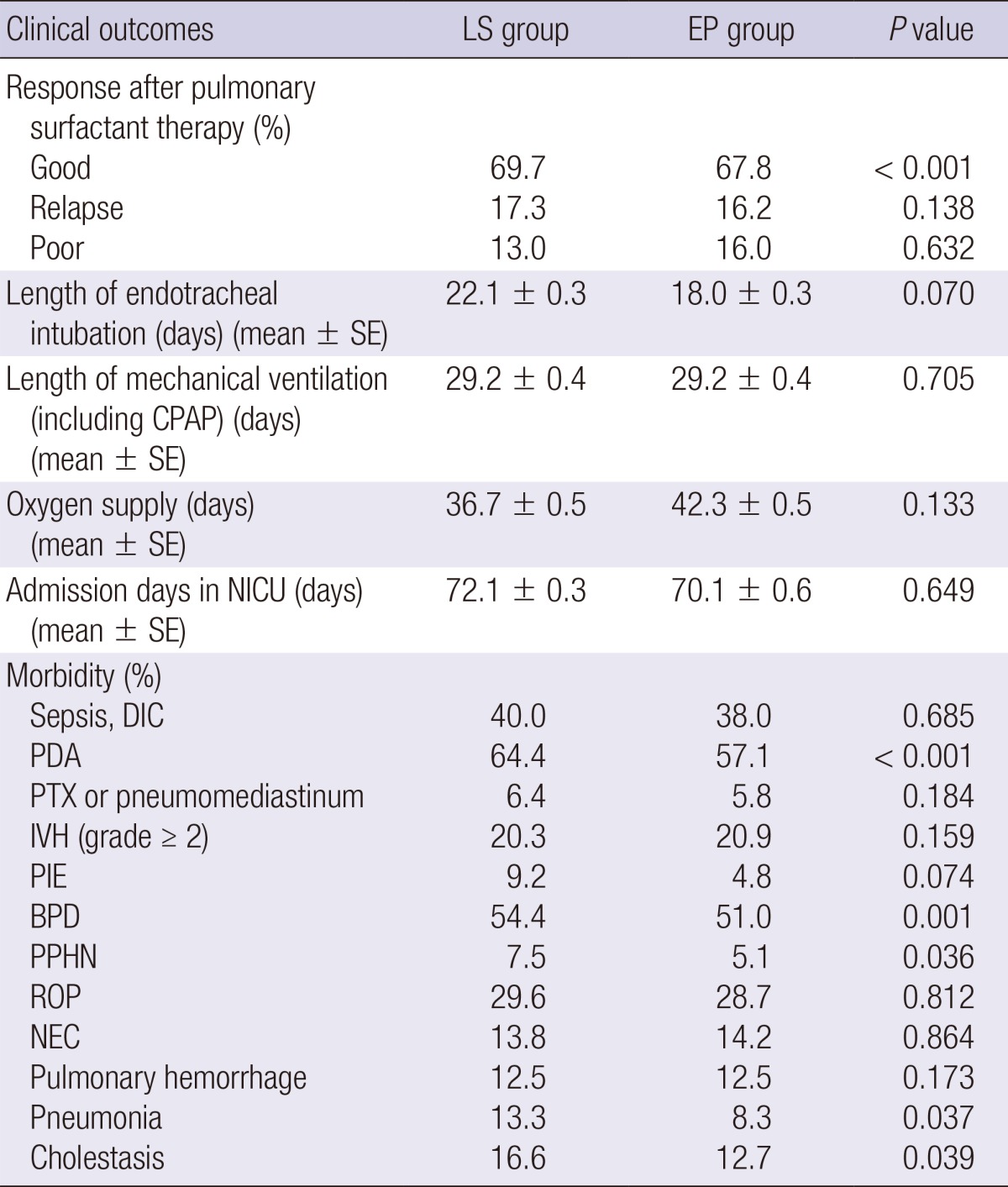
BPD, bronchopulmonary dysplasia; CPAP, continuous positive airway pressure; DIC, disseminated intravascular coagulation; EP, early prophylactic; IVH, intraventricular hemorrhage; LS, late selective; NEC, necrotizing enterocolitis; NICU, neonatal intensive care unit; PDA, patent ductus arteriosus; PIE, pulmonary interstitial emphysema; PPHN, persistent pulmonary hypertension; PTX, pneumothorax; ROP, retinopathy of prematurity.
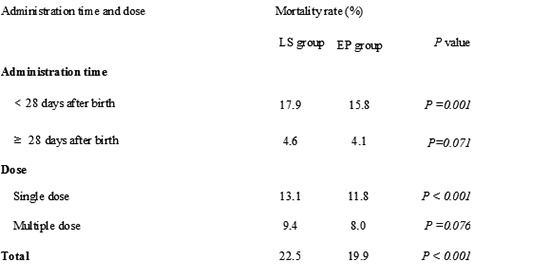
Regarding mortality in the two groups (Table 3), there was a significant decrease in overall mortality (19.9% in the EP group vs. 22.5% in the LS group) and mortality within 28 days after birth (15.8% in the EP group vs. 17.9% in the LS group) in the EP group compared to those in the LS group. In addition, there was a significant decrease in the mortality for cases with single and multiple dose of PS in the EP group compared to that in the LS group.
Table 3.
Comparison of mortality in late selective and early prophylactic groups for pulmonary surfactant use in Korea
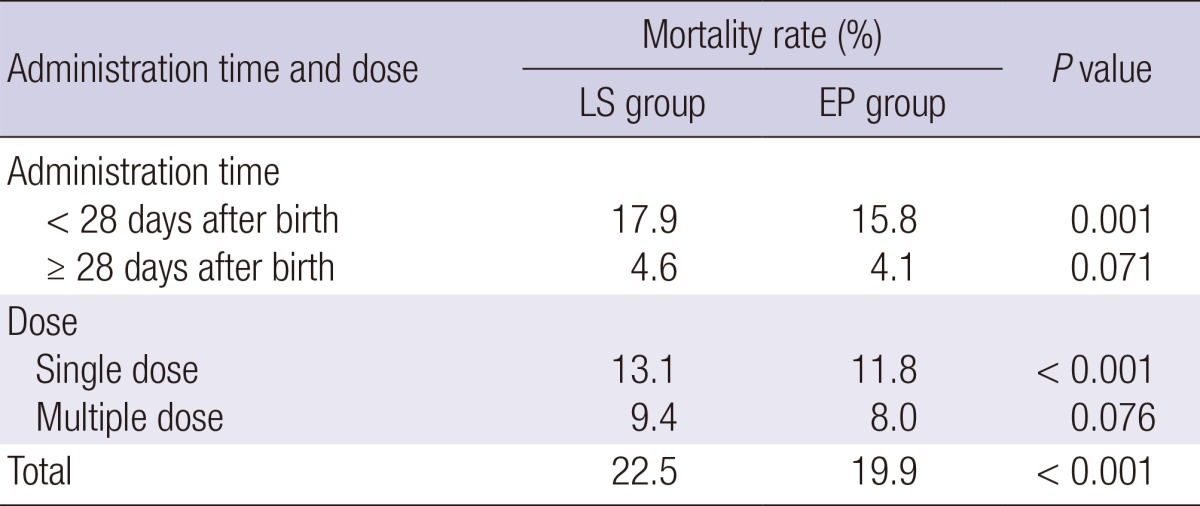
EP, early prophylactic use; LS, late selective use.
DISCUSSION
We have demonstrated a decrease in overall mortality in the EP group without an increase in the need for retreatment compared to that in the LS group. Furthermore, decreases in the duration of endotracheal intubation and the occurrence of morbidities, including PIE, PDA, BPD, PPHN, pneumonia, and cholestasis, were evident in the EP group. To the best of our knowledge, our study is the first nationwide Korean study to compare the outcomes of EP and LS PS therapies, and it provides evidence that EP PS therapy is important in very preterm infants to improve survival and reduce morbidities, even though it was a retrospective non-randomized controlled study.
With regard to the clinical use of EP PS therapy, there are several guidelines for very preterm infants, particularly in Europe and the US. The American Academy of Pediatrics recommends EP PS therapy (<2 hr after birth) in infants born at <30 weeks of gestation and a low rate of exposure to antenatal steroids to decrease mortality and the frequency of adverse respiratory outcomes (9). European consensus guidelines (8) recommends EP PS therapy (within 15 min of breath) for infants born at <26 weeks of gestation and infants born at 26-30 weeks of gestation when intubation is required in the delivery room or if the mother has not received prenatal corticosteroids. In addition, EP PS therapy should be given if there is evidence of RDS in infants (8, 18). Recently updated European guidelines suggests that EP PS therapy would be used to treat extremely preterm infants born at <26 weeks of gestation when FiO2 requirements are >0.30 and preterm infants born at >26 weeks of gestation when FiO2 requirements are >0.40 (19).
Korean guidelines were developed based on the US and European guidelines and consultation with the Korean Society of Neonatology (9, 18, 19, 20). The national medical insurance policy of the KMHW for PS therapy for RDS recommends that PS be administered to infants born at <30 weeks of gestation or with birth weight ≤1,250 g within 2 hr of birth in the delivery room or NICU without evidence of radiological evidence of RDS. These new guidelines were based on studies showing the efficacy of EP PS in decreasing mortality and morbidities in very preterm infants.
Several meta-analyses have reported the efficacy of EP PS on the mortality of very preterm infants. Jobe (12) reported a decrease in the mortality rate of 50% in the prophylactic group compared with the non-treatment group in a meta-analysis of 35 randomized controlled trials. Pramanik et al. (13) and Soll and Morley (14) also reported a beneficial effect of prophylactic PS treatment, particularly in infants born at <30 weeks of gestation, in agreement with our data. With regard to the frequency of adverse respiratory outcomes, several studies have reported a significant reduction in severe RDS, air-leak, and BPD with EP PS therapy, in accordance with our results. Soll and Morley (14) reported a decrease in RDS, mortality, pneumothorax, PIE, and combined outcome of BPD or death. Egberts et al. (21) reported a significant decrease in severe RDS, mortality, and BPD in vey preterm infants born at 24-31 weeks of gestation. Walti et al. (22) reported that EP PS therapy in infants born at 25-31 weeks of gestation decreased the occurrence of severe IVH and ROP. However, this was not observed in our study. Stevens et al. (11) reported a decrease in PDA, especially for PS treatment with FiO2 <0.45, in an EP group compared with a LS group, in accordance with a decrease in other respiratory outcomes in our results. Our data showed decrease of cholestasis in EP group, whereas Tan (23) reported no significant correlation between cholestasis and RDS. Our data demonstrated no difference between two groups regarding re-administration of PS in contrast with previous reports (24, 25), showing a decrease of re-administration of PS in the EP group.
The SUPPORT trial (26) recently emphasized the importance of CPAP application as an alternative method of support, similar to a previous method featuring endotracheal intubation and PS, in 1,316 very preterm (gestational age 24-28 weeks) infants in 2010. Dunn et al. (27) concluded that very preterm infants with either nasal CPAP or EP PS with rapid extubation (InSuRE) to nasal CPAP had similar outcomes to EP PS with subsequent mechanical ventilation, and that initial CPAP and LS PS might be an acceptable alternative, in accordance with other studies (2, 28). Our study is limited by the lack of analysis on the type of respiratory support, such as use of nasal CPAP and InSuRE, and lack of data on long-term outcomes.
Mortality of RDS has decreased markedly since PS therapy was brought to Korea (3, 4, 5, 6, 7). Furthermore, it has contributed greatly to the recent improved outcome of very and extremely low birth weight infant in Korea (29, 30, 31, 32). Therefore, establishment of EP PS therapy for RDS would bring further improved outcome especially in the mortality.
In conclusion, we have demonstrated the efficacy of EP PS therapy as developed by the national medical insurance policy of the KMHW for RDS in a national retrospective multi-center study. Furthermore, we identified decrease in overall mortality and the occurrence of morbidities including PIE, PDA, BPD, PPHN, pneumonia, and cholestasis with use of EP PS therapy. Further studies are needed to develop the method of EP PS therapy with routine CPAP or InSuRE compatible with each institutional environment.
Footnotes
The following 53 hospitals and individuals participated in this study by providing well-designed clinical data of two years (2010 and 2011) for this national study: Ajou University Hospital (Park MS, Lee JJ), Busan St. Mary's Medical Center (Kim SM), CHA Gangnam Medical Center, CHA University (Jeon JH), CHA Bundang Medical Center, CHA University (Lee KH, Cho HS), Cheil General Hospital & Women's Health Care Center (Shin SM), Cheju National University Hospital (Kim YD), Chonbuk National University Hospital (Cho SC), Chonnam National University Hospital (Choi YY, Song ES), Chosun University Hospital (Park SK), Chung Ang University Medical Center (Lee NM), Chungnam National University Hospital (Chang MY), Daegu Catholic University Medical Center (Kim WT), Daegu Fatima Hospital (Kim WD), Dong-A University Hospital (Kim MJ), Eulji General Hospital (Yoon HS), Ewha Womans University Mokdong Hospital (Park EA, Cho SJ), Gachon University Gil Hospital (Shon DW), GangneungAsan Hospital (Jin HS), Gyeongsang National University Hospital (Park CH), Hallym University Kangdong Sacred Heart Hospital (Hwang IT), Hallym University Kangnam Sacred Heart Hospital (Sung TJ), Hallym University Sacred Heart Hospital (Shim EJ), Hanyang University Medical Center (Park HK), Inha University Hospital (Jun YH), Inje University Ilsan Paik Hospital (Hwang JH), Inje University Sanggye Paik Hospital (Chey MJ), Inje University Pusan Paik Hospital (Shin JB), Inje University Seoul Paik Hospital (Park YW), Keimyung University Dongsan Medical Center (Lee SL, Kim CS), Konyang University Hospital (KoKO, Im JW), Korea University Anam Hospital (Lee EH), Korea University Ansan Hospital (Choi BM), Konkuk University Medical Center (Kim MH), Kyunghee University Hospital at Gangdong (Bae CW), Kyunghee University Medical Center (Choi YS), Kyungpook National University Hospital (Kim HM, Park SH), Kwangju Christian Hospital (Kim KS), Pusan National University Hospital (Byun SY), Samsung Medical Center (Park WS, Chang YS, Ahn SY), Seoul National University Bundang Hospital (Kim BI, Choi CW), Seoul National University Children's Hospital (Choi JH, Kim HS), Seoul National University Boramae Medical Center (Lee JA), Soonchunhyang University Bucheon Hospital (Kim SS), Hospital of Sungae (Kim ER), Soonchunhyang University Seoul Hospital (Lee WY), The Catholic University of Korea Seoul St. Mary's Hospital (Sung IK), The Catholic University of Korea St. Mary's Hospital (Kim SY), The Catholic University of Korea St. Vincent's Hospital (Lee JH), Ulsan University Hospital (Oh KW), University of Ulsan Asan Medical Center (Kim KS, Kim ER), Wonkwang University Hospital (Oh YK), Yonsei University Severance Children's Hospital (Namgung R, Eun HS), Yonsei University Gangnam Severance Hospital (Lee SM), Yonsei University Wonju Severance Christian Hospital (Lim BK).
The authors have no conflicts of interest to disclose.
References
- 1.Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980;1:55–59. doi: 10.1016/s0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- 2.Halliday HL. Surfactants: past, present and future. J Perinatol. 2008;28(Suppl 1):S47–S56. doi: 10.1038/jp.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae CW, Kwon YD, Ko SJ, Kim KS, Kim HM, Park WS, Byun SH, Son CS, Ahn HS, Lee SG, et al. Surfactant replacement therapy in neonates with respiratory distress syndrome: a collective evaluation of trials from 16 hospitals. J Korean Pediatr Soc. 1993;36:244–265. [Google Scholar]
- 4.Bae CW. Surfactant replacement therapy in RDS: a collaborative study of multi-center trials in Korea. J Korean Soc Neonatol. 1997;4:124–135. [Google Scholar]
- 5.Bae CW, Kim YM. Surfactant therapy for neonatal respiratory distress syndrome: experience in Korea over 15 years. Korean J Pediatr. 2004;47:940–948. [Google Scholar]
- 6.Bae CW, Hahn WH. Surfactant therapy for neonatal respiratory distress syndrome: a review of Korean experiences over 17 years. J Korean Med Sci. 2009;24:1110–1118. doi: 10.3346/jkms.2009.24.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae CW, Hahn WH, Chang JY, Kim SM. Surfactant replacement therapy for RDS: a collaborative study of 72 multi-center trials in Korea (2010) and a review of Korean experiences over 20 years. J Korean Soc Neonatol. 2011;18:409–411. [Google Scholar]
- 8.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, Saugstad OD, Simeoni U, Speer CP, Halliday HL, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants - 2010 update. Neonatology. 2010;97:402–417. doi: 10.1159/000297773. [DOI] [PubMed] [Google Scholar]
- 9.Engle WA American Academy of Pediatrics Committee on Fetus and Newborn. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics. 2008;121:419–432. doi: 10.1542/peds.2007-3283. [DOI] [PubMed] [Google Scholar]
- 10.Kirsten GF, Kirsten CL, Henning PA, Smith J, Holgate SL, Bekker A, Kali GT, Harvey J. The outcome of ELBW infants treated with NCPAP and InSurE in a resource-limited institution. Pediatrics. 2012;129:e952–e959. doi: 10.1542/peds.2011-1365. [DOI] [PubMed] [Google Scholar]
- 11.Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev. 2007;(4):CD003063. doi: 10.1002/14651858.CD003063.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jobe AH. Pulmonary surfactant therapy. N Engl J Med. 1993;328:861–868. doi: 10.1056/NEJM199303253281208. [DOI] [PubMed] [Google Scholar]
- 13.Pramanik AK, Holtzman RB, Merritt TA. Surfactant replacement therapy for pulmonary diseases. Pediatr Clin North Am. 1993;40:913–936. doi: 10.1016/s0031-3955(16)38616-3. [DOI] [PubMed] [Google Scholar]
- 14.Soll RF, Morley CJ. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2001;(2):CD000510. doi: 10.1002/14651858.CD000510. [DOI] [PubMed] [Google Scholar]
- 15.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 16.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 17.Bell RS. Neonatal necrotizing enterocolitis. N Engl J Med. 1970;283:153–154. doi: 10.1056/nejm197007162830318. [DOI] [PubMed] [Google Scholar]
- 18.Sweet D, Bevilacqua G, Carnielli V, Greisen G, Plavka R, Saugstad OD, Simeoni U, Speer CP, Valls-I-Soler A, Halliday H, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome. J Perinat Med. 2007;35:175–186. doi: 10.1515/JPM.2007.048. [DOI] [PubMed] [Google Scholar]
- 19.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, Saugstad OD, Simeoni U, Speer CP, Vento M, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants: 2013 update. Neonatology. 2013;103:353–368. doi: 10.1159/000349928. [DOI] [PubMed] [Google Scholar]
- 20.Kim SM, Yoon HS, Kim KS, Bae CW. The importance and the need of early pulmonary surfactant therapy in premature infant with respiratory distress syndrome. J Korean Soc Neonatol. 2009;16:101–109. [Google Scholar]
- 21.Egberts J, de Winter JP, Sedin G, de Kleine MJ, Broberger U, van Bel F, Curstedt T, Robertson B. Comparison of prophylaxis and rescue treatment with Curosurf in neonates less than 30 weeks' gestation: a randomized trial. Pediatrics. 1993;92:768–774. [PubMed] [Google Scholar]
- 22.Walti H, Paris-Llado J, Egberts J, Brand R, Bevilacqua G, Gardini F, Bréart G. Prophylactic administration of porcine-derived lung surfactant is a significant factor in reducing the odds for peri-intraventricular haemorrhage in premature infants. Biol Neonate. 2002;81:182–187. doi: 10.1159/000051532. [DOI] [PubMed] [Google Scholar]
- 23.Tan KL. High frequency oscillatory ventilation in neonates with respiratory distress. Ann Acad Med Singapore. 1991;20:219–222. [PubMed] [Google Scholar]
- 24.Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA, Charry L, Bastidas JA, Perez LA, Rojas C, et al. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics. 2009;123:137–142. doi: 10.1542/peds.2007-3501. [DOI] [PubMed] [Google Scholar]
- 25.Kattwinkel J, Bloom BT, Delmore P, Davis CL, Farrell E, Friss H, Jung AL, King K, Mueller D. Prophylactic administration of calf lung surfactant extract is more effective than early treatment of respiratory distress syndrome in neonates of 29 through 32 weeks' gestation. Pediatrics. 1993;92:90–98. [PubMed] [Google Scholar]
- 26.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, Yoder BA, Faix RG, Das A, Poole WK, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn MS, Kaempf J, de Klerk A, de Klerk R, Reilly M, Howard D, Ferrelli K, O'Conor J, Soll RF Vermont Oxford Network DRM Study Group. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics. 2011;128:e1069–e1076. doi: 10.1542/peds.2010-3848. [DOI] [PubMed] [Google Scholar]
- 28.Rojas-Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2012;3:CD000510. doi: 10.1002/14651858.CD000510.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn WH, Chang JY, Chang YS, Shim KS, Bae CW. Recent trends in neonatal mortality in very low birth weight Korean infants: in comparison with Japan and the USA. J Korean Med Sci. 2011;26:467–473. doi: 10.3346/jkms.2011.26.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang JY, Lee KS, Hahn WH, Chung SH, Choi YS, Shim KS, Bae CW. Decreasing trends of neonatal and infant mortality rates in Korea: compared with Japan, USA, and OECD nations. J Korean Med Sci. 2011;26:1115–1123. doi: 10.3346/jkms.2011.26.9.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung SH, Choi YS, Bae CW. Changes in neonatal epidemiology during the last 3 decades in Korea. Neonatal Med. 2013;20:249–257. [Google Scholar]
- 32.Cho JH, Choi SK, Chung SH, Choi YS, Bae CW. Changes in neonatal and perinatal vital statistics during last 5 decades in Republic of Korea: compared with OECD nations. Neonatal Med. 2013;20:402–412. [Google Scholar]


