Abstract
Motility in much of the gastrointestinal (GI) tract is coordinated by an electrical event known as slow waves, and several GI motility disorders are associated with slow wave arrhythmias. The GI smooth muscle cells (SMC) generate contraction, but slow waves are actively regenerated by specialized pacemaker cells called the interstitial cells of Cajal (ICC), which are coupled to the SMC. This unique electrical coupling presents an added layer of complexity to GI electromechanical models, and a major current barrier to further progress is the lack of a framework for ICC-SMC-contraction coupling. In this study, an initial framework for the electromechanical coupling was developed in a 2D model. At each solution step, the slow wave propagation was solved first and the intracellular calcium concentration in the SMC model was related to an adapted tension-extension-calcium relationship to simulate active contraction. With identification of more GI-specific constitutive laws, the ICC-SMC-contraction approach will underpin future GI electromechanical models of health and disease states.
Keywords: bidomain, ICC, SMC, slow waves, motility
I. Introduction
Motility of much of the gastrointestinal (GI) tract is coordinated by an underlying electrical event known as slow waves. GI slow waves are periodic and omnipresent, occurring at ~3 cycles per minute (cpm) in the human stomach and ~8 cpm in the small intestine [1]. Slow waves are actively regenerated by specialized pacemaker cells known as the interstitial cells of Cajal (ICC), and the subsequent depolarization of the surrounding gastric smooth muscle cells (SMC) via gap junctions is understood to coordinate GI motility, i.e., SMC contractions [2]. Dysfunction in either the ICC and/or SMC are increasingly being linked to a number of functional GI disorders, such as gastroparesis and dyspespia [3].
Existing GI mechanical models have typically modeled the movements of the GI luminal contents by imposing the mechanical deformation of the GI wall from imaging evidence [4]. The next critical step is to link slow wave activity to motility. Electromechanical models represent an active area of research in the cardiac field, offering a prime opportunity to translate the established multi-scale continuum modeling techniques to the GI field [5], [6] - in order to understand the complex interplay of distinct cellular mechanisms and systems across large spatiotemporal scales. However, as outlined above, the unique physiology of the GI tract also necessitates novel adaptations and approaches. This study presents a proof-of-concept framework and perspectives for: (i) cellular electromechanical coupling of ICC-SMC-contraction; (ii) simulation of entrainment of slow waves in a 1D model; (iii) a 2D model of GI electromechanical activity.
II. Cell Electrical and Electromechanical Coupling
A number of biophysically-based ICC and SMC slow wave models have recently been developed [6]–[8]. In further recent studies, the ICC and SMC models were electrically coupled via a gap junction conductance, through which the self-excitatory slow wave activity in the ICC model initiates the slow wave activity in the SMC model (Fig. 1a) [9], [10].
| (1) |
where Gcouple is the gap junction conductance between the membrane potentials of ICC (Vm(ICC)) and SMC (Vm(SMC)). The ratio of ICC-to-SMC (ηICC) at each continuum point also modulates the SMC slow wave activity [10].
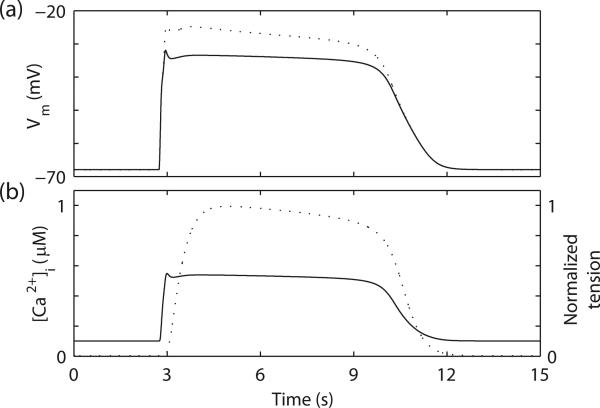
Fig. 1.
Simulated gastric slow waves and mechanical response. (a) Simulated slow waves in gastric smooth muscle cell (SMC) (solid line) and interstitial cell of Cajal (dashed line). (b) Simulated SMC [Ca2+]i (solid line) and the resultant normlized active force (dashed line).
Although many details of the intracellular processes involved in GI motility are still under investigation, the consensus is that contraction of SMC is initiated by myosin light chain (MLC) phosphorylation via the activation of calcium (Ca2+)-calmodulin-dependent MLC kinase (MLCK) [2]. The complexity lies in the additional physiological factors that co-regulate the sensitivity of the contractile apparatus to Ca2+, such as the spike activity in intestines, noradrenaline, and prostaglandins etc. [2]. A bimodular active force cellular mechanical model has been developed recently [11]. The first module of the mechanical model describes the activation of MLCK by interaction with [Ca2+]i with calmodulin; The second module of the mechanical model describes the cross-bridges ([AMp]) formation induced by the phosphorylated myosin. The active force generated is proportional to the sum of [AMp] and latch-bridges ([AM]), for a given total myosin concentration ([Mtotal]) (2).
| (2) |
where Fmax is the maximum achievable force. The active force was simulated by using the SMC [Ca2+]i as an input to the mechanical model (Fig. 1b).
III. Continuum Models Of Entrainment
Slow wave entrainment at higher spatial scales has been simulated using the bidomain equations in several studies [6], [12]. A system of two equations (3 and 4) constitute the basic bidomain model.
| (3) |
| (4) |
where Vm and φe are membrane and extracellular potentials, respectively. The ionic current (Iion) term from the ICC and/or SMC model relates the slow wave activity of the cell model to the bidomain equations. The tissue conductivity tensors (σ), with subscript i denotes the intracellular domain and subscript e denotes the extracellular domain.
ICC exhibit self-excitatory slow waves in a gradient of intrinsic frequencies along the GI tract, and the coupling of ICC slow waves, i.e., entrainment, underlies the major mechanism of slow wave propagation in the intact GI tissues [1]. Two entrainment models have been proposed. First, an IP3-dependent Ca2+-release model (5 and 6) was incorporated into the ICC model [9].
| (5) |
where,
| (6) |
where β is a chemical stimulus agent (such as Acetylcholine) that can modulate the sensitivity of IP3 to the changes in Vm. The temporary surge of IP3 due to an increase in Vm allows the ICC model to respond to a current source by producing a phase shift relative to its intrinsic slow wave activity. The rate constant of linear IP3 degradation (η), Hill coefficient (u), maximum rate of nonlinear IP3 degradation (Vm4), half saturation constant for nonlinear IP3 degradation (k4), maximum rate of Vm-dependent IP3 degradation (PMV ), half saturation constant for Vm-dependent IP3 degradation (kv), hill coefficient (r) were set to 2.7 × 10–5 mM s–1, 0.015 s– 1, 4, 3.3 × 10–5 mM s–1, 0.0005 mM, 0.33 × 10–5 mM s–1, −58 mV , and 8, respectively [9].
An alternative model of entrainment is a modified Vm-dependent dihydropyridine-resistant conductance (IV DDR) model in ICC [10]. The modified conductance represents a fraction of the whole cell IV DDR, originally denoted as IV DDRPU [10], and here denoted as IPU for short (7).
| (7) |
where GPU , dPU , and fPU are the maximum channel conductance, activation gate, and inactivation gate, respectively [10]. The fraction of the IPU in IV DDR conductance is denoted by dr. ECaPU is the Nernst potential of Ca2+ in the pacemaker unit in the ICC model. A Ca2+-extrusion mechanism was also added to the ICC cytoplasm to maintain long-term Ca2+ homeostasis in the ICC model (8).
| (8) |
where Camax was set to be 0.0315 μM ms–1; Ca50 and k were set to be 100 nM and 15, respectively.
Both mechanisms of entrainment were combined separately with the ICC model [9], [10], and slow waves were simulated in a 1D linear model (8 mm in length). A total of 101 grid-points, i.e., solution points, were distributed at equal spacing along the geometric element of the 1D model. Zero-flux boundary conditions were imposed on the two ends. To simulate propagation of slow waves, a linear gradient of ICC intrinsic frequencies (3-2.68 cpm) was applied to the 1D model. The slow wave activity at each grid point was self-excitatory at the prescribed frequency and occurs independently of each other in a decoupled setting, i.e., no entrainment. No stimulus current term was used to invoke an electrical propagation [6].
IV. 2D Model Of Electromechanical Activity
Propagation of slow waves was simulated using the bido-main equations on an 8×8 mm 2D model, which was defined by 8×8 linear elements, at the same spatial resolution as the 1D model. The physical dimension of each element was 1×1 mm. A linear gradient of ICC slow wave intrinsic frequencies (3-2.68 cpm) was distributed horizontally across the 2D model. Here, as a proof-of-concept step, a steady-state force-extension-Ca2+ relationship was used to relate the slow wave propagation to contraction, as previously described in a cardiac study [5].
| (9) |
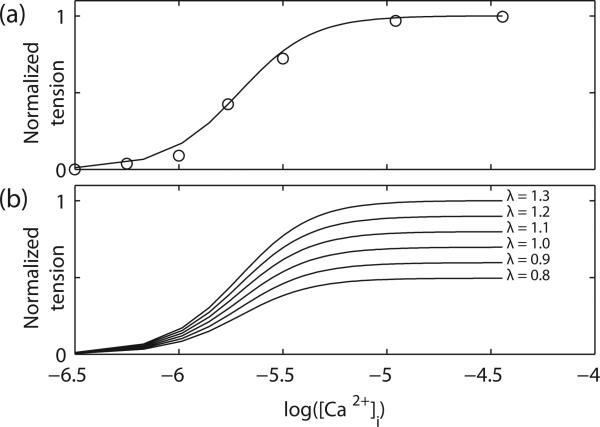
where Camax was set to be 0.6 μM and T0 is the maximum isometric force. The values of the non-dimensional slope parameter (β), Ca50, and Hill coefficient (h) were set to be 1.45, 1.95, and 2.5, respectively; these values were estimated by minimizing the root-mean-square error between a set of isometric force-[Ca2+]i recording from guinea-pig taenia coli [14], and the simulated isometric force using (9 (Fig. 2a). The isometric forces were then simulated over a range of extension-ratios (λ) (Fig. 2b).
Fig. 2.
Steady-state force-extension-Ca2+ relationship. (a) Simulated normalized isometric force-[Ca2+]i relationship. circle: experimental data [14]. The [Ca2+]i was presented in a logarithmic scale. (b) Simulated isometric force-[Ca2+]i relationship at different extension-ratios (λ).
The pole-zero law was used as the constitutive law to describe the passive mechanical behavior of the tissue [5]. Parameters for the limiting strains in the fiber and sheet directions were specified as the strains corresponding to maximum stress obtained during uniaxial tensile testing on human small intestine [16]. The 2nd Piola-Kirchhoff stress tensor was used to relate the force in the reference configuration to areas in the reference configuration. Zero displacements were imposed on the boundaries of the 2D model.
The LSODA and Euler solvers were used to solve for the intracellular and extracellular domains of slow wave activity, respectively, as previously described [9]. The cell models were encoded using the CellML standard [13]. The mechanical deformation was solved using the Newton-Raphson method. At each solution step, the electrical propagation was solved first and then the mechanical deformation was solved to update the mechanical parameters in the geometric elements.
The local deformation was quantified by calculating the area of each column of elements in the 2D model at each solution step. The average stress generated by the 2D model was also calculated at each solution step.
V. Results and Discussion
A. Entrainment Models
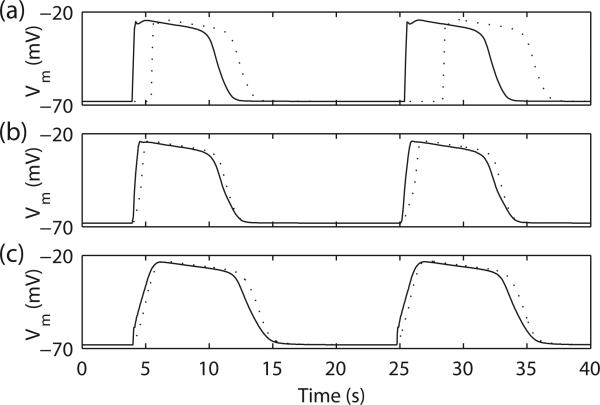
Decoupled slow waves activity is shown in Fig. 3a where entrainment does not occur. Simulations of the entrainment of slow waves demonstrated coupling of ICC with different intrinsic frequencies to a single frequency of 3 cpm. The IP3-dependent Ca2+-release model (Fig. 3b) produced a steeper rate-of-upstroke than the IPU model (Fig. 3c) (73 vs 23 mV s–1). In both cases, the entrainment models produced a constant velocity (~12 mm s–1), in accordance with physiological data [1]. Rather than a Vm-threshold based propagation mechanism, the entrainment models actively propagate slow waves through coupled ICC slow wave activity in the 1D model. While the decoupled slow waves may contribute to ectopic sources of entrainment in disease states.
Fig. 3.
Simulated 1D slow wave entrainment. (a) In the decoupled model (by setting σe and σi to 0.001 mS mm–1), the simulated slow waves on the two boundary nodes demonstrated different intrinsic frequencies, 3.00 cpm (solid line) and 2.68 cpm (dashed line). In the entrained model, (b) IP3-dependent Ca2+-release model and (c) IPU model, the slow wave with the lower intrinsic frequency (dashed line) was entrained to the slow wave with the higher intrinsic frequency (solid line).
B. Electromechanical Model
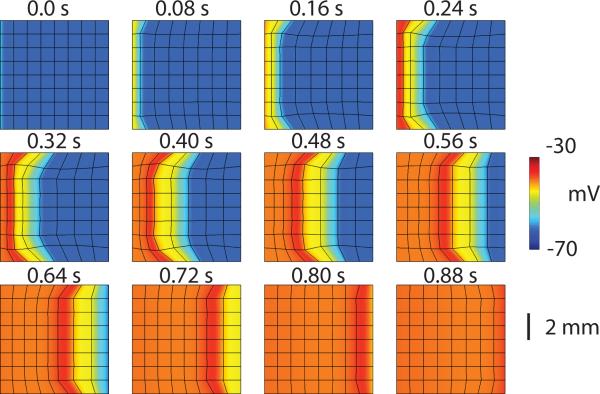
Electromechanical coupling was successfully achieved in a 2D multi-scale model, as shown in Fig. 4. The electromechanical activity resulted in a dynamic regional deformation of the 2D model that followed the slow wave propagation. The geometric elements in column 1 of the 2D model deformed first, followed sequentially by columns 2 to 8.
Fig. 4.
Simulated electromechanical activity. The black lines highlight the boundary of each geometric element (1×1 mm). The deformation of each element followed the direction of the simulated slow wave propagation.
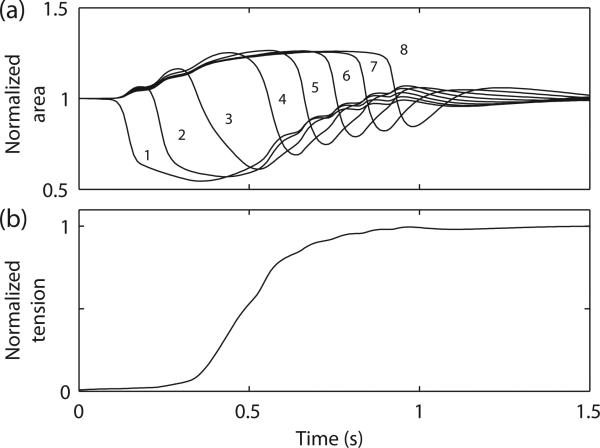
The deformations of the geometric elements in column 1 led to extension in the areas of the remaining elements in the 2D model. The peak deformation occurred in column 1 at 0.54 s (Fig. 5a). The stress developed monotonically in a sigmoidal profile (Fig. 5b). The development of stress in both the cell model (Fig. 1b) and the 2D model was delayed relative to the onset of slow wave activity.
Fig. 5.
Quantification of the mechanical activity. (a) Changes in the normalized area of the geometric elements columns 1-8. (b) Average normalized stress developed in the 2D model.
C. Limitations
There are a number of important limitations in the 2D model which warrant further discussion. The first limitation is the absence of an electromechanical feedback mechanism at the cellular level. For example, a voltage-sensitive sodium channel expressed in the human gut (Nav1.5) has recently been shown to have pronounced mechanosensitivity, providing valuable experimental data to inform future electromechanical models [15]. The incorporation of the cell mechanical model [11], and an electromechanical feedback mechanism will be an important next step.
The second limitation is the application of the pole-zero constitutive law in this study. A key feature described by the constitutive law is the difference in limiting strains in each of the main microstructural axes in the tissue; this has also been observed in human intestinal tissue [16], and incorporated into the 2D model. The remaining material parameters were left with cardiac-specific values. Future GI-specific strain field data from appropriate biaxial tests can be used to properly adapt the constitutive law to describe the passive mechanical properties of GI tissue. Additional parameter values in the constitutive law can be identified through optimization procedures. Similarly, the steady-state force-extension-Ca2+ relationship used to model the active force generation in this study could be replaced with the bimodular model [11].
The third limitation is the incomplete knowledge of the intracellular processes that contribute to GI motility. For example, it has been postulated that a Vm-threshold may exist for more vigorous mechanical contractions in the GI tract [1]. Much details of these processes are still under investigations. Future GI electromechanical models will need to incorporate these mechanisms as further details become available.
VI. Conclusions
This study presents a proof-of-concept gastrointestinal (GI) electromechanical model, demonstrating significant potential for relating GI slow waves to motility via a multi-scale framework. With more studies of GI tissue mechanical properties and incorporation of an electromechanical feedback mechanism, the model may be applied to show how mechanical deformation affects the propagation of slow waves; formation of slow wave arrhythmias may in turn exert changes to GI motility. This work provides a foundation for future studies incorporating electromechanical approaches to provide a quantitative understanding of GI motility in health and disease.
Acknowledgment
This project was supported by a University of Auckland Doctoral Scholarship, NUS Engineering Doctoral Scholarship, NUS NGS Doctoral Scholarship, grants from the New Zealand Health Research Council, and the U.S. National Institutes of Health (No. R01 DK64775).
Contributor Information
Peng Du, Department of Surgery, Auckland Bioengineering Institute, The University of Auckland, NZ.
Yong Cheng Poh, Division of Bio-engineering; National University of Singapore Graduate School of Integrative Sciences and Engineering, Singapore.
Jee Lean Lim, Department of Surgery, Auckland Bioengineering Institute, The University of Auckland, NZ.
Viveka Gajendiran, Division of Bio-engineering; National University of Singapore Graduate School of Integrative Sciences and Engineering, Singapore.
Greg O'Grady, Department of Surgery, Auckland Bioengineering Institute, The University of Auckland, NZ.
Martin L. Buist, Division of Bio-engineering; National University of Singapore Graduate School of Integrative Sciences and Engineering, Singapore
Andrew J. Pullan, Department of Engineering Science, Auckland Bioengineering Institute, The University of Auckland; The Riddet Institute, NZ; Department of Surgery, Vanderbilt University, Nashville, TN, USA
Leo K. Cheng, Department of Surgery, Auckland Bioengineering Institute, The University of Auckland, NZ
References
- 1.van Helden DF, Laver DR, Holdsworth J, Imtiaz MS. The generation and propagation of gastric slow waves. Clin. Exp. Pharmacol. Physiol. 2010;37:516–524. doi: 10.1111/j.1440-1681.2009.05331.x. [DOI] [PubMed] [Google Scholar]
- 2.Ozaki H, Stevens RJ, Blondfield DP, Publicover NG, Sanders KM. Simultaneous measurement of membrane potential, cytosolic Ca2+, and tension in intact smooth muscles. Am J Physiol. 1991 May;260(5 Pt 1):C917–25. doi: 10.1152/ajpcell.1991.260.5.C917. [DOI] [PubMed] [Google Scholar]
- 3.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol. Motil. 2008;20(1):54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 4.Pal A, Brasseur JG, Abrahamsson B. A stomach road or “Magenstrasse” for gastric emptying. J. Biomech. 2007;40(6):1202–10. doi: 10.1016/j.jbiomech.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Hunter PJ, McCulloch AD, ter Keurs HE. Modelling the mechanical properties of cardiac muscle. Prog Biophys Mol Biol. 1998;68(2-3):289–331. doi: 10.1016/s0079-6107(98)00013-3. [DOI] [PubMed] [Google Scholar]
- 6.Du P, O'Grady G, Davidson JB, Cheng LK, Pullan AJ. Multiscale modeling of gastrointestinal electrophysiology and experimental validation. Crit. Rev. Biomed. Eng. 2010;38(3):225–54. doi: 10.1615/critrevbiomedeng.v38.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrias A, Buist ML. A quantitative model of gastric smooth muscle cellular activation. Ann. Biomed. Eng. 2007;35(9):1595–607. doi: 10.1007/s10439-007-9324-8. [DOI] [PubMed] [Google Scholar]
- 8.Corrias A, Buist ML. Quantitative cellular description of gastric slow wave activity. Am J Physiol Gastrointest Liver Physiol. 2008 Apr;294(4):G989–95. doi: 10.1152/ajpgi.00528.2007. [DOI] [PubMed] [Google Scholar]
- 9.Du P, O'Grady G, Cheng LK, Pullan AJ. Tissue-specific mathematical models of slow wave entrainment in wild-type and 5-HT2B knockout mice with altered interstitial cells of Cajal networks. Biophys. J. 2010;98(9):1772–81. doi: 10.1016/j.bpj.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buist ML, Corrias A, Poh YC. A model of slow wave propagation and entrainment along the stomach. Ann. Biomed. Eng. 2010;38(9):3022–30. doi: 10.1007/s10439-010-0051-1. [DOI] [PubMed] [Google Scholar]
- 11.Gajendiran V, Buist ML. A quantitative description of active force generation in gastrointestinal smooth muscle. Int. J. Numer. Meth. Biomed. Engng. 2010;27(3):450–460. [Google Scholar]
- 12.Buist ML, Poh YC. An extended bidomain framework incorporating multiple cell types. Biophys. J. 2010;99(1):13–8. doi: 10.1016/j.bpj.2010.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd CM, Lawson JR, Hunter PJ, Nielsen PF. The CellML Model Repository. Bioinformatics. 2008;24(18):2122–3. doi: 10.1093/bioinformatics/btn390. [DOI] [PubMed] [Google Scholar]
- 14.Arner A. Mechanical characteristics of chemically skinned guinea-pig taenia coli. Pflugers Arch. 1982 Dec;395(4):277–84. doi: 10.1007/BF00580790. [DOI] [PubMed] [Google Scholar]
- 15.Beyder A, Rae JL, Bernard C, Strege PR, Sachs F, Farrugia G. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. Journal of Physiology. J. Physiol. 2010;588(24):4969–4985. doi: 10.1113/jphysiol.2010.199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egorov VI, Schastlivtsev IV, Prut EV, Baranov AO, Turusov RA. Mechanical properties of the human gastrointestinal tract. J. Biomech. 2002;35(10):1417–25. doi: 10.1016/s0021-9290(02)00084-2. [DOI] [PubMed] [Google Scholar]