Abstract
OBJECTIVES
The goal of this study was to assess the association between left atrial (LA) volume and function measured with feature-tracking cardiac magnetic resonance (CMR) and development of heart failure (HF) in asymptomatic individuals.
BACKGROUND
Whether alterations of LA structure and function precede or follow HF development remains incompletely understood. We hypothesized that significant alterations of LA deformation and architecture precede the development of HF in the general population.
METHODS
In a case-control study nested in MESA (Multi-Ethnic Study of Atherosclerosis), baseline LA volume and function assessed using CMR feature-tracking were compared between 112 participants with incident HF (mean age 68.4 ± 8.2 years; 66% men) and 224 age- and sex-matched controls (mean age 67.7 ± 8.9 years; 66% men). Participants were followed up for 8 years. All individuals were in normal sinus rhythm at the time of imaging, without any significant valvular abnormalities and free of clinical cardiovascular diseases.
RESULTS
Individuals with incident HF had greater maximal and minimal LA volume indexes (LAVImin) than control subjects (40 ± 13 mm3/m2 vs. 33 ± 10 mm3/m2 [p <0.001] for maximal LA index and 25 ± 11 mm3/m2 vs. 17 ± 7 mm3/m2 [p <0.001] for LAVImin). The HF case subjects also had smaller global peak longitudinal atrial strain (PLAS) (25 ± 11% vs. 38 ± 16%; p <0.001) and lower LA emptying fraction (40 ± 11% vs. 48 ± 9%; p <0.001) at baseline. After adjustment for traditional cardiovascular risk factors, left ventricular mass, and N-terminal pro–B-type natriuretic peptide, global PLAS (odds ratio: 0.36 per SD [95% confidence interval: 0.22 to 0.60]) and LAVImin (odds ratio: 1.65 per SD [95% confidence interval: 1.04 to 2.63]) were independently associated with incident HF.
CONCLUSIONS
Deteriorations in LA structure and function preceded development of HF. Lower global PLAS and higher LAVImin, measured using CMR feature-tracking, were independent markers of incident HF in a multiethnic population of asymptomatic individuals.
Keywords: feature-tracking MRI, heart failure, left atrial function, left atrial strain
The majority of individuals in the community with left ventricular (LV) dysfunction (systolic or isolated diastolic) are in the preclinical phase of heart failure (HF) (1). Methods to assess the risk of progression to symptomatic HF would be clinically valuable. Left atrial (LA) size is a known prognostic marker in multiple conditions such as HF (2-5), myocardial infarction (6), atrial fibrillation (7), and thromboembolic events (8,9). More recently, evidence for LA function being a prognostic indicator in the outcome of cardiovascular diseases has emerged (10-13).
The left atrium acts as a reservoir during systole, as a conduit during early diastole, and works as an active pump during late diastole. During diastole, the left atrium and the left ventricle are directly connected and, in the absence of valvular disease, LA and LV function are tightly coupled.
There are several established methods for non-invasive evaluation of LA volume such as using 2- or 3-dimensional echocardiography (14,15), speckle-tracking echocardiography (16), dual-source computed tomography (17), or cardiac magnetic resonance (CMR) (15,18,19). LA strain assessment has previously been mainly performed using speckle-tracking echocardiography (9,16,20). However, given the thin LA wall, the use of speckle-tracking echocardiography can be a challenging technique for the assessment of LA function. CMR has therefore been proposed as the gold standard modality to measure atrial and ventricular volumes (18). CMR is also an established method for measurements of cardiac deformation given its excellent ability to define endocardial and epicardial borders for adequate strain analysis. CMR feature-tracking has been described recently as an accurate method of wall motion assessment (21-23). However, to our knowledge, CMR feature-tracking has not been used for LA strain or volume evaluation in individuals at risk of developing HF. The present study was performed in a large multiethnic population to assess the association between LA function and subsequent HF development. We hypothesized that reduced LA function, as assessed using CMR feature-tracking, would be associated with a higher incidence of HF.
MATERIALS AND METHODS
Study design
The present study was designed as a nested case-control study within MESA (Multi-Ethnic Study of Atherosclerosis). The MESA study protocol has previously been described in detail (24). Briefly, between July 2000 and August 2002, a total of 6,814 individuals between 45 and 84 years of age from 4 different self-reported ethnic backgrounds (white, African-American, Hispanic, and Chinese) were enrolled. These individuals were recruited from 6 U.S. communities in California, Illinois, Maryland, Minnesota, New York, and North Carolina. All participants were free of any clinically apparent cardiovascular disease.
To evaluate cardiac structure and function, 5,004 individuals (73.4%) underwent CMR at baseline. CMR was performed using 1.5-T magnets; the full CMR protocol and methods of analysis have been described previously (25).
Follow-up
Every 6 to 9 months, a telephone interviewer contacted each participant to ask about hospital admissions, cardiovascular outpatient diagnoses, and deaths. Medical records and information were successfully obtained on an estimated 98% of reported hospitalized cardiovascular events and 95% of reported outpatient cardiovascular diagnostic encounters.
Identification of case and control subjects
The endpoint in this study was incident symptomatic HF. Cases of HF in MESA have been classified as definite or probable. Both types required HF symptoms and/or signs such as dyspnea or edema. In addition to these criteria, definite HF also required at least 1 other finding such as pulmonary edema or congestion according to chest radiograph, dilated ventricle or poor LV function by echocardiography or ventriculography, or evidence of LV diastolic dysfunction. Probable HF required a physician diagnosis of HF and medical treatment for HF. After 8 years of follow-up, 112 cases of probable and definite HF who had available CMR data at baseline were identified and entered into the study as the case subjects (Fig. 1). Based on the measured LV ejection fraction (EF) at the time of the HF diagnosis, cases were categorized into systolic HF or heart failure with normal ejection fraction (HFNEF) if the measured EF was <50% or ≥50%, respectively.
Figure 1. Flow Chart of the Study.
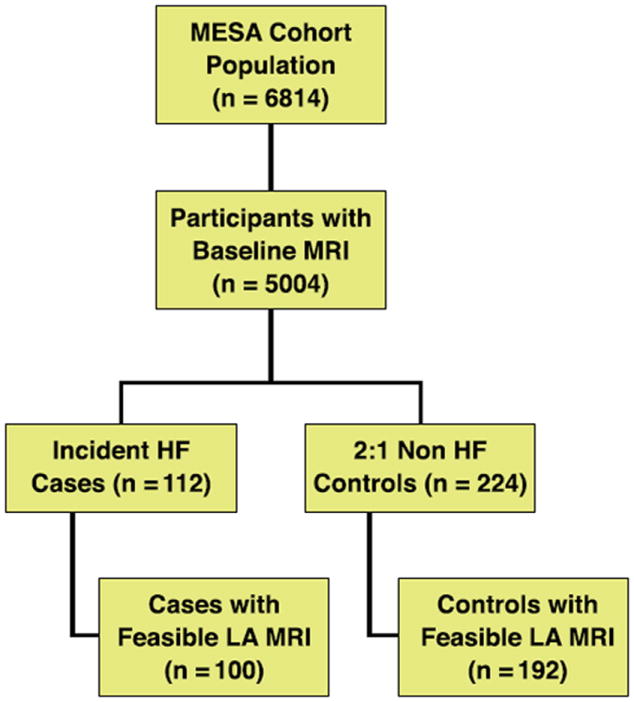
A nested case-control study with 112 case and 224 control subjects. HF = heart failure; LA = left atrial; MESA = Multi-Ethnic Study of Atherosclerosis; MRI = magnetic resonance imaging.
For each case subject, 2 control subjects matched for age and sex were randomly selected from the 5,004 participants who had CMR performed at baseline (Fig. 1).
Covariates
Standardized questionnaires were used at baseline to collect information about age, sex, ethnic background, cigarette smoking, use of anti-hypertensive medication, or high cholesterol. Cigarette smoking was categorized to current, former, or never. Body mass index was calculated as weight divided by height squared (kg/m2) from weight measured to the nearest 0.5 kg and height to the nearest 0.1 cm. Blood pressure was measured 3 times using a Dinamp model Pro 100 automated oscillometric sphygmomanometer (Critikon, Inc., Tampa, Florida) while the participants were resting in a seated position. The average of the last 2 measurements was used in the analysis. Fasting blood glucose and total and high-density lipoprotein cholesterol levels were measured after fasting for 12 h. Fasting glucose was obtained by a thin-film adaptation of the glucose oxidase method (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, New York). Diabetes was defined as a fasting glucose level ≥126 mg/dl or use of hypoglycemic medication. N-terminal pro–B-type natriuretic peptide (NT-proBNP) was analyzed centrally at a core laboratory (University of Vermont, Burlington, Vermont).
Image analysis
We used multimodality tissue tracking (MTT) software version 5.0 (Toshiba, Tokyo, Japan) to obtain LA strain and volume quantification from baseline 4-chamber and 2-chamber cine CMR images. A single experienced operator places points along the endocardial and epicardial borders in the left atrium. Using the marked points, the software draws endocardial and epicardial borders and then searches for the most closely matching borders in the subsequent frames. The operator then follows endocardial and epicardial contours generated by the software during the cardiac cycle for quality control. Maximum, minimum, and pre-atrial contracture LA volumes were measured using the area–length method from apical 4-and 2-chamber views (Fig. 2). All measured LA volumes were subsequently indexed according to body surface area. The parameters of LA volume included in our study were:
Maximum LA volume (LAVmax): LA volume at end systole before mitral valve opening.
Minimum LA volume (LAVmin): LA volume at end diastole right after mitral valve closure.
Pre–atrial contraction LA volume (LAVPreA): LA volume before atrial contraction.
Conduit volume: LAVmax – LAVPreA.
Conduit volume fraction: 100 × (LAVmax – LAVPreA)/LAVmax.
Total LA emptying fraction: 100 × (LAVmax – LAVmin)/LAVmax.
Figure 2. Tracking LA Wall Motions Using Cine CMR Images.
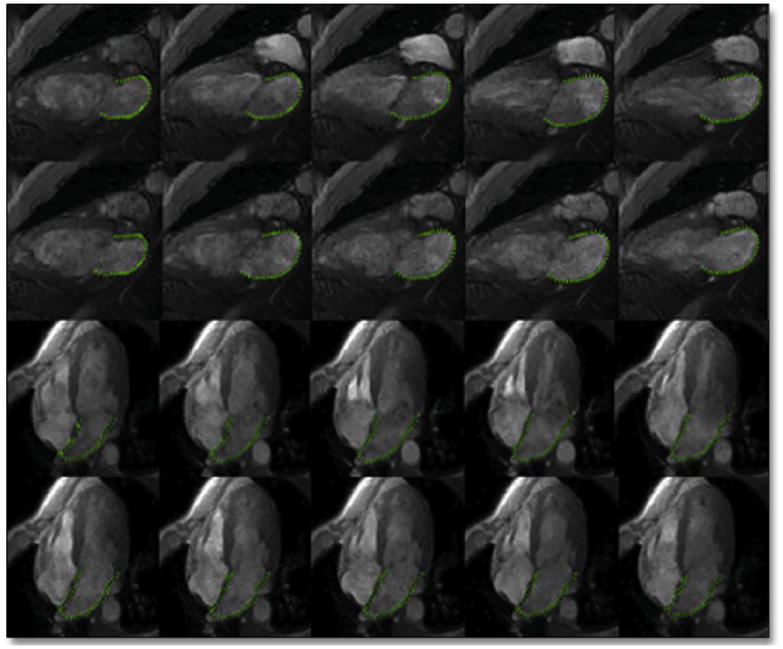
Multidisciplinary tissue tracking software was used on untagged cine cardiac magnetic resonance (CMR) images in 2-chamber and 4-chamber views to track LA wall motions in systole and diastole. Abbreviations as in Figure 1.
Strain measurement
The software generates longitudinal strain curves for each atrial wall segment as shown in Figure 3. Global longitudinal atrial strain was generated by averaging all LA segmental values at each time frame. Global peak longitudinal atrial strain (PLAS) and longitudinal strain before atrial contraction (preA-S) were measured from the global longitudinal strain curve.
Figure 3. Volume and Strain Curves.
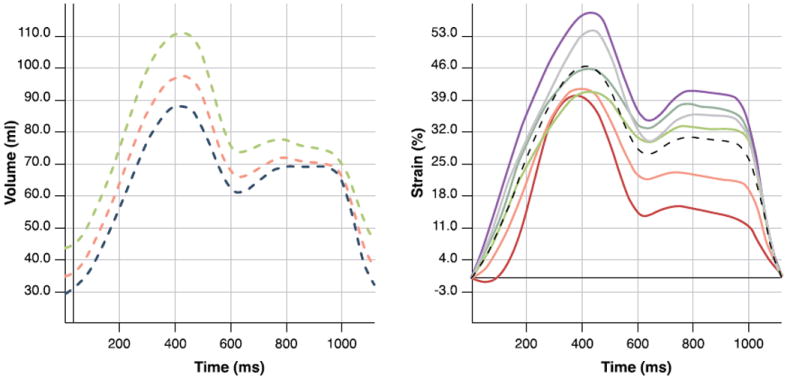
Volume/time (left) and strain/time (right) curves showing atrial volume and global longitudinal strain at different phases.
Validation of volume measurements using MTT software
To validate the measured volumes according to MTT software, using the same CMR images, we also measured minimum and maximum LA volumes using the area–length method in all case and control subjects. Image analysis was performed on a Windows workstation using QMass software version 7.0 (Medis Medical Imaging Systems BV, Leiden, the Netherlands). To measure LA area, endocardial contours were drawn in the left atrium in end systole and diastole. The longitudinal axis of the left atrium was defined by measuring the distance from the center of the mitral valve annulus to the posterior LA wall (Fig. 4). We used the biplane area–length formula to measure the LA volumes: volume = (0.848 · area4ch · area2ch)/([length2ch + length4ch]/2) (15).
Figure 4. Measuring LA Volume Using the Area–Length Method.
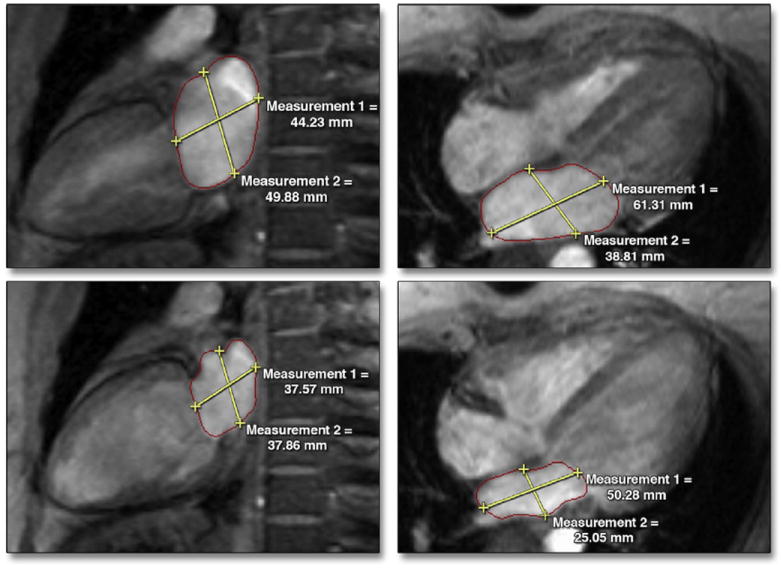
Maximum and minimum LA areas along with longitudinal and transverse diameters were measured in the 2-chamber (left) and 4-chamber (right) views. Abbreviation as in Figure 1.
Statistical analysis
Continuous variables are presented as mean ± SD. Categorical variables are presented as frequencies and percentages. Differences between group means were evaluated with Student t tests (continuous variables) or chi-square analysis (categorical variables) as appropriate. Logarithmic transformation was applied to NT-proBNP before entry into the models because of its skewed distribution. Nonconditional logistic regression was used to calculate odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) in 3 models. Model 1 was adjusted for age, sex, ethnicity, body mass index, diabetes mellitus, cigarette smoking, systolic blood pressure, heart rate, total cholesterol, and high-density lipoprotein levels (traditional cardiovascular risk factors). In model 2, an additional adjustment was made for LV mass. We additionally added NT-proBNP into model 3. We adjusted for LV mass and NT-proBNP because both markers have been shown to be associated with incident HF (26,27). In addition, these 2 variables are well-known markers of volume or pressure overload in the left ventricle that directly affect LA volume and function (28,29). Receiver-operating characteristic curves were generated to assess the overall performance of these 3 models and LA parameters in predicting HF. Area under the curve (AUC) derived from receiver-operating characteristic curve analysis were calculated and compared using a previously described method from DeLong et al. (30).
Interobserver and intraobserver reproducibility for LA parameters were assessed in 20 randomly selected subjects from the case and control groups. Two readers (M.H. and a trained lab technologist with 10 years of experience in CMR data analysis) remeasured LA parameters using the same method. The readers were blinded to the other measurements. Also in the same sample, LA parameters were remeasured by the original reader blinded to the first measurement. Intraclass correlation coefficient analysis was performed to evaluate interobserver and intraobserver agreement. All statistical analyses were performed using Stata software version 11.2 (Stata Corp., College Station, Texas).
RESULTS
It was feasible to measure LA function parameters in 100 (89%) and 192 (86%) case subjects and control subjects, respectively (Fig. 1). The differences in baseline characteristics of incident HF case subjects and non-HF control subjects have been summarized in Table 1. Incident HF case subjects had higher systolic blood pressure, body mass index, and heart rate at baseline. Hypertension, diabetes mellitus, and cigarette smoking were also more common at baseline in incident HF cases.
Table 1.
Baseline Characteristics of HF Case Subjects and Non-HF Control Subjects
| Non-HF Controls (n = 224) | HF Cases (n = 112) | p Value | ||
|---|---|---|---|---|
| Age, yrs | 67.7 ± 8.9 | 68.4 ± 8.2 | 0.517 | |
|
| ||||
| Male | 66.5 | 66.7 | ||
|
| ||||
| Ethnicity | 0.035 | |||
| White | 38.1 | 42.9 | ||
| Chinese | 15.1 | 4.5 | ||
| Black | 25.7 | 31.2 | ||
| Hispanic | 21.1 | 21.4 | ||
|
| ||||
| Systolic blood pressure, mm Hg | 130 ± 23 | 138 ± 21 | 0.005 | |
|
| ||||
| Diastolic blood pressure, mm Hg | 73 ± 11 | 73 ± 11 | 0.878 | |
|
| ||||
| Heart rate, beats/min | 62 ± 10 | 66 ± 11 | 0.005 | |
|
| ||||
| Body mass index, kg/m2 | 27.2 ± 4.2 | 28.8 ± 5.0 | 0.002 | |
|
| ||||
| Current smoker | 11 | 19 | 0.012 | |
|
| ||||
| Total cholesterol, mg/dl | 190 ± 33 | 190 ± 35 | 0.970 | |
|
| ||||
| HDL cholesterol, mg/dl | 49 ± 15 | 49 ± 14 | 0.735 | |
|
| ||||
| Diabetes | 14 | 30 | <0.001 | |
|
| ||||
| LV ejection fraction | 69 ± 8 | 64 ± 12 | <0.001 | |
|
| ||||
| LV mass index, g/m2 | 148 ± 39 | 187 ± 58 | <0.001 | |
Values are mean ± SD or %.
HDL = high-density lipoprotein; HF = heart failure; LV = left ventricular.
LA volumes
LA volume measurements in case and control subjects are summarized in Table 2. Baseline LAVmax was higher in incident HF patients compared with control subjects (77 ± 25 ml in case subjects vs. 60 ± 21 ml in control subjects; p < 0.001). LAVmin and LAVPreA indexes were also significantly higher in HF case subjects than in control subjects.
Table 2.
Left Atrial Parameters in HF Case Subjects and Non-HF Control Subjects
| Non-HF Controls (n = 224) | HF Cases (n = 112) | p Value | |
|---|---|---|---|
| LAVmax, ml | 60 ± 21 | 77 ± 25 | <0.001 |
|
| |||
| LAVImax, ml/m2 | 33 ± 10 | 40 ± 13 | <0.001 |
|
| |||
| LAVmin, ml | 32 ± 14 | 48 ± 22 | <0.001 |
|
| |||
| LAVImin, ml/m2 | 17 ± 7 | 25 ± 11 | <0.001 |
|
| |||
| LAEF, % | 48 ± 9 | 40 ± 11 | <0.001 |
|
| |||
| CVEF, % | 23 ± 8 | 17 ± 8 | <0.001 |
|
| |||
| Global PLAS, % | 38 ± 16 | 25 ± 11 | <0.001 |
|
| |||
| PreA-S % | 23 ± 11 | 16 ± 8 | <0.001 |
Values are mean ± SD.
CVEF = conduit volume fraction; LAEF = left atrial emptying fraction; LAVmax = left atrial maximum volume; LAVmin = left atrial minimum volume; LAVImax = left atrial maximum volume index; LAVImin = left atrial minimum volume index; PLAS = peak longitudinal atrial strain; preA-S = longitudinal strain before atrial contraction; other abbreviations as in Table 1.
LA strain
Incident HF patients had a significantly lower global PLAS at baseline compared with control subjects (25 ± 11% vs. 38 ± 16%, respectively; p < 0.001). Global preA-S was also lower in HF cases (16 ± 8% in case subjects vs. 23 ± 11% in control subjects; p < 0.001). Among all case and control subjects, all those with global PLAS <14% developed HF during the 8-year follow-up period.
Association of LA function and HF
The relationship between LA volumes and incident HF was tested in logistic regression models. Table 3 summarizes ORs (95% CIs) associated with measured LA parameters and incident HF. In model 1 (adjusted for traditional risk factors), all LA parameters were significantly associated with development of HF. Among the volume measurements, the LAVmin index had the highest OR (2.75 [95% CI: 1.9 to 4.0]). Low LA emptying fraction, conduit volume fraction, and peak and preA-S at baseline were also associated with higher incidence of HF; global PLAS had the strongest OR (0.28 [95% CI: 0.18 to 0.44]). In model 2, the results were similar after additionally adjusting for the LV mass index. Both strain measurements remained significantly associated with HF development in model 3, with global PLAS having the strongest OR (0.36 [95% CI: 0.22 to 0.60]). However, in model 3, after adding log NT-proBNP to the model, the only statistically significant volume measurement associated with HF was the LAVmin index (OR: 1.65 [95% CI: 1.04 to 2.63]).
Table 3.
Left Atrial Function and Incident HF
| Odds Ratio* (95% CI)
|
|||
|---|---|---|---|
| Model 1† | Model 2‡ | Model 3§ | |
| LAVImax, ml/m2 | 2.09 (1.50–2.89) | 1.72 (1.22–2.43) | 1.46 (0.96–2.27) |
|
| |||
| LAVImin, ml/m2 | 2.75 (1.89–3.99) | 2.17 (1.46–3.21) | 1.65 (1.04–2.63) |
|
| |||
| LAEF, % | 0.38 (0.27–0.55) | 0.48 (0.33–0.71) | 0.68 (0.45–1.03) |
|
| |||
| LACV, % | 0.48 (0.34–0.67) | 0.59 (0.42–0.83) | 0.69 (0.46–1.03) |
|
| |||
| Global PLAS, % | 0.28 (0.18–0.44) | 0.35 (0.22–0.55) | 0.36 (0.22–0.60) |
|
| |||
| PreA-S, % | 0.37 (0.25–0.57) | 0.44 (0.30–0.67) | 0.48 (0.30–0.77) |
Odds ratios are presented per SD change in measurements.
Model 1 was adjusted for age, sex, ethnicity, heart rate, body mass index, diabetes mellitus, systolic blood pressure, total cholesterol, high-density lipoprotein, and cigarette smoking.
Model 2 was additionally adjusted for LV mass.
Model 3 was additionally adjusted for log N-terminal pro–B-type natriuretic peptide.
To further explore the value of LA function in HF, we performed receiver operating curve analyses. We added all left atrium–measured parameters to traditional risk factors for HF development (Table 4). The AUC was highest for PLAS and traditional risk factors compared with traditional risk factors alone (0.81 vs. 0.71, respectively; p = 0.004). We also compared the AUC by incrementally adding LV mass index, NT-proBNP, and global PLAS to traditional risk factors in Table 5. Compared with traditional risk factors alone, AUC values for HF development showed the greatest augmentation after incremental addition of the LV mass index, log NT-proBNP, and global PLAS in the overall case and control populations (0.76 vs. 0.71 [p = 0.04], 0.82 vs. 0.76 [p < 0.001], and 0.86 vs. 0.82 [p < 0.001], respectively).
Table 4.
AUC for LA Parameters According to HF Development
| AUC | p Value | |
|---|---|---|
| Base model | 0.71 | — |
|
| ||
| Base model + LAVImax | 0.77 | 0.029 |
|
| ||
| Base model + LAVImin | 0.79 | 0.003 |
|
| ||
| Base model + LAEF | 0.79 | 0.006 |
|
| ||
| Base model + LACV | 0.77 | 0.013 |
|
| ||
| Base model+preA-S | 0.79 | 0.005 |
|
| ||
| Base model + PLAS | 0.81 | <0.001 |
Table 5.
AUC According to Incident HF
| AUC | p Value | |
|---|---|---|
| Base model | 0.71 | — |
|
| ||
| Base model + LV mass | 0.76 | 0.04 |
|
| ||
| Base model + LV mass + NT-proBNP | 0.82 | <0.001 |
|
| ||
| Base model + LV mass + NT-proBNP + PLAS | 0.86 | <0.001 |
Systolic HF versus HF with normal EF
Of 112 cases, 98 had measured EF at the time of HF diagnosis. Thirty-nine cases (39.8%) developed HFNEF, and 59 (60.2%) developed systolic HF. LA parameters at baseline did not differ between these 2 categories (Table 6).
Table 6.
LA Parameters at Baseline in Individuals With HF and HFNEF and Those With Systolic HF
| Systolic HF (n = 59) | HFNEF (n = 39) | p Value | |
|---|---|---|---|
| LAVImax, ml/m2 | 39.4 ± 11.7 | 40.8 ± 13.9 | 0.61 |
|
| |||
| LAVmin index, ml/m2 | 24.4 ± 9.6 | 24.5 ± 11.2 | 0.99 |
|
| |||
| LAEF, % | 38.3 ± 9.5 | 42.3 ± 11.5 | 0.09 |
|
| |||
| LACV, % | 16.5 ± 7.1 | 18.2 ± 7.3 | 0.27 |
|
| |||
| Global PLAS, % | 23.2 ± 9.6 | 26.3 ± 13.2 | 0.20 |
|
| |||
| PreA-S, % | 14.44 ± 6.4 | 17.99 ± 9.9 | 0.053 |
Reproducibility of LA variables using MTT software
Reproducibility of LA measurements was evaluated in 20 randomly selected subjects. Intraclass correlation coefficients were 0.95 and 0.97 for LAVmax, 0.92 and 0.93 for LAVmin, 0.92 and 0.95 for global PLAS, and 0.90 and 0.91 for preA-S for interobserver and intraobserver reproducibility, respectively.
Measured LA volumes using area–length method compared with use of MTT software
LAVmin and LAVmax were similar when measured using the area–length method compared with those obtained using MTT software. The mean LAVmax and LAVmin were 66.5 ± 24.0 ml and 37.0 ± 18.6 ml when measured using MTT software compared with 69.5 ± 24.6 ml and 38.9 ± 18.9 ml when measured using the area–length method, respectively. Intraclass correlation coefficients were 0.94 (95% CI: 0.92 to 0.97) for LAVmax and 0.91 (95% CI: 0.87 to 0.95) for LAVmin.
DISCUSSION
This nested case-control study performed within a large longitudinal multiethnic population of asymptomatic individuals demonstrated a strong association between altered LA deformation as measured using CMR feature-tracking and HF development. We found that individuals who develop HF have reduced global PLAS and increased LAVmin index well before the onset of symptomatic clinical manifestation. In our study, this association was independent of traditional cardiovascular risk factors, LV mass, and NT-proBNP. These findings emphasize the role of LA dysfunction in identifying asymptomatic individuals at higher risk for HF development. Given the fact that LVEF is normal in more than one-half of HF patients (31), the assessment of LA function is valuable in the evaluation of LV diastolic performance. Although the left atrial volume index (LAVI) measurement is now included as a parameter in the European Society of Cardiology guidelines to diagnose HF with preserved EF (32), no measures of LA mechanical behavior have so far been proposed as markers of risk for this condition.
In most previous studies, 2-dimensional echocardiography has been used for volume measurements of the left atrium. However, CMR has been shown to have excellent reproducibility and accuracy in the measurement of cardiac volumes, making it the gold standard modality for the assessment of cardiac chambers (33). One study comparing LA volume measured using CMR and echocardiography showed that echocardiography consistently underestimated volumes compared with CMR (19).
To our knowledge, CMR has never been used to assess LA strain as a prognostic marker in HF. In this study, we demonstrated the value of CMR feature-tracking to assess LA function, including volumes and strain.
LA volume and HF
Recently, there has been emerging evidence supporting the prognostic role of LA volume in predicting incident HF (5,34,35). Takemoto et al. (5) showed that LAVI ≥32 ml/m2 in elderly adults referred for echocardiography was independently associated with higher incident HF (hazard ratio: 1.97; 95% CI: 1.4 to 2.7). In another large prospective study, older (>65 years of age) individuals who developed HF during follow-up had a higher LA linear diameter at baseline (34). Also, in another population study, LAVI, as measured using echocardiography, was associated with the severity of diastolic dysfunction after adjusting for age, sex, presence of cardiovascular disease, EF, and LV mass index (36). In these studies, the associations of LAVI were tested only after adjustment for several covariables but not for NT-proBNP. In our study, all volume measurements were still significantly associated with HF after adjusting for traditional risk factors and LV mass index. However, after adjusting for NT-proBNP, only the LAVmin index was independently associated with HF. Our findings complement 2 other studies emphasizing the association of LAVmin and LV dysfunction. In 1 study of patients with HFNEF, Russo et al. (37) demonstrated that the LAVmin increases even in mild diastolic dysfunction, whereas an increase in LAVmax was only seen in later stages of systolic dysfunction. In the other study of 41 patients undergoing cardiac catheterization, among the LA volumes, LAVmin was the most sensitive identifier of LV end-diastolic pressure (38).
We found no statistically significant differences in baseline LA parameters between cases with systolic HF versus those with HFNEF. It has been shown previously that patients with HFNEF have larger LA volumes and reduced total, passive, and active LA emptying fractions (4,37). LA function is also reduced with exercise in patients with HFNEF compared with asymptomatic hypertensive and healthy individuals (39). However, to our knowledge, this is the first study comparing LA function between individuals with systolic HF and HFNEF before symptomatic HF development.
LA strain and HF
Global PLAS has been proposed as a useful parameter in the assessment of LA function (40,41). This parameter reflects the passive stretching of the left atrium during LV systole and represents LA compliance and reservoir function. It has also been previously demonstrated that global PLAS correlates strongly with LV filling pressure (20,42). In 1 study involving 36 patients with systolic HF, among all parameters of LA function, global PLAS was the strongest predictor of elevated pulmonary capillary wedge pressure with an AUC of 0.93 and a sensitivity and specificity of 100% and 93%, respectively, using a cutoff value <15.1% (20). LA strain has also been associated with cardiovascular outcomes other than HF. In previous studies, LA strain measured using speckle-tracking echocardiography has been associated with the risk of new-onset atrial fibrillation (43) and stroke (44). Also, in a previous longitudinal study of 312 adults with a mean follow-up of 3.1 ± 1.4 years, global PLAS was the strongest predictor of cardiovascular outcomes (45). Our results therefore are consistent with these previous findings, as global PLAS measured using CMR feature-tracking was the strongest parameter of LA performance associated with incident HF in this multiethnic population of asymptomatic individuals.
Study limitations
The strengths and limitations of our study include being a longitudinal study with 8 years of follow-up in asymptomatic individuals from a multiethnic population. Other strengths include a wide age range of the study population, in contrast to most other studies on LA function, and also availability of comprehensive and standardized clinical data as well as biomarkers. To our knowledge, our study is among the first to measure LA strain using CMR.
Several study limitations should also be noted. The design was a nested case-control study. However, we matched our control group for age and sex, and our statistical analysis was adjusted for possible confounders. This was a relatively small study, with 112 case subjects (100 with analyzable images) and 224 control subjects (192 with analyzable images). However, based on the study by Morton et al. (23), a sample size of 29 has 90% power and an alpha error of 0.05 to detect a change of 5% in strain. Although feature-tracking CMR in our study had a great interobserver and intraobserver reproducibility, its test–retest variability in the measurement of LA function was not examined. Given the thin wall of the left atrium, the CMR images were not interpretable in about 13% of the study population due to the failure in LA wall tracking. Nevertheless, this rate is comparable to, or lower than the rate in other studies using speckle-tracking echocardiography in measurements of LA function (4,16).
CONCLUSIONS
Among this diverse population with no symptomatic cardiovascular disease, there was an independent inverse association between global LA longitudinal strain measured using CMR and incident HF. Among the LA volume measurements, only LA minimal volume index was independently associated with incident HF. Moreover, our study demonstrated that measurements of LA longitudinal strain and volumes using CMR feature-tracking are feasible and reproducible in population studies. Our study, which was performed using CMR as an accurate but expensive modality for the assessment of myocardial wall motion, complements previous studies performed with echocardiography. Our findings emphasize the importance of LA function, especially global PLAS and LAVImin, in HF development among asymptomatic healthy individuals. Further studies investigating the longitudinal changes in LA function would be helpful for quantifying the independent prognostic power of LA function in predicting HF.
Acknowledgments
The authors thank all the investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
This research was supported by contracts N01-HC-95159 through N01-HC-95166 and N01-HC-95168 from the National Heart, Lung, and Blood Institute and UL1-RR-024156 and UL1-RR-025005 from the National Center for Research Resources, National Institutes of Health. Sherif Nagueh, MD, served as Guest Editor for this paper
ABBREVIATIONS AND ACRONYMS
- AUC
area under the curve
- CI
confidence interval
- CMR
cardiac magnetic resonance
- EF
ejection fraction
- HF
heart failure
- HFNEF
heart failure with normal ejection fraction
- LA
left atrium
- LAV
left atrial volume
- LAVmax
maximum left atrial volume
- LAVmin
minimum left atrial volume
- LAVI
left atrial volume index
- LAVPreA
pre–atrial contraction left atrial volume
- LV
left ventricle
- NT-proBNP
N-terminal pro–B-type natriuretic peptide
- OR
odds ratio
- PLAS
peak longitudinal atrial strain
- preA-S
longitudinal strain before atrial contraction
Footnotes
All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 2.Rossi A, Cicoira M, Zanolla L, et al. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1425–30. doi: 10.1016/s0735-1097(02)02305-7. [DOI] [PubMed] [Google Scholar]
- 3.Rossi A, Temporelli PL, Quintana M, et al. Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta-analysis of longitudinal data (MeRGE Heart Failure) Eur J Heart Fail. 2009;11:929–36. doi: 10.1093/eurjhf/hfp112. [DOI] [PubMed] [Google Scholar]
- 4.Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–5. doi: 10.1161/CIRCIMAGING.108.813071. [DOI] [PubMed] [Google Scholar]
- 5.Takemoto Y, Barnes ME, Seward JB, et al. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol. 2005;96:832–6. doi: 10.1016/j.amjcard.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Moller JE, Hillis GS, Oh JK, et al. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–12. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 7.Osranek M, Bursi F, Bailey KR, et al. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three-decade follow-up. Eur Heart J. 2005;26:2556–61. doi: 10.1093/eurheartj/ehi483. [DOI] [PubMed] [Google Scholar]
- 8.Buber J, Luria D, Sternik L, et al. Left atrial contractile function following a successful modified Maze procedure at surgery and the risk for subsequent thromboembolic stroke. J Am Coll Cardiol. 2011;58:1614–21. doi: 10.1016/j.jacc.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 9.Azemi T, Rabdiya VM, Ayirala SR, McCullough LD, Silverman DI. Left atrial strain is reduced in patients with atrial fibrillation, stroke or TIA, and low risk CHADS(2) scores. J Am Soc Echocardiogr. 2012;25:1327–32. doi: 10.1016/j.echo.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the Heart and Soul Study. J Am Coll Cardiol. 2012;59:673–80. doi: 10.1016/j.jacc.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel RK, Jardine AG, Mark PB, et al. Association of left atrial volume with mortality among ESRD patients with left ventricular hypertrophy referred for kidney transplantation. Am J Kidney Dis. 2010;55:1088–96. doi: 10.1053/j.ajkd.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozdogan O, Kayikcioglu M, Asci G, et al. Left atrial volume predicts mortality in low-risk dialysis population on long-term low-salt diet. Am Heart J. 2010;159:1089–94. doi: 10.1016/j.ahj.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Montefusco A, Biasco L, Blandino A, et al. Left atrial volume at MRI is the main determinant of outcome after pulmonary vein isolation plus linear lesion ablation for paroxysmal-persistent atrial fibrillation. J Cardiovasc Med (Hagerstown) 2010;11:593–8. doi: 10.2459/JCM.0b013e32833831e4. [DOI] [PubMed] [Google Scholar]
- 14.Kircher B, Abbott JA, Pau S, et al. Left atrial volume determination by biplane two-dimensional echocardiography: validation by cine computed tomography. Am Heart J. 1991;121:864–71. doi: 10.1016/0002-8703(91)90200-2. [DOI] [PubMed] [Google Scholar]
- 15.Rodevan O, Bjornerheim R, Ljosland M, Maehle J, Smith HJ, Ihlen H. Left atrial volumes assessed by three- and two-dimensional echocardiography compared to MRI estimates. Int J Card Imaging. 1999;15:397–410. doi: 10.1023/a:1006276513186. [DOI] [PubMed] [Google Scholar]
- 16.Saraiva RM, Demirkol S, Buakhamsri A, et al. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J Am Soc Echocardiogr. 2010;23:172–80. doi: 10.1016/j.echo.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Bastarrika G, Zudaire B, Ferreira M, Arraiza M, Saiz-Mendiguren R, Rabago G. Assessment of left atrial volumes and function in orthotopic heart transplant recipients by dual-source CT: comparison with MRI. Invest Radiol. 2010;45:72–6. doi: 10.1097/RLI.0b013e3181c4f535. [DOI] [PubMed] [Google Scholar]
- 18.Maceira AM, Cosin-Sales J, Roughton M, Prasad SK, Pennell DJ. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:65. doi: 10.1186/1532-429X-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitlock M, Garg A, Gelow J, Jacobson T, Broberg C. Comparison of left and right atrial volume by echocardiography versus cardiac magnetic resonance imaging using the area-length method. Am J Cardiol. 2010;106:1345–50. doi: 10.1016/j.amjcard.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 20.Cameli M, Lisi M, Mondillo S, et al. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound. 2010;8:14. doi: 10.1186/1476-7120-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hor KN, Baumann R, Pedrizzetti G, et al. Magnetic resonance derived myocardial strain assessment using feature tracking. J Vis Exp. 2011;48:2356. doi: 10.3791/2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuster A, Morton G, Hussain ST, et al. The intra-observer reproducibility of cardiovascular magnetic resonance myocardial feature tracking strain assessment is independent of field strength. Eur J Radiol. 2013;82:296–301. doi: 10.1016/j.ejrad.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Morton G, Schuster A, Jogiya R, Kutty S, Beerbaum P, Nagel E. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson. 2012;14:43. doi: 10.1186/1532-429X-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 25.Natori S, Lai S, Finn JP, et al. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 26.Choi EY, Bahrami H, Wu CO, et al. N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2012;5:727–34. doi: 10.1161/CIRCHEARTFAILURE.112.968701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurt M, Tanboga IH, Aksakal E, et al. Relation of left ventricular end-diastolic pressure and N-terminal pro-brain natriuretic peptide level with left atrial deformation parameters. Eur Heart J Cardiovasc Imaging. 2012;13:524–30. doi: 10.1093/ejechocard/jer283. [DOI] [PubMed] [Google Scholar]
- 29.Tschope C, Kasner M, Westermann D, Gaub R, Poller WC, Schultheiss HP. The role of NT-proBNP in the diagnostics of isolated diastolic dysfunction: correlation with echocardiographic and invasive measurements. Eur Heart J. 2005;26:2277–84. doi: 10.1093/eurheartj/ehi406. [DOI] [PubMed] [Google Scholar]
- 30.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 31.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 32.Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 33.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 34.Gardin JM, McClelland R, Kitzman D, et al. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study) Am J Cardiol. 2001;87:1051–7. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- 35.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the Cardiovascular Health Study) Am J Cardiol. 2006;97:83–9. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 36.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 37.Russo C, Jin Z, Homma S, et al. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. 2012;98:813–20. doi: 10.1136/heartjnl-2011-301388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Posina K, McLaughlin J, Rhee P, et al. Relationship of phasic left atrial volume and emptying function to left ventricular illing pressure: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2013;15:99. doi: 10.1186/1532-429X-15-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan YT, Wenzelburger F, Lee E, et al. Reduced left atrial function on exercise in patients with heart failure and normal ejection fraction. Heart. 2010;96:1017–23. doi: 10.1136/hrt.2009.189118. [DOI] [PubMed] [Google Scholar]
- 40.Cameli M, Lisi M, Giacomin E, et al. Chronic mitral regurgitation: left atrial deformation analysis by two-dimensional speckle tracking echocardiography. Echocardiography. 2011;28:327–34. doi: 10.1111/j.1540-8175.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- 41.Mondillo S, Cameli M, Caputo ML, et al. Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr. 2011;24:898–908. doi: 10.1016/j.echo.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 42.Wakami K, Ohte N, Asada K, et al. Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr. 2009;22:847–51. doi: 10.1016/j.echo.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 43.Hirose T, Kawasaki M, Tanaka R, et al. Left atrial function assessed by speckle tracking echocardiography as a predictor of new-onset non-valvular atrial fibrillation: results from a prospective study in 580 adults. Eur Heart J Cardiovasc Imaging. 2012;13:243–50. doi: 10.1093/ejechocard/jer251. [DOI] [PubMed] [Google Scholar]
- 44.Shih JY, Tsai WC, Huang YY, et al. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J Am Soc Echocardiogr. 2011;24:513–9. doi: 10.1016/j.echo.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Cameli M, Lisi M, Focardi M, et al. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am J Cardiol. 2012;110:264–9. doi: 10.1016/j.amjcard.2012.03.022. [DOI] [PubMed] [Google Scholar]


