Abstract
Enterovirus B81 (EV-B81) is a newly identified serotype within the species enterovirus B (EV-B). To date, only eight nucleotide sequences of EV-B81 have been published and only one full-length genome sequence (the prototype strain) has been made available in the GenBank database. Here, we report the full-length genome sequences of two EV-B81 strains isolated in the Tibet Autonomous Region of China during acute flaccid paralysis surveillance activities, and we also conducted an antibody seroprevalence study in two prefectures of Tibet. The sequence comparison and phylogenetic dendrogram analysis revealed high variability among the global EV-B81 strains and frequent intertypic recombination in the non-structural protein region of EV-B serotypes, suggesting high genetic diversity of EV-B81. However, low positive rates and low titers of neutralizing antibodies against EV-B81 were detected. Nearly 68% of children under the age of five had no neutralizing antibodies against EV-B81. Hence, the extent of transmission and the exposure of the population to this EV type are very limited. Although little is known about the biological and pathogenic properties of EV-B81 because of few research in this field owing to the limited number of isolates, our study provides basic information for further studies of EV-B81.
Enteroviruses (EVs) belong to the family Picornaviridae within the new order Picornavirales. The genome of the small non-enveloped viruses is a single-stranded, positive-sense RNA molecule of approximately 7500 nucleotides consisting of a single open reading frame flanked by 5′ and 3′ untranslated regions (UTRs). A single polyprotein translated from the RNA strand is first cleaved into three polyprotein precursors: P1, P2, and P3. P1 is processed to yield four structural proteins: Viral protein 1–4 (VP1–VP4); P2 and P3 are precursors of the nonstructural proteins 2A–2C and 3A–3D, respectively.
Most EV infections are asymptomatic or cause only mild symptoms. However, some EVs can also cause a broad spectrum of other clinical illnesses, including acute flaccid paralysis (AFP); acute hemorrhagic conjunctivitis; encephalitis; aseptic meningitis; and hand, foot, and mouth disease1,2,3,4. Human EVs now comprise more than 110 serotypes, which are currently classified into four species, EV-A to EV-D, on the basis of their molecular and biological characteristics5. Although generally reliable, the neutralization test–a traditional method for EV typing–has gradually been replaced because it is labor-intensive and time-consuming; furthermore, it may fail to identify an isolate because of aggregation of virus particles, antigenic drift, or the presence of multiple viruses in the specimen6. Current EV classification is based on the high nucleotide sequence divergence within the VP1 capsid-coding region, which has been shown to correspond with serotype neutralization6,7. According to the recommended specific criteria for the interpretation of VP1 sequence data, EVs are classified into the same type if they have more than 75% nucleotide similarity (85% amino acid similarity) and into different types if they have less than 70% nucleotide similarity in this region. While 70–75% nucleotide similarity in VP1 region has been considered as a “grey zone” of molecular typing of EVs, and in this instance, additional information such as complete P1 region sequences nucleotide similarity or neutralization profile should be obtained to decide the EV serotype7. A large number of new EV types have been discovered after molecular typing methods became available.
Enterovirus B81 (EV-B81) is a newly identified serotype within the species EV-B. The prototype strain (USA/CA68-10389) of EV-B81 was isolated in the USA in 19688. Subsequently, several other EV-B81 strains were isolated from AFP patients or healthy individuals during AFP case surveillance in China9, Bangladesh10, India1,11, Gabon12, and Cameroon13. To date, the only full-length genome sequence available for EV-B81 has been that of the prototype strain. Besides the prototype strain, only one entire VP1 sequence and several partial VP1 sequences of this EV type were available in the GenBank database. In this study, we analyzed the full-length genome sequences of two strains of EV-B81 isolated in the Tibet Autonomous Region of China.
Results
Serotyping and molecular typing of the Tibetan isolates
The Tibetan isolates (strain 99279/XZ/CHN/1999 and strain 99298c/XZ/CHN/1999, hereafter referred to as 99279 and 99298c, respectively) were initially characterized using a standard pool of EV typing antisera (RIVM, the Netherlands) distributed by the World Health Organization. However, neither of the isolates could be neutralized by any of the antisera (data not shown). Therefore, the isolates were initially identified as “untypeable” non-polio EVs. The VP1 capsid-coding regions of the two Tibetan isolates were then partially sequenced using molecular typing methods and analyzed by an online enterovirus genotyping tool14. Both isolates were identified as EV-B81.
Full-length genomic characterization of Chinese EV-B81 strains
The full-length genomes of the two EV-B81 strains were sequenced. Both were 7417 nucleotides in length, encoding a polypeptide of 2191 amino acids. The coding sequences were flanked by a non-coding 5′ UTR of 741 nucleotides and a non-coding 3′ UTR of 100 nucleotides followed by a poly (A) tail composed of a long sequence of adenine nucleotides. Alignment of the full-length genomes of the two Tibetan EV-B81 strains with the genome of the EV-B81 prototype strain (USA/CA68-10389) showed that they all had the same genomic organization and collinear order of genomic regions. However, in the 5′ UTR, Tibet EV-B81 strains contained three nucleotide insertions at positions 101, 102, and 118 and a nucleotide deletion at position 179. In the 3′ UTR, they contained a nucleotide insertion at position 7328 and a nucleotide deletion at position 7341. The overall base compositions of strains 99279 and 99298c were 28.03% and 28.11% A, 24.36% and 24.35% G, 23.82% and 23.73% C, and 23.79% and 23.81% U, respectively. The polypeptide cleavage sites were predicted based on the full-length genome sequence of the EV-B81 prototype strain. Table 1 shows the nucleotide sequence and deduced amino acid sequence identities between the Tibetan EV-B81 strains and the EV-B81 prototype strain and other prototype strains within the EV-B species. The nucleotide sequences available for the distinct EV-B81 strains differ considerably. The complete genome nucleotide sequence similarity between these two Tibetan EV-B81 strains is 99.6%, and they displayed 79.1% and 79.2% nucleotide identity and 94.7% and 95.2% amino acid identity with the prototype EV-B81 strain, respectively.
Table 1. The nucleotide sequence and deduced amino acid sequence identities between two Tibetan enterovirus B81 (EV-B81) strains (99279 and 99298c) and the EV-B81 prototype strain and other prototype strains belonging to enterovirus B (EV-B).
| Region | % nucleotide identity (% amino acid identity) | |||
|---|---|---|---|---|
| EV-B81 strain 99279 | EV-B81 strain 99298c | |||
| Prototype of EV-B81 | Prototypes of other EV-B | Prototype of EV-B81 | Prototypes of other EV-B | |
| 5′ UTR | 78.3 | 66.3–89.4 | 78.4 | 66.3–89.3 |
| VP4 | 78.2 (95.6) | 67.6–79.7 (71.0–82.6) | 78.2 (95.6) | 67.6–79.7 (71.0–82.6) |
| VP2 | 79.6 (96.5) | 64.5–73.4 (73.3–84.2) | 79.6 (96.5) | 64.3–73.5 (73.3–83.9) |
| VP3 | 79.8 (100.0) | 62.1–73.0 (67.5–87.4) | 79.8 (100.0) | 62.0–72.8 (67.5–87.4) |
| VP1 | 79.1 (94.7) | 53.8–68.8 (53.3–79.5) | 79.2 (95.4) | 53.9–69.2 (53.6–79.5) |
| 2A | 79.3 (93.3) | 75.1–82.8 (85.3–96.6) | 79.3 (93.3) | 75.1–82.8 (86.0–97.3) |
| 2B | 77.5 (94.0) | 72.6–79.8 (91.0–96.0) | 77.2 (94.0) | 72.6–79.8 (91.0–96.0) |
| 2C | 80.9 (96.9) | 78.0–85.7 (95.1–98.1) | 81.1 (97.2) | 78.2–85.8 (95.4–98.4) |
| 3A | 75.2 (92.1) | 73.7–90.2 (88.7–97.7) | 75.2 (92.1) | 73.7–90.2 (88.7–97.7) |
| 3B | 77.2 (100.0) | 72.7–89.3 (90.9–100.0) | 77.2 (100.0) | 72.7–89.3 (90.9–100.0) |
| 3C | 77.9 (95.6) | 75.0–88.7 (92.8–99.4) | 78.1 (95.6) | 75.2–88.7 (92.8–99.4) |
| 3D | 79.4 (96.7) | 77.8–86.2 (94.5–98.0) | 79.6 (96.7) | 77.9–86.1 (94.5–98.0) |
| 3′ UTR | 84.6 | 75.9–90.2 | 84.6 | 75.9–90.2 |
Phylogenetic analysis of the Chinese EV-B81 strains and other EV-B genomes
Phylogenetic trees were generated from the 252-nucleotide (nucleotide 2567–2818) partial VP1 coding region of the two Tibetan EV-B81 strains and eight other EV-B81 strains available in the GenBank database (Fig. 1). The two Tibetan strains displayed great genetic distance to another Chinese strain (strain 142-98-YN/CHN/1998 isolated in the Yunnan province in 1998) and clustered with the strain BAN-10491 isolated from Bangladesh. Three strains isolated from India clustered together, and most branches displayed long genetic distances.
Figure 1. Phylogenetic relationships based on partial VP1 region sequences of enterovirus B81 (EV-B81).
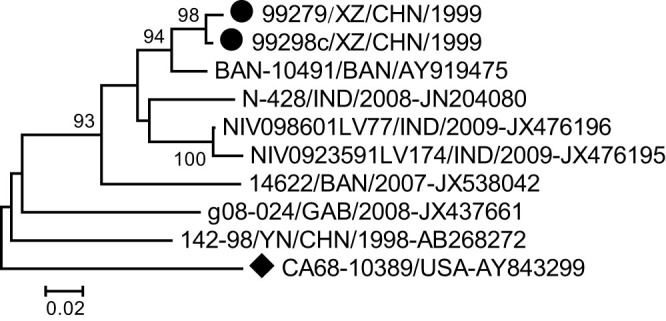
Two Tibetan EV-B81 strains isolated in this study (indicated by circles) and other EV-B81 strains (available in the GenBank database) were analyzed based on the 252-nucleotide (nucleotide 2567–2818) partial VP1 coding region sequence. The strain indicated by a diamond is the EV-B81 prototype strain.
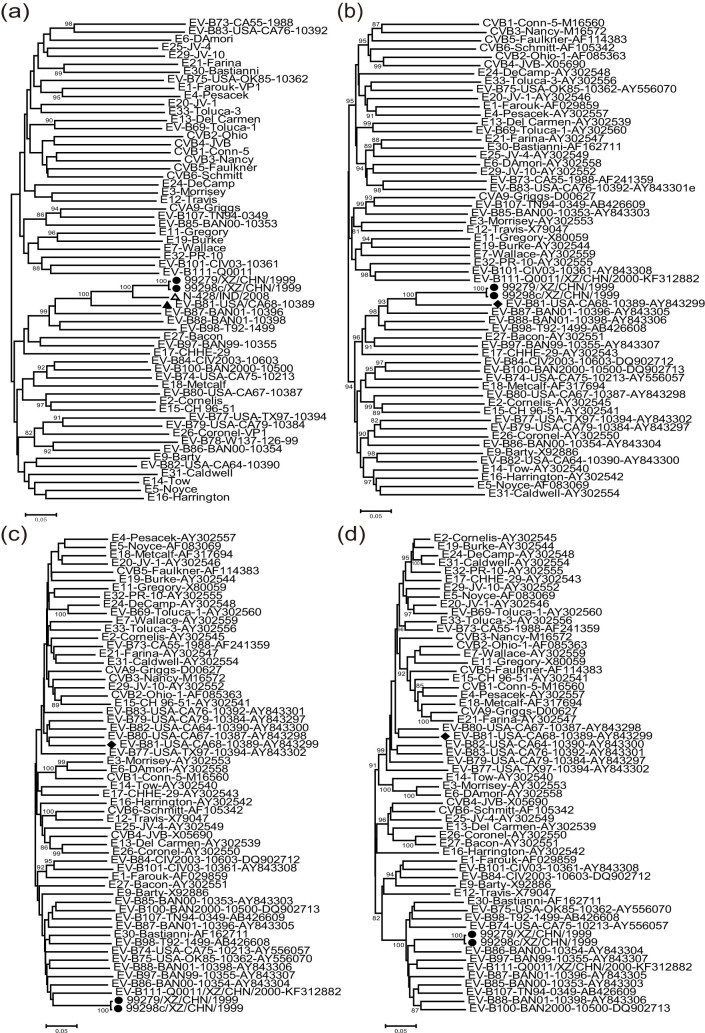
To investigate the genetic relationship between the Tibetan EV-B81 strains, the EV-B81 prototype strain, and other EV-B prototype strains available in the GenBank database, we constructed phylogenetic trees based on the VP1, P1, P2, and P3 regions of the genome. The phylogenetic tree based on the VP1 region also contained an EV-B81 strain (N-428/IND/2008) isolated from India, for which the entire VP1 sequence was available in the GenBank database (Fig. 2). In the VP1 and P1 capsid regions, the two Tibetan EV-B81 strains clustered together with the EV-B81 prototype strain and the Indian EV-B81 strain, confirming the preliminary molecular typing results. In the VP1 region, the nucleotide identity between the Tibetan strains 99279 and 99298c and the Indian strain was 88.8% and 89.1%, respectively. However, in the non-capsid regions, the phylogenetic trees differed greatly from those in the capsid regions. In the P2 and P3 regions, the two Tibetan EV-B81 strains shared the highest similarity with the prototype strains of EV-B111 and EV-B86, respectively. This surprised us because EV-B111 is a recently reported type of enterovirus15. The phylogenetic analysis indicated that recombinations between Chinese EV-B81 strains and other EV-B serotypes might have occurred.
Figure 2. Phylogenetic relationships based on the VP1, P1, P2, and P3 genome regions of enterovirus B (EV-B).
Two Tibetan EV-B81 strains (indicated by solid circles) and 55 other EV-B prototype strains were analyzed by nucleotide sequence alignment using the Neighbor-Joining algorithms implemented in the MEGA 5.0 program. Numbers at the nodes indicate bootstrap support for that node (percent of 1000 bootstrap replicates). The open triangle indicates the India EV-B81 which has the entire VP1 sequence in the GenBank database, and the solid diamond indicates EV-B81 prototype strain. The scale bars represent the genetic distance. All panels have the same scale. (a) VP1 coding sequences; (b) P1 coding sequences; (c) P2 coding sequences; and (d) P3 coding sequences.
Recombinant structure of the Chinese EV-B81 strains
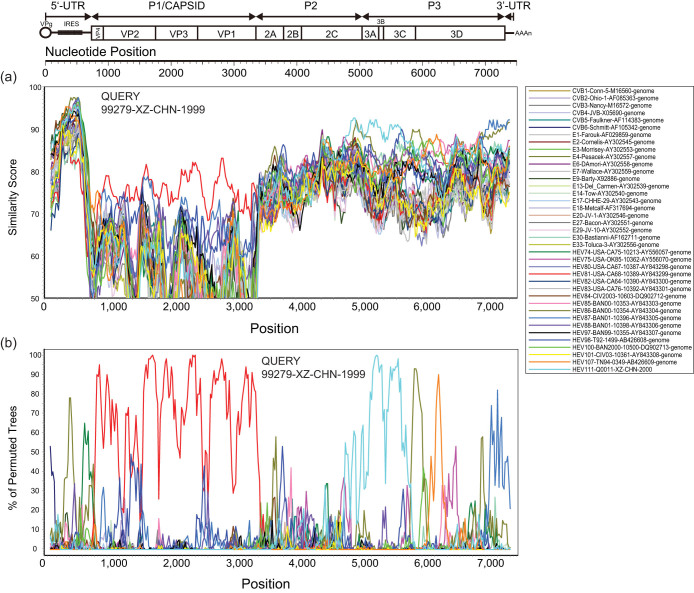
Similarity plots and bootscanning analyses were performed to confirm the recombinations between the Tibetan EV-B81 strains and other EV-B prototype strains. Because the two Tibetan EV-B81 strains share high nucleotide identity in their full-length genomes (99.6%), only one of the strains (99279) was used as a query sequence. It was compared with the EV-B81 prototype strain (USA/CA68-10389) and other EV-B prototype strains. In the P1 coding region, strain 99279 possessed the highest similarity with the EV-B81 prototype strain as expected (Fig. 3). However, in the 5′ UTR, P2, P3, and 3′ UTR regions, strain 99279 was apparently not related to the EV-B81 prototype strain, which further confirmed the occurrence of recombination in these regions. Interestingly, a relatively high similarity within the 3′ end of the 2C region to the 5′ end of the 3C region was found between strain 99279 and the EV-B111 prototype strain, which was supported by the bootscanning analysis. And the bootscanning analysis also suggested the possibility of recombination of small-sized fragment in 5′ UTR, 3D, and 3′ UTR region between Chinese EV-B81 strains and other EV-B serotypes such as EV-B86, EV-B107, and EV-B87. (Fig. 3).
Figure 3. Recombination analyses of complete enterovirus B (EV-B) genomes.
(a) Similarity plot and (b) bootscanning analysis. A sliding window of 200 nucleotides was used, moving in 20-nucleotide steps. The Tibetan EV-B81 strain 99279/XZ/CHN/1999 was used as a query sequence (indicated in the upper right corner of the image).
Seroprevalence of EV-B81 in Tibet
Among the 50 serum samples surveyed, 16 were seropositive for EV-B81 (>1:8), with a total positive rate of 32.0% and geometric mean titers (GMTs) of 1:27 among the positive sera samples. The composition ratios for the anti-EV-B81 antibody titers of <1:8, 1:8–1:64, and >1:64 were 68%, 32%, and 0, respectively. Compared with seroepidemiology studies of other EVs in China, the positive rate and GMTs of EV-B81 are apparently lower than that of other EVs such as EV-A71 and CVA16 in the same age group (1–5 years old)16,17.
Although both Lhasa City and Shigatse Prefecture showed low seroprevalence rates and low titers of anti-EV-B81 antibodies, there were some differences between the two areas. In Lhasa City, the seroprevalence rate and GMTs of the positive sera samples were 16% and 1:22.6, respectively, and in Shigatse Prefecture, they were 48% and 1:28.5, respectively. The positive rate in Shigatse is significantly higher than those in Lhasa City, while there is no significant difference between the GMTs of the two prefectures (seroprevalence rate: p = 0.0153, GMTs: p = 0.521).
Discussion
EV-B81 is a new type of enterovirus belonging to the species EV-B. The prototype of EV-B81 was isolated in the USA in 1968. Subsequently, several other EV-B81 strains were isolated from different countries in Asia and Africa, indicating global distribution of the serotype. Of the eight EV-B81 strains reported in the GenBank database to date, six were isolated from Southeast Asia, including from India, Bangladesh, and provinces in southwestern China, suggesting the possible circulation of EV-B81 in this area. Detailed information about the prototype strain, such as information about its host, was not indicated in the reference publications, but almost all the other EV-B81 strains were isolated from patients with AFP except for a strain from Cameroon13. Hence, there may be some correlation between EV-B81 and AFP. However, little research has been done on EV-B81 worldwide, and more data are necessary to unveil the biological and pathological properties of EV-B81.
The first EV-B81 strain reported in China was isolated from the Yunnan province in 1998, and no other case has since been reported in China9. In this study, we characterized the full-length genomes of two EV-B81 strains isolated in 1999 from Mangkang Prefecture of Tibet, located in the southeast of the Qinghai-Tibet Plateau. Although EV-B81 strains were reported in both Tibet and the Yunnan province of China, they showed great genetic diversity, the level of genetic diversity between two Tibetan EV-B81 strains and Yunnan strains was 20.4% and 20.9%, respectively, suggesting that EV-B81 from different lineages circulated separately in these two regions. For the entire VP1 sequences, the nucleotide identities between the two Tibetan EV-B81 strains 99279 and 99298c and the EV-B81 prototype strain were 79.1% and 79.2%, respectively, further indicating that EV-B81 strains have high nucleotide sequence diversity.
Recently, increasing numbers of studies have shown that intertypic recombination is common within the species EV-A, EV-B, and EV-C18,19,20,21,22. The two EV-B81 strains characterized in this study are no exception. The clustering of the two strains in the phylogenetic trees based on the P2 and P3 regions was inconsistent with the clustering in the tree based on the P1 region, which suggested intertypic recombination between the two EV-B81 strains and other EV-B serotypes (probably EV-B86 or EV-B111) in the P2 and P3 regions. The result was further corroborated by similarity plot and bootscanning analyses. Previous studies have indicated that recombination between different serotypes may occur when different viruses infect and replicate in the same cell, and that recombination usually occurs among EV serotypes within a species18,22. Hence, we can assume that the two Tibetan EV-B81 strains may have co-circulated with other EV-B serotypes, especially EV-B111, for a period before they were isolated. The EV-B111 prototype was also isolated in the Tibet Autonomous Region in the same period. However, more data are needed to define the exact serotype of the donor sequence.
Most EV infections are asymptomatic and are hence an underestimated epidemic. To investigate the prevalence of EV-B81 in the Tibet Autonomous Region, we conducted a seroepidemiology survey. We found that the seropositive rate and GMT were higher in Shigatse Prefecture than in Lhasa City (p < 0.05), indicating a geographical difference in EV-B81 infection patterns. However, the seropositive rates and titers of anti-EV-B81 antibodies were overall very low compared with other EVs prevalent in China such as EV-A71 and CVA1616, suggesting that the extent of transmission and the exposure of the population to EV-B81 were limited. However, considering the small sample sizes (n = 50) of the survey and the limited number of EV-B81 strains, the possibility of cross reactivity between EV-B81 and antibody against other closely related EVs, in particular members within EV-B, could not be exclude, so further research will be required to draw firm conclusions.
In conclusion, we report the full-length genome sequences of two EV-B81 strains isolated during AFP surveillance in the Tibet Autonomous Region, China. Sequence analysis revealed high genetic diversity in the two strains compared with the EV-B81 prototype, as well as intertypic recombination in the non-structural protein region of both strains. Although little is known about the biological and pathogenic properties of EV-B81 because of few research in this field owing to a limited number of isolates, our study provides a basis for further study of EV-B81.
Methods
Sample collection
This study did not involve human participants or human experimentation; the only human materials used were stool samples collected from AFP patients or their close contacts at the instigation of the Ministry of Health P. R. of China for public health purposes, and written informed consent for the use of their clinical samples was obtained from their parents of all the patients involved in this study. This study was approved by the second session of the Ethics Review Committee of the National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention.
The two EV-B81 strains (strain 99279 and 99298c) were isolated from stool samples from two children living in the same village in Mangkang Prefecture of the Tibet Autonomous Region, China. The samples were collected in 1999, during the course of poliovirus surveillance activities in support of the global polio eradication initiative. Strain 99279 was isolated from a 3-year-old boy with AFP, and strain 99298c was isolated from a 5-year-old girl who was an asymptomatic contact of an AFP patient.
For a seroprevalence study of EV-B81 antibodies, 50 healthy children ≤ 5 years of age were surveyed. Fifty serum samples were collected randomly in 2010, with informed parental consent, by the Tibet Center for Disease Control and Prevention: 25 samples were collected in Lhasa City and 25 samples were collected in Shigatse Prefecture. No children had any sign of disease at the time of sample collection.
Viral isolation and primary identification
Stool samples from AFP patients were collected and processed according to standard procedures recommended by the World Health Organization23. The samples were then inoculated into two cell lines, human rhabdomyosarcoma (RD) and a mouse cell line carrying the human poliovirus receptor (L20B) used to observe the development of EV-like cytopathic effects; the virus grew only in the RD cell line. Isolates were initially characterized by a micro-neutralization assay using poliovirus type-specific rabbit polyclonal antisera and pooled horse antisera against the most frequently isolated echoviruses and coxsackieviruses (National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands)23.
Molecular typing
For molecular typing, viral RNA was extracted from the viral isolates using a QIAamp Viral RNA Mini Kit (Qiagen. Germany) and stored at −80°C until use. Reverse transcription polymerase chain reaction (RT-PCR) was performed to amplify the VP1 coding region using PrimeScript One Step RT-PCR Kit Ver.2 (TaKaRa, Dalian, China) with primer pairs 008 and 0137. The PCR products were purified using the QIAquick PCR purification kit (Qiagen, Germany) and then subjected to nucleotide sequencing. Sequencing was performed in both directions using an ABI 3130 Genetic Analyzer (Applied Biosystems, USA), and every nucleotide position was sequenced at least once from each strand. The sequences were analyzed with the Basic Local Alignment Search Tool server at the National Center for Biotechnology Information and the EV serotype was determined according to a previously described molecular typing method7.
Neutralizing antibody detection
Neutralizing antibodies against EV-B81 were detected with a neutralization test using the microtechnique and the human RD cell line as previously described, with some modifications16. Serum samples were inactivated at 56°C for 30 min before use, and sample dilutions of 1:4 to 1:1024 were assayed. Virus samples (50 μL) with a tissue culture infective dose (TCID50) of 100 were mixed with the appropriate serum dilution (50 μL) and incubated at 36°C in a CO2 incubator. After incubation for 7 days, the highest dilution of serum that protected 50% of the cultures was recorded. A serum sample was considered positive if the neutralization antibody level was presented at a dilution of 1:8, and the GMT was calculated.
Full-length genomic sequencing
Two long-distance PCR amplifications were performed using the SuperScript III One-Step PCR-PCR system (Invitrogen). Reaction mixtures (50 μL) contained template RNA (5 μL), reaction buffer (25 μL), forward [0001S48 (GGGGACAAGTTTGTACAAAAAAGCAGGCTTT)24 or E490 (TGIGTIYTITGYRTICCITGGAT)25] and reverse [E492 (GGRTTIGTIGWYTGCCA)25 or 7500A (GGGGACCACTTTGTACAAGAAAGCTGGG(T)24)24] primers (1.0 ng/μL), and RT/Platinum Taq High Fidelity enzyme (5 U). cDNA synthesis and pre-denaturation were carried out with one cycle of 50°C (30 min) and 94°C (2 min). Amplification was carried out with 40 cycles of 94°C (15 s), 60°C (30 s), and 68°C (5 min), followed by a final incubation at 68°C (5 min). The primers used for sequencing of the full-length genome were designed by a “primer-walking” strategy.
Phylogenetic and bioinformatics analyses
The nucleotide and deduced amino acid sequences of strains 99279 and 99298c were compared with those of the prototype EV-B strains by pairwise alignment using the MEGA program (version 5.03)26. Phylogenetic trees were constructed by the neighbor-joining method implemented in the MEGA program using the Kimura 2-parameter model. Regions containing alignment gaps were omitted from the analysis. The branch lengths of the dendrogram were determined from the topologies of the trees and were obtained by majority rule consensus among 1000 bootstrap replicates. Bootstrap values greater than 80% were considered statistically significant for grouping. Similarity plot and bootscanning analyses were performed using the SimPlot program (version 3.5.1; Stuart Ray, Johns Hopkins University, Baltimore, MD, USA)27. For similarity plot analyses, a 200-nucleotide window was moved in 20-nucleotide steps, and bootscanning analyses were run with the neighbor-joining method.
Statistical analysis
The titers of neutralization antibodies were log-transformed to calculate the GMTs. Chi-square test was used to compare the seroprevalence rates between Shigatse Prefecture and Lhasa City. Mann-Whitney U test was used to analyze their difference of GMTs. All titers below 1:8 were assumed to be 1:4 for calculation. Differences with an error probability of P < 0.05 were regarded as significant. All statistical analyses were performed with IBM SPSS Statistics software (version 19.0).
Nucleotide sequence accession numbers
The complete genomic sequences of the EV-B81 strains (99279 and 99298c) described in this study were deposited in the GenBank database under the accession numbers KJ755189 and KJ755190, respectively.
Author Contributions
Y.Z. and W.X. conceived and designed the experiments. L.H., M.H., S.Z., D.Y., D.W., X.L. and T.W. performed the experiments. L.H. and Y.Z. analyzed the data. L.H. and Y.Z. wrote the main manuscript text and LH prepared Fig. 1–3. All authors reviewed the manuscript.
Acknowledgments
We would like to acknowledge the staff of the national polio eradication program in the Tibet Autonomous Region Center for Disease Control and Prevention (CDC) for investigating AFP cases and collecting stool specimens from the patients and their close contacts for use in this study. The study was supported by the National Natural Science Foundation of China (project no. 30900063, 81101303, and 81373049) and the National Key Technology R&D Program of China (project no. 2013ZX10004-202).
References
- Rao C. D., Yergolkar P. & Shankarappa K. S. Antigenic diversity of enteroviruses associated with nonpolio acute flaccid paralysis, India, 2007–2009. Emerg Infect Dis 18, 1833–1840 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J. et al. Cytoplasmic redistribution and cleavage of AUF1 during coxsackievirus infection enhance the stability of its viral genome. FASEB J 27, 2777–2787 (2013). [DOI] [PubMed] [Google Scholar]
- Kelly T. A. et al. Underreporting of viral encephalitis and viral meningitis, ireland, 2005–2008. Emerg Infect Dis 19, 1428–1436 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. et al. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J Clin Virol 44, 262–267 (2009). [DOI] [PubMed] [Google Scholar]
- Picornaviridae. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. (Elsevier, San Diego, 2011).
- Oberste M. S. et al. Comparison of classic and molecular approaches for the identification of untypeable enteroviruses. J Clin Microbiol 38, 1170–1174 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M. S., Maher K., Kilpatrick D. R. & Pallansch M. A. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol 73, 1941–1948 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M. S. et al. Molecular identification of 13 new enterovirus types, EV79–88, EV97, and EV100–101, members of the species Human Enterovirus B. Virus Res 128, 34–42 (2007). [DOI] [PubMed] [Google Scholar]
- Bingjun T. et al. Molecular typing and epidemiology of non-polio enteroviruses isolated from Yunnan Province, the People's Republic of China. J Med Virol 80, 670–679 (2008). [DOI] [PubMed] [Google Scholar]
- Oberste M. S. et al. Characterizing the picornavirus landscape among synanthropic nonhuman primates in Bangladesh, 2007 to 2008. J Virol 87, 558–571 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxmivandana R., Yergolkar P., Gopalkrishna V. & Chitambar S. D. Characterization of the non-polio enterovirus infections associated with acute flaccid paralysis in South-Western India. PLoS One 8, e61650 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeuh-Mba S. A. et al. High frequency and diversity of species C enteroviruses in Cameroon and neighboring countries. J Clin Microbiol 51, 759–770 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayukekbong J. et al. Shift of Enterovirus species among children in Cameroon--identification of a new enterovirus, EV-A119. J Clin Virol 58, 227–232 (2013). [DOI] [PubMed] [Google Scholar]
- Kroneman A. et al. An automated genotyping tool for enteroviruses and noroviruses. J Clin Virol 51, 121–125 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Molecular typing and characterization of a new serotype of human enterovirus (EV-B111) identified in China. Virus Res 183, 75–80 (2014). [DOI] [PubMed] [Google Scholar]
- Zhu Z. et al. Retrospective seroepidemiology indicated that human enterovirus 71 and coxsackievirus A16 circulated wildly in central and southern China before large-scale outbreaks from 2008. Virol J 7, 300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. et al. Seroepidemiology of human enterovirus71 and coxsackievirusA16 among children in Guangdong province, China. BMC Infect Dis 13, 322 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. et al. Emergence and transmission pathways of rapidly evolving evolutionary branch c4a strains of human enterovirus 71 in the central plain of china. PLoS One 6, e27895 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashev A. N. et al. Recombination strategies and evolutionary dynamics of the Human enterovirus A global gene pool. J Gen Virol 95, 868–873 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang T. et al. Epidemics and Frequent Recombination within Species in Outbreaks of Human Enterovirus B-Associated Hand, Foot and Mouth Disease in Shandong China in 2010 and 2011. PLoS One 8, e67157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P. & Welch J. Frequency and dynamics of recombination within different species of human enteroviruses. J Virol 80, 483–493 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. et al. A Sabin 2-related poliovirus recombinant contains a homologous sequence of human enterovirus species C in the viral polymerase coding region. Arch Virol 155, 197–205 (2010). [DOI] [PubMed] [Google Scholar]
- Isolation and identification of polioviruses. WHO Polio laboratory manual, 4th edn. (World Health Organization, Geneva, 2004). [Google Scholar]
- Yang C. F. et al. Circulation of endemic type 2 vaccine-derived poliovirus in Egypt from 1983 to 1993. J Virol 77, 8366–8377 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M. S. et al. Species-specific RT-PCR amplification of human enteroviruses: a tool for rapid species identification of uncharacterized enteroviruses. J Gen Virol 87, 119–128 (2006). [DOI] [PubMed] [Google Scholar]
- Tamura K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28, 2731–2739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen M. O., Carr J. K., Burke D. S. & McCutchan F. E. Identification of breakpoints in intergenotypic recombinants of HIV type 1 by bootscanning. AIDS research and human retroviruses 11, 1423–1425 (1995). [DOI] [PubMed] [Google Scholar]