Abstract
Liver wound healing is a coordinated response to injury caused by infections (hepatitis) or toxins (alcohol) or other processes where activation of hepatic stellate cells are a central component. During stellate cell activation, a major phenotypic transformation occurs which leads to increased production of increased extracellular matrix proteins and smooth muscle α-actin the results is organ dysfunction due to gross architectural disruption and impaired blood flow.
Endothelin-1 (ET-1) is produced in increased amounts and the cellular source of ET-1 shifts from endothelial cells to stellate cells during liver injury thus setting a feedback loop which accentuates further activation, stellate cell proliferation, and production of extracellular matrix proteins. Therapy directed at intervening the ET-1 signaling pathway has significant therapeutic potential in patients with liver disease.
Introduction
Liver wound healing is an orchestrated process characterized by fibrogenesis, remodeling, and gross distortion of liver architecture. Persistent and progressive accumulation of extracellular matrix proteins concurrent with a regenerative response gradually transforms the liver to a fibrotic, and cirrhotic structure.
Although a number of cell types have been advanced as important to the wounding response, hepatic stellate cells, which are retinoid rich, perisinusoidal cell of mesenchymal origin have a central role. After injury, this cell undergoes “activation”. The activation process is typical of all forms of liver injury and is characterized by morphological and functional features which include loss of retinoids, development of rough endoplasmic reticulum and acquisition of “smooth muscle-like” cytoskeleton, [1-3] (Figure 1). One of the major functional attributes of activated stellate cells is the production of extracellular matrix, including types I, III, IV collagens, fibronectin, proteoglycans in excessive amounts (Figure 1); studies have shown that stellate cells from fibrotic animals exhibited a 30 to 40 fold increase in type I and type III collagen mRNA relative to normal stellate cells and hepatocytes respectively [4,5]. It has also been shown that stellate cells specifically acquire smooth muscle α actin during activation process (Figure 1), marking them as liver specific myofibroblasts [3,5,6].
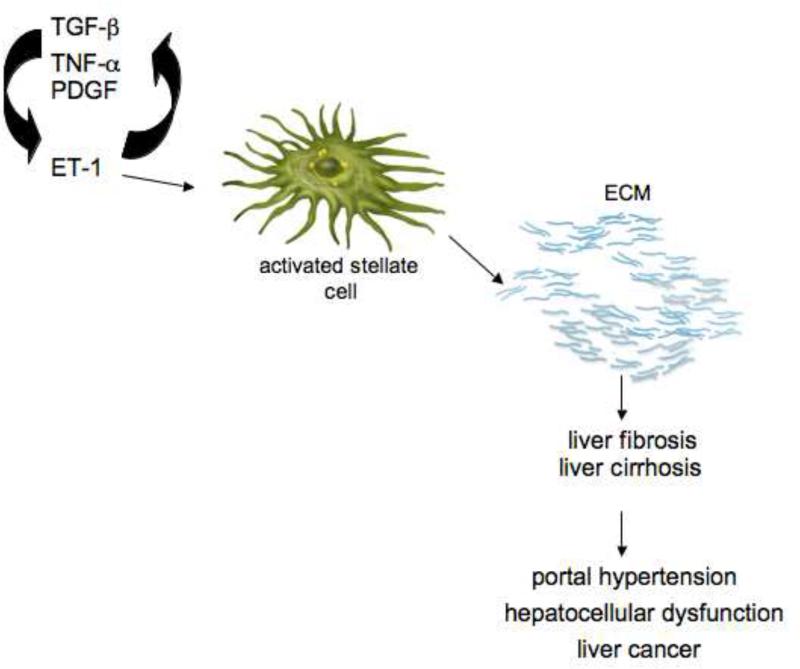
Figure 1. The role of ET-1 in stellate cell activation and hepatic fibrogenesis.
Stellate cell activation is characterized by typical phenotypic features including the loss of retinoids, proliferation, development of a robust rough endoplasmic reticulum and acquisition of a “smooth muscle-like” cytoskeleton. ET-1 is one of a wide array of factors that appear to contribute to stellate cell activation and directly stimulates expression of smooth muscle α-actin. On the left side of the image, various cytokines stimulate ET-1 synthesis in activated stellate cells, ET-1 then has autocrine effects on stellate cells themselves, which fuel the fibrogenic cascade to stimulate synthesis of ECM proteins. The ultimate result of the wounding response is fibrosis, and in the liver, cirrhosis with complications that include portal hypertension, hepatocellular dysfunction, and even hepatocellular cancer. Abbreviations: TGF-β = transforming growth factor-β; TNF-α = tumor necrosis factor-α, PDGF = platelet- derived growth factor
A growing body of evidence has now suggested a crucial link between endothelin (ET) and the liver wound healing response; endothelin levels are elevated in diverse forms of injury and wound healing [7-10]. Abundant literature also suggests a critical role of ET in patients with liver cirrhosis.
Endothelin Biology
The family of ET's [11] are potent vasoconstrictor peptides made up of the three following peptides: ET-1, ET-2 and ET-3. Each peptide is a unique 21- amino acid residue that binds to G-protein coupled receptor (GPCR) including, the ETA and ETB [11,12]. Typically, ET is produced by endothelial cells and exerts paracrine effects on adjacent smooth muscle cells. However in pathological situations, ET appears to be synthesized in a broader range of cell types [9,13,14].
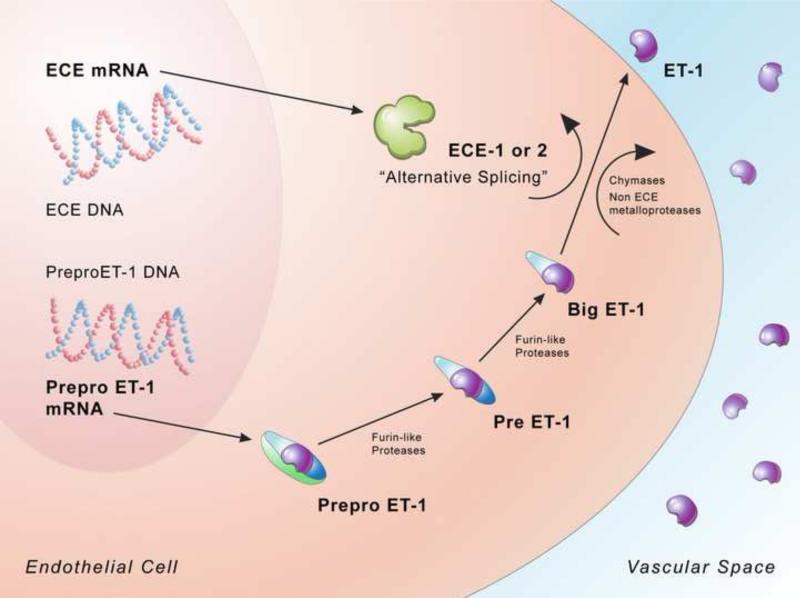
ET-1 synthesis is regulated at the level of synthesis of precursors as well as by their processing [15-17]. Pre-pro ET-1, the canonical precursor is induced by a variety of extracellular stimuli (e.g. shear stress) vasopressor hormones (e.g. vasopressin) and cytokines (e.g. interleukin-1) and a variety of vasoactive peptides (e.g epinephrine and angiotensin II) [18-20]. Proteolytic processing of prepro ET-1 by furin-like enzymes leads to production of intermediate ET, ultimately big ETs which are 38 to 40 amino acids residue (Figure 2). The big ETs have little or no biological effects and are cleaved at Trp-21- Val/Ile-22 by specific endothelin-converting enzymes (ECE), to yield a the 21amino acid peptide which are biologically active [20] (Figure 2).
Figure 2. Endothelin-1 biosynthetic pathway.
Pre-pro ET-1, the canonical precursor is synthesized as a product of transcription from preproendothelin , a 203-amino acid peptide that cleaved at dibasic sites by furin-like endopeptidases to form intermediate ETs, and then big ET's which are 38 to 40 amino acid residues. The intermediate and big ET's have no biological activity but undergo cleavage at Trp-21- Val/Ile-22 by specific endothelin-converting enzymes (ECE), to yield a the mature 21aa ET-1 which is biologically active (from Khimji AK and Rockey DC. Endothelin-biology and disease. Cell Signal 2010 11: 1615 with permission).
The ECE are neutral membrane-bound metalloproteases, a 120 kD endopeptidase-24 family of proteins, which belong to M13 group of proteins that includes neutral endopeptidases, kell blood group antigens (Kell), a peptide from phosphate regulating gene (PEX), X-converting enzyme (XCE), “secreted” endopeptidases, and the ECEs [21] found in brain [22-24]. Three isoforms of ECE have been reported [25], namely ECE-1, ECE-2 and ECE-3; ECE-1 and ECE-2 are most prominent [24]. ECE's. M13 family members contain type II integral membrane proteins with zinc metalloprotease activity [21], and their function is inhibited by phosphoramidon [24]. Four variants of ECE-1 have been reported in humans [26], namely ECE-1a, ECE-1b, ECE-1c and ECE-1d which are a result of alternate splicing of ECE-1 mRNA. ECE-1 appears to be localized to the plasma cell membrane and its optimal activity is at pH 7; it processes big ET's both intracellularly and on the cell surface [27]. It is distributed predominantly in smooth muscle cells. ECE-1 can also hydrolyze other proteins including bradykinin, substance P, and insulin [27]. ECE-2 is localized to the trans-Golgi network and is expressed abundantly in neural tissues and endothelial cells. Its optimal activity is at pH 5 [21,28]; the acidic activity marks ECE-2 as an intracellular enzyme [28]. Substrate selectivity experiments indicate that both ECE-1 and ECE-2 show preference for big ET-1 over big ET-2 or big ET-3 [29]. To date, evidence points to the existence of ECE isoforms or proteases other than ECE-1 and ECE-2 in the final ET processing step, since mice lacking both ECE-1 and ECE-2 produce mature ET-1 [30]. Some data suggest the presence of an ECE-1 independent pathway that process big endothelin, possibly involving tissue chymases and non-ECE metalloproteinases [31].
ET binds to two prominent G-coupled receptor subtypes, ETA and ETB which mediate a range of it's biologic effects [32]. ETA receptors are predominantly expressed in vascular smooth muscle cells with the rank order affinities of the peptide as ET-1> ET-2 >> ET-3, while ETB receptors are widely distributed and have equal affinity for all ET subtypes [12,33]. ETA and ETB receptors on smooth muscle cells mediate vasoconstriction while the ETB receptor appears to mediate diverse responses depending on the cell type expressing the receptor. For example stimulation of ETB receptors on endothelial cells stimulates Nitric oxide (NO) production and release and vascular smooth muscle relaxation. Interestingly, a variant of ETB receptors also produces smooth muscle vasoconstriction [34].
The ETA receptor is isoform selective and binds ET-1 and ET-2 with higher affinity than ET-3. In contrast, the ETB receptor is not isoform specific and binds ET-1, ET-2 and ET-3 with equal affinity. In addition sarafatoxin (S6c), a peptide extracted from the venom of Atractaspis engaddenensis (Israeli burrowing asp) also serves as a ligand for ETB receptor. Based primarily on functional assays [35]; two ETB receptor subtypes have been proposed based on pharmacologic studies, ETB1 and ETB2, however, the molecular basis for existence of these subtypes is still lacking [36]. The ETC, a third type of ET receptor has been cloned from Xenopus laevis and displays a higher affinity for ET-3 than ET-1 and when stimulated causes release of NO [37].
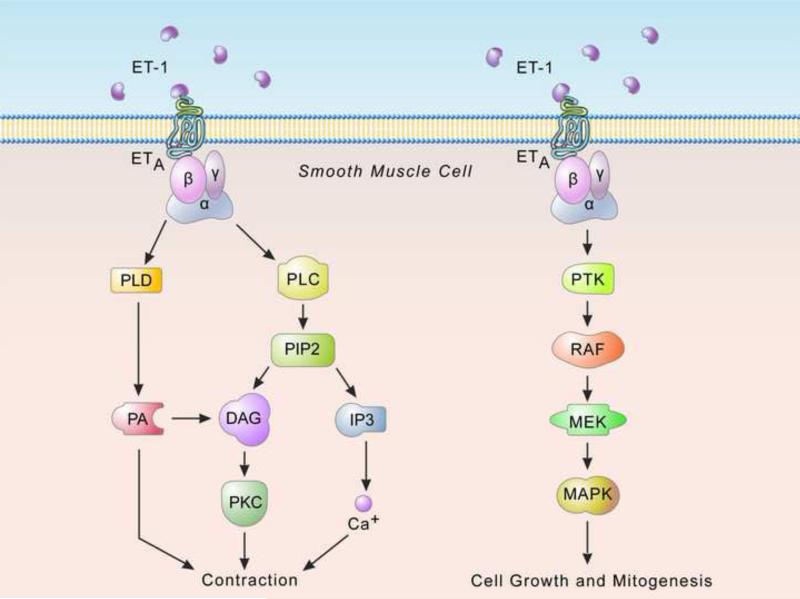
ET signaling has been studied extensively (Figure 3). Upon binding to it's cognate receptors, ET leads to activation of phospholipase C [32], cleavage of phosphatidyl inositol 4, 5-biphosphate and subsequent release of second messengers inositol 1. 4, 5-triphosphate (IP3) and diacylglycerol (DAG) (Figure 3). The IP3 stimulates release of Ca2+ from endoplasmic reticulum leading to activation of Ca2+ calmodulin-dependant myosin light chain kinase, which in turn stimulates myosin Mg-ATPase and actomyosin cross bridging and contraction [38]. In addition other ET-1 stimulated signaling pathways have also been reported as for example: phosphatidyl choline-specific phospholipase (PC-PLD) and phosphatidic acid (PA) pathway in smooth muscle cells (Figure 3), ET-1 also stimulates protein tyrosine kinases (PTK) (Figure 3) such as RAF and RAS, particularly in neoplastic cells.
Figure 3. Endothelin signaling.
ET binds to two prominent G-coupled receptor subtypes, ETA and ETB. ET receptor stimulation is followed by activation of a variety of different downstream cascades. For example, shown on the left, ETA induced activation of phosphatidyl inositol specific phospholipase C (PI-PLC) leads to the formation of inositol triphosphate and diacylglcerol (DAG) from phosphatidylinositol. Inositol 1, 4, 5 triphosphate (IP3) which then diffuses to specific endoplasmic reticulum receptors and releases stored Ca2+ into the cytosol. This causes a rapid elevation in intracellular Ca2+ which in turn causes cellular contraction. In addition, ETA stimulation also induces activation of phosphatidyl choline-specific phospholipase (PC-PLD) yielding phosphatidic acid (PA) (shown on the left), PA is dephosphorylated by PA phosphorylase to DAG and DAG is also metabolized to PA by DAG kinase. ET-1 also stimulates protein tyrosine kinases (PTK) (shown on the right) such as RAS, particularly in neoplastic cells. Activation of PTKs in this pathway results in induction of the RAF/MEK/MAPK pathway, subsequently stimulating transcription of protooncogenes such as c-FOS, c-MYC, c-JUN, which in turn activate cell growth and metastasis (from Khimji AK and Rockey DC. Endothelin-biology and disease. Cell Signal 2010 11: 1615 with permission).
Endothelin and liver wound healing
Numerous lines of evidence support the importance of ET in the liver wound healing process. For example, circulating levels of ET-1 and ET-3 are increased in patients with cirrhosis, as well as in animal models of hepatic wound healing [7,8,39,40] and the source of ET in the injured liver is the organ itself [9,41]. Intrahepatic immunoreactive ET-1 levels have been found to correlate with the severity of liver disease [41]. Further, inhibition of ET signaling with ET receptor antagonists reduces the hepatic fibrogenic response [35,42-44].
These data emphasize the importance of ET in the hepatic wound healing response. Substantial effort has been directed at understanding both the signaling mechanisms underlying the effect of ET in the liver, as well as how ET (ET-1 in particular) is upregulated in injured liver. The mechanism underlying the increased production of ET in the injured liver is complex, but is coming into better focus. In the normal liver, ET is produced primarily by sinusoidal endothelial cells; however, after injury, synthesis of ET-1 shifts from endothelial cells to hepatic stellate cells [9,45-47].
Another area of focus has been on the effect of ET-1 in the liver. A number of early studies demonstrated that the primary “target” of ET-1 in the liver is the hepatic stellate cell. [48-50]. This conclusion was drawn after it was shown that stellate cells possess abundant ET receptors, far in excess of other liver cells particularly after liver injury [48-50]. The study of ET receptors is of importance because ET receptors on hepatic stellate cells appear to have important biological effects- including proliferation, cell spreading, contractility, and even fibrogenesis- all features of the activated phenotype (see below). Interestingly ET receptor mRNA is unchanged or slightly decreased in stellate cells undergoing activation in culture or after carbon tetrachloride (CCl4) induced injury. Further in one study, endothelin receptors appeared to be downregulated in stellate cells in response to TGF-β [51]. However, binding sites for ET-1 in stellate cells are markedly increased after their activation [48]. Additionally, ETB receptors were upregulated in stellate cells after their activation [52]. This is in contrast with other work, in which it has been shown that there is dramatic upregulation of ET receptors in rat livers after endotoxin treatment [53]. The reason for the discrepancy in reports of endothelin receptors expression in stellate cells is unclear and may be related to divergent culture conditions or different biologic systems.
Any discussion of the role of stellate cells in wound healing requires an understanding of hepatic stellate cell “activation”. This process, which essentially involves transdifferentiation of the cell from a quiescent state to an activated, myofibroblast-like cell is associated with loss of vitamin A droplets, increased rough endoplasmic reticulum, dynamic cytoskeletal changes (i.e. such as expression of smooth muscle α-actin, and fibrogenesis [54,55]. Activation of hepatic stellate cells appears to be a key step in the development of liver cirrhosis and fibrosis. Activated stellate cells have many functional roles, prominent among which are the production of pathological amounts of extracellular matrix (ECM) proteins [56-58]. These cells also proliferate and secrete a variety of cytokines that not only stimulate stellate cells in an autocrine manner, but also are important in perpetuating the entire wounding response [59]. Additionally, the acquisition of a robust actin based cytoskeleton in activated stellate cells imparts a contractile phenotype and appears to be directly correlated with force generation and regulation of sinusoidal blood flow which is disturbed in portal hypertension [42,60].
Stellate cell activation is stimulated by several important factors in the wounding environment including growth factors, cytokines, chemokines, oxidative stress, and the ECM itself [61]. ET-1 is one of the wide array of factors that appear to contribute to stellate cell activation [35]. ET-1 directly stimulates expression of smooth muscle α-actin in cultured stellate cells [62]. Further, the ET receptor antagonist, bosentan, inhibited fibrogenesis in a chronic liver injury model, consistent with an important role for ET-1 in wound healing [35].
Available data further suggest complex interplay between TGF-β and ET-1 in modulating hepatic stellate cell activation in each a paracrine and autocrine manner [45]. ET-1 has been found to increase TGF-β1 mRNA and also stimulates release of TGF-β1 in stellate cells [63]. The autocrine functional effects of ET-1 in stellate cell activation have been emphasized in a signaling loop that involves a fibronectin-Shc-SRC-ERK dependant mechanism [47]. In liver injury, it has also been shown that TGF-β dependent ET-1 signaling proceed through differential signaling pathways depending on the mechanism of liver injury that leads to stellate cell activation [64]. For example, in one study, in CCl4 induced injury, TGF-β dependent ET-1 signaling proceeded through a p38 MAPK pathway [64], while after bile duct ligation, signaling was mediated through a TGF-β dependant extracellular regulated kinases (ERK) pathway [64]. Although the precise mechanism of interaction between ET-1 and TGF-β has not been elucidated in stellate cells, it may be a result of cross talk between the ET-1 and TGF-β receptor as has been demonstrated in vascular smooth muscle cells [65].
One of the most prominent phenotypes in stellate cells is their production of extracellular matrix [66] which has been shown to be stimulated by ET-1, the latter of which appears to be at least partially TGF-β dependant [45]. Additionally, other ET-1 induced phenotypes like such as keratinocyte transdifferentiation and contractility [67,68] also appear to be TGF-β dependant. ET-1 may interact with other cytokines during liver injury, although this area is less well studied than TGF-β, some data suggest cross talk between ET-1 and PDGF in liver wound healing [69]. PDGF mediates chemotactic recruitment, and early proliferative responses of hepatic stellate cells in liver fibrosis [70,71]. However, the precise mechanism of interaction between ET-1 and PDGF has not been fully determined.
One of the most prominent roles of ET-1 in liver wound healing is to induce cellular contraction. The pathways by which ETs induce cellular contraction appears to be complex. The canonical signaling pathway by which ET-1 induces cellular contraction in smooth muscle cells is via Ca2+ dependant signaling [72] (Figure 3). In this pathway, stimulation of ETA receptors leads to activation of phospholipase C and formation of inositol 1, 4, 5 triphosphate (IP3) and diacylglycerol (DAG) from phosphatidyl inositol [32]. Further, IP3 interacts with specific receptors localized in the endoplasmic reticulum, releasing Ca2+ from Ca2+ stores into the cytosol [32,72,73] (Figure 3). A rapid elevation cytosolic Ca2+ then activates the Ca2+/calmodulin dependant myosin light chain kinase (MLCK) which in turn leads to increased phosphorylation of regulatory myosin light chain (rMLC). Phosphorylation of rMLC leads to activation of myosin ATPase, actomyosin bridging and cellular contraction [74,75]. Evidence suggests that stellate cells employ Ca2+ dependent contraction [76,77], which may be enhanced after activation [78].
A unique attribute of ET-1 induced contraction, described in particular for non-smooth muscle cells, including activated hepatic stellate cells (myofibroblasts) appears to be via a Ca2+independent pathway, thought to be activated via protein kinase C (PKC) [77], integrin-linked kinase (ILK) ([79,80], Raf-1 [81] and Rho-associated kinase (ROCK) [82]. PKC and ROCK have been associated with reduced activity of myosin light chain phosphatase (MLCP) and prolonged activation of rMLC, and thus contraction [83]. In stellate cells, ET-1 is capable of inducing cellular contractility independent of Ca2+, in addition to more classical contraction pathways [77,80,84].
A further important point is that activated stellate cells (i.e., myofibroblasts) have been shown to exhibit an enhanced contractile phenotype after liver injury [35,85]. This has important implications for disease in the liver, via several putative mechanisms. First, stellate cells are known to reside in a perisinusoidal fashion [86], akin to pericytes in the periphery; pericytes appear to regulate blood flow. Thus, it has been proposed that stellate cells function as liver specific pericytes, and regulate sinusoidal blood flow [87-89]. This function appears to be important in regulation of intrahepatic resistance, and further may contribute to increased resistance in portal hypertension [87]. Interestingly, the degree of increase in contractility appears to be proportional to the expression of the putative contractile protein, smooth muscle α-actin [35].
Stellate cell activation is also characterized by fibrogenesis – i.e., by the production of increased quantities of ECM proteins including types I, III, and IV collagens, fibronectin, laminin and proteoglycans [4] with type I collagen constituting a high proportion in liver fibrosis [90]. Abundant evidence indicates that activated hepatic stellate cells are a major source of ECM proteins produced during fibrogenesis [91]. Activation is also associated with dysregulation of matrix-degrading enzymes matrix-metalloproteinases (MMP) and their inhibitors (such as tissue inhibitors of metalloproteinases (TIMPs) [63]. For example, MMPs may have suppressed activity in the injured liver due to increased expression of TIMPs [63] indicating that ECM deposition occurs as a result of excessive production as well as decreased degradation. Indeed, it appears that the ECM plays a dynamic role in modulating the wound healing events through it's continuous synthesis and degradation [92,93] thus providing scaffold for tissue construction as well as deterioration of vital function of the organ [94].
A large body of experimental literature suggests a relationship between ET-1 and fibrogenesis (Figure 1). It has been shown that ET receptor antagonists inhibit liver fibrosis [35,43,95-97]. For example, bosentan, a mixed ET receptor antagonist used in rat liver fibrosis suppressed the activation of stellate cells and reduced levels of type I collagen synthesis [35]. An ETA receptor antagonist also reduced fibrosis [95]. TAK-044, an ETA receptor antagonist treatment reduced collagen synthesis, as evidenced by decreased hepatic hydroxyproline content, mRNA expression of collagen-alpha type I, and tissue inhibitors of matrix metalloproteinases 1 and 2, and mRNA and protein expression of a TGF-β in CCl4 and LPS induced cirrhosis in rats, respectively [96] [98].
The mechanism for amelioration of hepatic fibrogenesis has been suggested to be via direct effects on the fibrogenic cascade in stellate cells as well as by interplay with other fibrogenic mediators such as TGF-β [99], platelet derived growth factor (PDGF) [69,71] and tumour necrosis factor (TNF-α) [100] (Figure 1) and interferon gamma ( Li, T- abstract- AASLD 2008).
Another important role of ET-1 in the wounding environment appears to be cell proliferation. ET-1 is known to be important in smooth muscle cell proliferation, as well as in neoplastic cells [101-103]. Although, proliferative effects of ET-1 have been reported on quiescent stellate cells [9,104] analogous to other cell types [101,102], It's proliferative effects on cells in vivo situation is controversial [66,105,106]. For example, studies on human hepatic stellate cell, derived from outgrowth of normal-liver explant tissue, have shown that ET-1 had mitogenic effects primarily on quiescent cells, yet it had anti-proliferative effects on activated cells [66,105]. The mechanism of this anti-proliferative effect appears to be mediated through ETB receptors, which are prominent in activated cells [66]. It has been suggested that NF-kappaB and cyclooxygenase-2 (COX-2) [107] signaling through ETB receptors may facilitate the anti-proliferative effects through a cAMP mechanism [108]. The mechanism underlying the discrepancy in ETs effect on quiescent (proliferative) and activated cells (anti-proliferative) remains unknown, but may be related to culture conditions under which stellate cells were grown. Further, different proportions and effects of ETA and ETB receptors in these cells at different stages of myofibroblastic differentiation may determine the proliferative response of ET-1 in stellate cells.
Although beyond the scope of this review, ET-1 appears to be important on other forms of wound healing, including in the heart, lung, skin, kidney, and vasculature [109-113].
Future
Given the apparent benefit of ET receptor antagonists in liver wound healing, it is possible that these could be effective in humans with fibrosis. Currently, very few human studies are available. ETA antagonist, BQ-123 and ETB antagonist, BQ-788 were studied in patients with a history of variceal bleeding [114], and did not show any effect on hepatic vein pressure gradient [114]. Another study suggested that an ETA endothelin antagonist might improve vascular tone in patients with cirrhosis [115]. Studies published to date have been performed with smaller number of patients and perhaps the use of sub-therapeutic doses of ET receptor antagonists. A further impediment to development of clinical trials has been concern about drug induced hepatototoxicity; fatal acute hepatitis and liver injury in patients receiving ET receptor antagonists has been reported and thus has led most clinicians to avoid the use of ET receptor antagonists in patients with liver disease [116,117]. ET receptor antagonists studied in clinical trials for different conditions are highlighted in Table I. Although there is concern about toxicity of ET receptor antagonists in patients with cirrhosis, the preclinical data suggest that there is significant translational potential for antagonism of the endothelin system in patients with cirrhosis.
Table 1. current status of ET receptor antagonists.
Use of ET receptor antagonists for pulmonary hypertension has become well established. Animal models suggest benefit in other disorders, and they have been studied in those highlighted; however, proof of their effectiveness at this point is generally modest.
| Compound | ET receptor target | Clinical trial disease | Reference |
|---|---|---|---|
| Bosentan | ETA/B | pulmonary hypertension | [118] |
| congestive heart failure | [118] | ||
| coronary artery disease | [119] | ||
| scleroderma | [120] | ||
| cerebral vasospasm | [118] | ||
| Sitaxsentan | ETA | pulmonary hypertension | [116] |
| chronic heart failure | [121] | ||
| Ambrisentan | ETA | pulmonary hypertension | [122] |
| chronic heart failure | [121] | ||
| Atrsentan | ETA | prostate cancer | [123] |
| S-0139 | ETA | cerebrovascular ischaemia | [124] |
| BQ-123 | ETA | cirrhosis and portal hypertension | [114] |
| BQ-788 | ETB | cirrhosis and portal hypertension | [114] |
Acknowledgements
This work was supported by the NIH (Grant R01 DK 50574 to DCR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: The principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockey DC, Friedman SL. Cytoskeleton of liver perisinusoidal cells (lipocytes) in normal and pathological conditions. Cell Motil Cytoskeleton. 1992;22:227–234. doi: 10.1002/cm.970220402. [DOI] [PubMed] [Google Scholar]
- 3.Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193–203. [PubMed] [Google Scholar]
- 4.Maher JJ, McGuire RF. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990;86:1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bissell DM, Friedman SL, Maher JJ, Roll FJ. Connective tissue biology and hepatic fibrosis: Report of a conference. Hepatology. 1990;11:488–498. doi: 10.1002/hep.1840110322. [DOI] [PubMed] [Google Scholar]
- 6.Desmouliere A. Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int. 1995;19:471–476. doi: 10.1006/cbir.1995.1090. [DOI] [PubMed] [Google Scholar]
- 7.Moore K, Wendon J, Frazer M, Karani J, Williams R, Badr K. Plasma endothelin immunoreactivity in liver disease and the hepatorenal syndrome. N Engl J Med. 1992;327:1774–1778. doi: 10.1056/NEJM199212173272502. [DOI] [PubMed] [Google Scholar]
- 8.Asbert M, Gines A, Gines P, Jimenez W, Claria J, Salo J, Arroyo V, Rivera F, Rodes J. Circulating levels of endothelin in cirrhosis. Gastroenterology. 1993;104:1485–1491. doi: 10.1016/0016-5085(93)90360-o. [DOI] [PubMed] [Google Scholar]
- 9.Rockey DC, Fouassier L, Chung JJ, Carayon A, Vallee P, Rey C, Housset C. Cellular localization of endothelin-1 and increased production in liver injury in the rat: Potential for autocrine and paracrine effects on stellate cells. Hepatology. 1998;27:472–480. doi: 10.1002/hep.510270222. [DOI] [PubMed] [Google Scholar]
- 10.Ota T, Hirai R, Urakami A, Soga H, Nawa S, Shimizu N. Endothelin-1 levels in portal venous blood in relation to hepatic tissue microcirculation disturbance and hepatic cell injury after ischemia/reperfusion. Surg Today. 1997;27:313–320. doi: 10.1007/BF00941805. [DOI] [PubMed] [Google Scholar]
- 11.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 12.Elshourbagy NA, Korman DR, Wu HL, Sylvester DR, Lee JA, Nuthalaganti P, Bergsma DJ, Kumar CS, Nambi P. Molecular characterization and regulation of the human endothelin receptors. J Biol Chem. 1993;268:3873–3879. [PubMed] [Google Scholar]
- 13.Lariviere R, Lebel M. Endothelin-1 in chronic renal failure and hypertension. Can J Physiol Pharmacol. 2003;81:607–621. doi: 10.1139/y03-012. [DOI] [PubMed] [Google Scholar]
- 14.Wanecek M, Weitzberg E, Rudehill A, Oldner A. The endothelin system in septic and endotoxin shock. Eur J Pharmacol. 2000;407:1–15. doi: 10.1016/s0014-2999(00)00675-0. [DOI] [PubMed] [Google Scholar]
- 15.Lee ME, Bloch KD, Clifford JA, Quertermous T. Functional analysis of the endothelin-1 gene promoter. Evidence for an endothelial cell-specific cis-acting sequence. J Biol Chem. 1990;265:10446–10450. [PubMed] [Google Scholar]
- 16.Lee ME, Dhadly MS, Temizer DH, Clifford JA, Yoshizumi M, Quertermous T. Regulation of endothelin-1 gene expression by fos and jun. J Biol Chem. 1991;266:19034–19039. [PubMed] [Google Scholar]
- 17.Quehenberger P, Bierhaus A, Fasching P, Muellner C, Klevesath M, Hong M, Stier G, Sattler M, Schleicher E, Speiser W, Nawroth PP. Endothelin 1 transcription is controlled by nuclear factor-kappab in age-stimulated cultured endothelial cells. Diabetes. 2000;49:1561–1570. doi: 10.2337/diabetes.49.9.1561. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura H, Nishimura J, Sakihara C, Kobayashi S, Takahashi S, Kanaide H. Expression and function of endothelins, endothelin receptors, and endothelin converting enzyme in the porcine trachea. Am J Respir Cell Mol Biol. 1997;17:471–480. doi: 10.1165/ajrcmb.17.4.2832. [DOI] [PubMed] [Google Scholar]
- 19.Xavier FE, Yogi A, Callera GE, Tostes RC, Alvarez Y, Salaices M, Alonso MJ, Rossoni LV. Contribution of the endothelin and renin-angiotensin systems to the vascular changes in rats chronically treated with ouabain. Br J Pharmacol. 2004;143:794–802. doi: 10.1038/sj.bjp.0705994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanagisawa M, Inoue A, Takuwa Y, Mitsui Y, Kobayashi M, Masaki T. The human preproendothelin-1 gene: Possible regulation by endothelial phosphoinositide turnover signaling. J Cardiovasc Pharmacol. 1989;13(Suppl 5):S13–17. discussion S18. [PubMed] [Google Scholar]
- 21.Macours N, Poels J, Hens K, Francis C, Huybrechts R. Structure, evolutionary conservation, and functions of angiotensin- and endothelin-converting enzymes. Int Rev Cytol. 2004;239:47–97. doi: 10.1016/S0074-7696(04)39002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohnaka K, Takayanagi R, Nishikawa M, Haji M, Nawata H. Purification and characterization of a phosphoramidon-sensitive endothelin-converting enzyme in porcine aortic endothelium. Off. J Biol Chem. 1993;268:26759–26766. [PubMed] [Google Scholar]
- 23.Turner AJ, Murphy LJ. Molecular pharmacology of endothelin converting enzymes. Biochem Pharmacol. 1996;51:91–102. doi: 10.1016/0006-2952(95)02036-5. [DOI] [PubMed] [Google Scholar]
- 24.Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M. Ece-1: A membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78:473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- 25.D'Orleans-Juste P, Plante M, Honore JC, Carrier E, Labonte J. Synthesis and degradation of endothelin-1. Can J Physiol Pharmacol. 2003;81:503–510. doi: 10.1139/y03-032. [DOI] [PubMed] [Google Scholar]
- 26.Valdenaire O, Rohrbacher E, Mattei MG. Organization of the gene encoding the human endothelin-converting enzyme (ece-1). J Biol Chem. 1995;270:29794–29798. doi: 10.1074/jbc.270.50.29794. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt M, Kroger B, Jacob E, Seulberger H, Subkowski T, Otter R, Meyer T, Schmalzing G, Hillen H. Molecular characterization of human and bovine endothelin converting enzyme (ece-1). FEBS Lett. 1994;356:238–243. doi: 10.1016/0014-5793(94)01277-6. [DOI] [PubMed] [Google Scholar]
- 28.Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic ph optimum. J Biol Chem. 1995;270:15262–15268. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- 29.Kedzierski RM, Yanagisawa M. Endothelin system: The double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 30.Yanagisawa H, Hammer RE, Richardson JA, Emoto N, Williams SC, Takeda S, Clouthier DE, Yanagisawa M. Disruption of ece-1 and ece-2 reveals a role for endothelin-converting enzyme-2 in murine cardiac development. J Clin Invest. 2000;105:1373–1382. doi: 10.1172/JCI7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano A, Kishi F, Minami K, Wakabayashi H, Nakaya Y, Kido H. Selective conversion of big endothelins to tracheal smooth muscle-constricting 31-amino acid-length endothelins by chymase from human mast cells. J Immunol. 1997;159:1987–1992. [PubMed] [Google Scholar]
- 32.Simonson MS, Dunn MJ. Cellular signaling by peptides of the endothelin gene family. Faseb J. 1990;4:2989–3000. doi: 10.1096/fasebj.4.12.2168326. [DOI] [PubMed] [Google Scholar]
- 33.Masaki T. Historical review: Endothelin. Trends Pharmacol Sci. 2004;25:219–224. doi: 10.1016/j.tips.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Clozel M, Gray GA. Are there different etb receptors mediating constriction and relaxation? J Cardiovasc Pharmacol. 1995;26(Suppl 3):S262–264. [PubMed] [Google Scholar]
- 35.Rockey DC, Chung JJ. Endothelin antagonism in experimental hepatic fibrosis. Implications for endothelin in the pathogenesis of wound healing. J Clin Invest. 1996;98:1381–1388. doi: 10.1172/JCI118925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Battistini B, Warner TD, Fournier A, Vane JR. Characterization of etb receptors mediating contractions induced by endothelin-1 or irl 1620 in guinea-pig isolated airways: Effects of bq-123, fr139317 or pd 145065. Br J Pharmacol. 1994;111:1009–1016. doi: 10.1111/j.1476-5381.1994.tb14844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karne S, Jayawickreme CK, Lerner MR. Cloning and characterization of an endothelin-3 specific receptor (etc receptor) from xenopus laevis dermal melanophores. J Biol Chem. 1993;268:19126–19133. [PubMed] [Google Scholar]
- 38.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 39.Moller S, Henriksen JH. [plasma endothelin immunoreactivity in liver disease and the hepatorenal syndrome]. Ugeskr Laeger. 1993;155:728–729. [PubMed] [Google Scholar]
- 40.Trevisani F, Colantoni A, Gerbes AL, Gulberg V, Sica G, Caraceni P, De Notariis S, Morselli-Labate AM, Ligabue A, Gasbarrini G, Bernardi M. Daily profile of plasma endothelin-1 and -3 in pre-ascitic cirrhosis: Relationships with the arterial pressure and renal function. J Hepatol. 1997;26:808–815. doi: 10.1016/s0168-8278(97)80246-2. [DOI] [PubMed] [Google Scholar]
- 41.Alam I, Bass NM, Bacchetti P, Gee L, Rockey DC. Hepatic tissue endothelin-1 levels in chronic liver disease correlate with disease severity and ascites. Am J Gastroenterol. 2000;95:199–203. doi: 10.1111/j.1572-0241.2000.01684.x. [DOI] [PubMed] [Google Scholar]
- 42.Feng HQ, Weymouth ND, Rockey DC. Endothelin antagonism in portal hypertensive mice: Implications for endothelin receptor-specific signaling in liver disease. Am J Physiol Gastrointest Liver Physiol. 2009;297:G27–33. doi: 10.1152/ajpgi.90405.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thirunavukkarasu C, Yang Y, Subbotin VM, Harvey SA, Fung J, Gandhi CR. Endothelin receptor antagonist tak-044 arrests and reverses the development of carbon tetrachloride induced cirrhosis in rats. Gut. 2004;53:1010–1019. doi: 10.1136/gut.2003.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavasin MA, Semus H, Pitts K, Peng Y, Sandoval J, Chapo J, Plato CF. Acute effects of endothelin receptor antagonists on hepatic hemodynamics of cirrhotic and noncirrhotic rats. Can J Physiol Pharmacol. 88:636–643. doi: 10.1139/Y10-038. [DOI] [PubMed] [Google Scholar]
- 45.Shao R, Shi Z, Gotwals PJ, Koteliansky VE, George J, Rockey DC. Cell and molecular regulation of endothelin-1 production during hepatic wound healing. Mol Biol Cell. 2003;14:2327–2341. doi: 10.1091/mbc.02-06-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shao R, Rockey DC. Effects of endothelins on hepatic stellate cell synthesis of endothelin-1 during hepatic wound healing. J Cell Physiol. 2002;191:342–350. doi: 10.1002/jcp.10110. [DOI] [PubMed] [Google Scholar]
- 47.Zhan S, Chan CC, Serdar B, Rockey DC. Fibronectin stimulates endothelin-1 synthesis in rat hepatic myofibroblasts via a src/erk-regulated signaling pathway. Gastroenterology. 2009;136:2345–2355. e2341–2344. doi: 10.1053/j.gastro.2009.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Housset C, Rockey DC, Bissell DM. Endothelin receptors in rat liver: Lipocytes as a contractile target for endothelin 1. Proc Natl Acad Sci U S A. 1993;90:9266–9270. doi: 10.1073/pnas.90.20.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21:397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 50.Schuppan D, Popov Y. Hepatic fibrosis: From bench to bedside. J Gastroenterol Hepatol. 2002;17(Suppl 3):S300–305. doi: 10.1046/j.1440-1746.17.s3.18.x. [DOI] [PubMed] [Google Scholar]
- 51.Gabriel A, Kuddus RH, Rao AS, Gandhi CR. Down-regulation of endothelin receptors by transforming growth factor beta1 in hepatic stellate cells. J Hepatol. 1999;30:440–450. doi: 10.1016/s0168-8278(99)80103-2. [DOI] [PubMed] [Google Scholar]
- 52.Chi X, Anselmi K, Watkins S, Gandhi CR. Prevention of cultured rat stellate cell transformation and endothelin-b receptor upregulation by retinoic acid. Br J Pharmacol. 2003;139:765–774. doi: 10.1038/sj.bjp.0705303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandhi CR, Kuddus RH, Nemoto EM, Murase N. Endotoxin treatment causes an upregulation of the endothelin system in the liver: Amelioration of increased portal resistance by endothelin receptor antagonism. J Gastroenterol Hepatol. 2001;16:61–69. doi: 10.1046/j.1440-1746.2001.02419.x. [DOI] [PubMed] [Google Scholar]
- 54.Schmitt-Graff A, Kruger S, Bochard F, Gabbiani G, Denk H. Modulation of alpha smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am J Pathol. 1991;138:1233–1242. [PMC free article] [PubMed] [Google Scholar]
- 55.Khimji AK, Rockey DC. Endothelin--biology and disease. Cell Signal. 22:1615–1625. doi: 10.1016/j.cellsig.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Friedman SL. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blomhoff R, Wake K. Perisinusoidal stellate cells of the liver: Important roles in retinol metabolism and fibrosis. Faseb J. 1991;5:271–277. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- 59.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 60.Reynaert H, Thompson MG, Thomas T, Geerts A. Hepatic stellate cells: Role in microcirculation and pathophysiology of portal hypertension. Gut. 2002;50:571–581. doi: 10.1136/gut.50.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pinzani M, Marra F, Carloni V. Signal transduction in hepatic stellate cells. Liver. 1998;18:2–13. doi: 10.1111/j.1600-0676.1998.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 62.Yata Y, Gotwals P, Koteliansky V, Rockey DC. Dose-dependent inhibition of hepatic fibrosis in mice by a tgf-beta soluble receptor: Implications for antifibrotic therapy. Hepatology. 2002;35:1022–1030. doi: 10.1053/jhep.2002.32673. [DOI] [PubMed] [Google Scholar]
- 63.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khimji AK, Shao R, Rockey DC. Divergent transforming growth factor-beta signaling in hepatic stellate cells after liver injury: Functional effects on ece-1 regulation. Am J Pathol. 2008;173:716–727. doi: 10.2353/ajpath.2008.071121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang SN, Burch ML, Tannock LR, Evanko S, Osman N, Little PJ. Transforming growth factor-beta regulation of proteoglycan synthesis in vascular smooth muscle: Contribution to lipid binding and accelerated atherosclerosis in diabetes. J Diabetes. 2:233–242. doi: 10.1111/j.1753-0407.2010.00089.x. [DOI] [PubMed] [Google Scholar]
- 66.Koda M, Bauer M, Krebs A, Hahn EG, Schuppan D, Murawaki Y. Endothelin-1 enhances fibrogenic gene expression, but does not promote DNA synthesis or apoptosis in hepatic stellate cells. Comp Hepatol. 2006;5:5. doi: 10.1186/1476-5926-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shephard P, Hinz B, Smola-Hess S, Meister JJ, Krieg T, Smola H. Dissecting the roles of endothelin, tgf-beta and gm-csf on myofibroblast differentiation by keratinocytes. Thromb Haemost. 2004;92:262–274. doi: 10.1160/TH03-11-0669. [DOI] [PubMed] [Google Scholar]
- 68.Shephard P, Martin G, Smola-Hess S, Brunner G, Krieg T, Smola H. Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-beta and interleukin-1. Am J Pathol. 2004;164:2055–2066. doi: 10.1016/s0002-9440(10)63764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogawa S, Ochi T, Shimada H, Inagaki K, Fujita I, Nii A, Moffat MA, Katragadda M, Violand BN, Arch RH, Masferrer JL. Anti-pdgf-b monoclonal antibody reduces liver fibrosis development. Hepatol Res. 40:1128–1141. doi: 10.1111/j.1872-034X.2010.00718.x. [DOI] [PubMed] [Google Scholar]
- 70.Kinnman N, Goria O, Wendum D, Gendron MC, Rey C, Poupon R, Housset C. Hepatic stellate cell proliferation is an early platelet-derived growth factor-mediated cellular event in rat cholestatic liver injury. Lab Invest. 2001;81:1709–1716. doi: 10.1038/labinvest.3780384. [DOI] [PubMed] [Google Scholar]
- 71.Cao S, Yaqoob U, Das A, Shergill U, Jagavelu K, Huebert RC, Routray C, Abdelmoneim S, Vasdev M, Leof E, Charlton M, Watts RJ, Mukhopadhyay D, Shah VH. Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing pdgf/tgf-beta signaling in hepatic stellate cells. J Clin Invest. 120:2379–2394. doi: 10.1172/JCI41203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clarke JG, Benjamin N, Larkin SW, Webb DJ, Davies GJ, Maseri A. Endothelin is a potent long-lasting vasoconstrictor in men. Am J Physiol. 1989;257:H2033–2035. doi: 10.1152/ajpheart.1989.257.6.H2033. [DOI] [PubMed] [Google Scholar]
- 73.Clerk A, Sugden PH. Regulation of phospholipases c and d in rat ventricular myocytes: Stimulation by endothelin-1, bradykinin and phenylephrine. J Mol Cell Cardiol. 1997;29:1593–1604. doi: 10.1006/jmcc.1997.0395. [DOI] [PubMed] [Google Scholar]
- 74.Pfitzer G. Invited review: Regulation of myosin phosphorylation in smooth muscle. J Appl Physiol. 2001;91:497–503. doi: 10.1152/jappl.2001.91.1.497. [DOI] [PubMed] [Google Scholar]
- 75.Stull JT, Lin PJ, Krueger JK, Trewhella J, Zhi G. Myosin light chain kinase: Functional domains and structural motifs. Acta Physiol Scand. 1998;164:471–482. doi: 10.1111/j.1365-201x.1998.tb10699.x. [DOI] [PubMed] [Google Scholar]
- 76.Shimizu I, Mizobuchi Y, Yasuda M, Shiba M, Ma YR, Horie T, Liu F, Ito S. Inhibitory effect of oestradiol on activation of rat hepatic stellate cells in vivo and in vitro. Gut. 1999;44:127–136. doi: 10.1136/gut.44.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laleman W, Van Landeghem L, Severi T, Vander Elst I, Zeegers M, Bisschops R, Van Pelt J, Roskams T, Cassiman D, Fevery J, Nevens F. Both ca2+ -dependent and -independent pathways are involved in rat hepatic stellate cell contraction and intrahepatic hyperresponsiveness to methoxamine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G556–564. doi: 10.1152/ajpgi.00196.2006. [DOI] [PubMed] [Google Scholar]
- 78.Reinehr R, Sommerfeld A, Haussinger D. Cd95 ligand is a proliferative and antiapoptotic signal in quiescent hepatic stellate cells. Gastroenterology. 2008;134:1494–1506. doi: 10.1053/j.gastro.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 79.Muranyi A, MacDonald JA, Deng JT, Wilson DP, Haystead TA, Walsh MP, Erdodi F, Kiss E, Wu Y, Hartshorne DJ. Phosphorylation of the myosin phosphatase target subunit by integrin-linked kinase. Biochem J. 2002;366:211–216. doi: 10.1042/BJ20020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shafiei MS, Rockey DC. The role of integrin-linked kinase in liver wound healing. J Biol Chem. 2006;281:24863–24872. doi: 10.1074/jbc.M513544200. [DOI] [PubMed] [Google Scholar]
- 81.Borman MA, MacDonald JA, Muranyi A, Hartshorne DJ, Haystead TA. Smooth muscle myosin phosphatase-associated kinase induces ca2+ sensitization via myosin phosphatase inhibition. J Biol Chem. 2002;277:23441–23446. doi: 10.1074/jbc.M201597200. [DOI] [PubMed] [Google Scholar]
- 82.Ono K, Tsujimoto G, Sakamoto A, Eto K, Masaki T, Ozaki Y, Satake M. Endothelin-a receptor mediates cardiac inhibition by regulating calcium and potassium currents. Nature. 1994;370:301–304. doi: 10.1038/370301a0. [DOI] [PubMed] [Google Scholar]
- 83.Somlyo AV, Bradshaw D, Ramos S, Murphy C, Myers CE, Somlyo AP. Rho-kinase inhibitor retards migration and in vivo dissemination of human prostate cancer cells. Biochem Biophys Res Commun. 2000;269:652–659. doi: 10.1006/bbrc.2000.2343. [DOI] [PubMed] [Google Scholar]
- 84.Perri RE, Langer DA, Chatterjee S, Gibbons SJ, Gadgil J, Cao S, Farrugia G, Shah VH. Defects in cgmp-pkg pathway contribute to impaired no-dependent responses in hepatic stellate cells upon activation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G535–542. doi: 10.1152/ajpgi.00297.2005. [DOI] [PubMed] [Google Scholar]
- 85.Rockey DC, Housset CN, Friedman SL. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest. 1993;92:1795–1804. doi: 10.1172/JCI116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin a-storing cells in extrahepatic organs. Int Rev Cytol. 1980;66:303–353. doi: 10.1016/s0074-7696(08)61977-4. [DOI] [PubMed] [Google Scholar]
- 87.Ekataksin W, Kaneda K. Liver microvascular architecture: An insight into the pathophysiology of portal hypertension. Semin Liver Dis. 1999;19:359–382. doi: 10.1055/s-2007-1007126. [DOI] [PubMed] [Google Scholar]
- 88.McCuskey RS. Morphological mechanisms for regulating blood flow through hepatic sinusoids. Liver. 2000;20:3–7. doi: 10.1034/j.1600-0676.2000.020001003.x. [DOI] [PubMed] [Google Scholar]
- 89.Clemens MG, Zhang JX. Regulation of sinusoidal perfusion: In vivo methodology and control by endothelins. Semin Liver Dis. 1999;19:383–396. doi: 10.1055/s-2007-1007127. [DOI] [PubMed] [Google Scholar]
- 90.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and tgf-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: Collagens and glycoproteins. Semin Liver Dis. 1990;10:1–10. doi: 10.1055/s-2008-1040452. [DOI] [PubMed] [Google Scholar]
- 92.Haralson MA. Extracellular matrix and growth factors: An integrated interplay controlling tissue repair and progression to disease. Lab Invest. 1993;69:369–372. [PubMed] [Google Scholar]
- 93.Ravanti L, Kahari VM. Matrix metalloproteinases in wound repair (review). Int J Mol Med. 2000;6:391–407. [PubMed] [Google Scholar]
- 94.Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13:377–383. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 95.Cho JJ, Hocher B, Herbst H, Jia JD, Ruehl M, Hahn EG, Riecken EO, Schuppan D. An oral endothelin-a receptor antagonist blocks collagen synthesis and deposition in advanced rat liver fibrosis. Gastroenterology. 2000;118:1169–1178. doi: 10.1016/s0016-5085(00)70370-2. [DOI] [PubMed] [Google Scholar]
- 96.Anselmi K, Subbotin VM, Nemoto E, Gandhi CR. Accelerated reversal of carbon tetrachloride-induced cirrhosis in rats by the endothelin receptor antagonist tak-044. J Gastroenterol Hepatol. 2002;17:589–597. doi: 10.1046/j.1440-1746.2002.02705.x. [DOI] [PubMed] [Google Scholar]
- 97.Ali H, Dashwood M, Dawas K, Loizidou M, Savage F, Taylor I. Endothelin receptor expression in colorectal cancer. J Cardiovasc Pharmacol. 2000;36:S69–71. doi: 10.1097/00005344-200036051-00023. [DOI] [PubMed] [Google Scholar]
- 98.Urbanowicz W, Sogni P, Moreau R, Tazi KA, Barriere E, Poirel O, Martin A, Guimont MC, Cazals-Hatem D, Lebrec D. Tezosentan, an endothelin receptor antagonist, limits liver injury in endotoxin challenged cirrhotic rats. Gut. 2004;53:1844–1849. doi: 10.1136/gut.2003.036517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gandhi CR, Kuddus RH, Uemura T, Rao AS. Endothelin stimulates transforming growth factor-beta1 and collagen synthesis in stellate cells from control but not cirrhotic rat liver. Eur J Pharmacol. 2000;406:311–318. doi: 10.1016/s0014-2999(00)00683-x. [DOI] [PubMed] [Google Scholar]
- 100.Ruetten H, Thiemermann C. Endothelin-1 stimulates the biosynthesis of tumour necrosis factor in macrophages: Et-receptors, signal transduction and inhibition by dexamethasone. J Physiol Pharmacol. 1997;48:675–688. [PubMed] [Google Scholar]
- 101.Hafizi S, Allen SP, Goodwin AT, Chester AH, Yacoub MH. Endothelin-1 stimulates proliferation of human coronary smooth muscle cells via the et(a) receptor and is co-mitogenic with growth factors. Atherosclerosis. 1999;146:351–359. doi: 10.1016/s0021-9150(99)00178-1. [DOI] [PubMed] [Google Scholar]
- 102.Shahar I, Fireman E, Topilsky M, Grief J, Schwarz Y, Kivity S, Ben-Efraim S, Spirer Z. Effect of endothelin-1 on alpha-smooth muscle actin expression and on alveolar fibroblasts proliferation in interstitial lung diseases. Int J Immunopharmacol. 1999;21:759–775. doi: 10.1016/s0192-0561(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 103.Bagnato A, Spinella F, Rosano L. The endothelin axis in cancer: The promise and the challenges of molecularly targeted therapy. Can J Physiol Pharmacol. 2008;86:473–484. doi: 10.1139/Y08-058. [DOI] [PubMed] [Google Scholar]
- 104.Pinzani M, Milani S, De Franco R, Grappone C, Caligiuri A, Gentilini A, Tosti-Guerra C, Maggi M, Failli P, Ruocco C, Gentilini P. Endothelin 1 is overexpressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534–548. doi: 10.1053/gast.1996.v110.pm8566602. [DOI] [PubMed] [Google Scholar]
- 105.Mallat A, Fouassier L, Preaux AM, Gal CS, Raufaste D, Rosenbaum J, Dhumeaux D, Jouneaux C, Mavier P, Lotersztajn S. Growth inhibitory properties of endothelin-1 in human hepatic myofibroblastic ito cells. An endothelin b receptor-mediated pathway. J Clin Invest. 1995;96:42–49. doi: 10.1172/JCI118052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mallat A, Fouassier L, Preaux AM, Mavier P, Lotersztajn S. Antiproliferative effects of et-1 in human liver ito cells: An etb- and a cyclic amp-mediated pathway. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S132–134. [PubMed] [Google Scholar]
- 107.Gallois C, Habib A, Tao J, Moulin S, Maclouf J, Mallat A, Lotersztajn S. Role of nfkappab in the antiproliferative effect of endothelin-1 and tumor necrosis factor-alpha in human hepatic stellate cells. Involvement of cyclooxygenase-2. J Biol Chem. 1998;273:23183–23190. doi: 10.1074/jbc.273.36.23183. [DOI] [PubMed] [Google Scholar]
- 108.Mallat A, Gallois C, Tao J, Habib A, Maclouf J, Mavier P, Preaux AM, Lotersztajn S. Platelet-derived growth factor-bb and thrombin generate positive and negative signals for human hepatic stellate cell proliferation. Role of a prostaglandin/cyclic amp pathway and cross-talk with endothelin receptors. J Biol Chem. 1998;273:27300–27305. doi: 10.1074/jbc.273.42.27300. [DOI] [PubMed] [Google Scholar]
- 109.Piacentini L, Gray M, Honbo NY, Chentoufi J, Bergman M, Karliner JS. Endothelin-1 stimulates cardiac fibroblast proliferation through activation of protein kinase c. J Mol Cell Cardiol. 2000;32:565–576. doi: 10.1006/jmcc.2000.1109. [DOI] [PubMed] [Google Scholar]
- 110.Rubanyi GM, Polokoff MA. Endothelins: Molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- 111.Edwards RM, Trizna W, Ohlstein EH. Renal microvascular effects of endothelin. Am J Physiol. 1990;259:F217–221. doi: 10.1152/ajprenal.1990.259.2.F217. [DOI] [PubMed] [Google Scholar]
- 112.Bergler-Klein J, Pacher R, Berger R, Bojic A, Stanek B. Neurohumoral and hemodynamic effects of the selective endothelin antagonist darusentan in advanced chronic heart failure. J Heart Lung Transplant. 2004;23:20–27. doi: 10.1016/s1053-2498(03)00062-7. [DOI] [PubMed] [Google Scholar]
- 113.Zamora MR, O'Brien RF, Rutherford RB, Weil JV. Serum endothelin-1 concentrations and cold provocation in primary raynaud's phenomenon. Lancet. 1990;336:1144–1147. doi: 10.1016/0140-6736(90)92766-b. [DOI] [PubMed] [Google Scholar]
- 114.Tripathi D, Therapondos G, Ferguson JW, Newby DE, Webb DJ, Hayes PC. Endothelin-1 contributes to maintenance of systemic but not portal haemodynamics in patients with early cirrhosis: A randomised controlled trial. Gut. 2006;55:1290–1295. doi: 10.1136/gut.2005.077453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Helmy A, Newby DE, Jalan R, Hayes PC, Webb DJ. Enhanced vasodilatation to endothelin antagonism in patients with compensated cirrhosis and the role of nitric oxide. Gut. 2003;52:410–415. doi: 10.1136/gut.52.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lavelle A, Sugrue R, Lawler G, Mulligan N, Kelleher B, Murphy DM, Gaine SP. Sitaxentan-induced hepatic failure in two patients with pulmonary arterial hypertension. Eur Respir J. 2009;34:770–771. doi: 10.1183/09031936.00058409. [DOI] [PubMed] [Google Scholar]
- 117.Barst RJ, Rich S, Widlitz A, Horn EM, McLaughlin V, McFarlin J. Clinical efficacy of sitaxsentan, an endothelin-a receptor antagonist, in patients with pulmonary arterial hypertension: Open-label pilot study. Chest. 2002;121:1860–1868. doi: 10.1378/chest.121.6.1860. [DOI] [PubMed] [Google Scholar]
- 118.Clozel M. Endothelin receptor antagonists: Current status and perspectives. J Cardiovasc Pharmacol. 2000;35:S65–68. doi: 10.1097/00005344-200000002-00015. [DOI] [PubMed] [Google Scholar]
- 119.Remuzzi G, Perico N, Benigni A. New therapeutics that antagonize endothelin: Promises and frustrations. Nat Rev Drug Discov. 2002;1:986–1001. doi: 10.1038/nrd962. [DOI] [PubMed] [Google Scholar]
- 120.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 121.Kirkby NS, Hadoke PW, Bagnall AJ, Webb DJ. The endothelin system as a therapeutic target in cardiovascular disease: Great expectations or bleak house? Br J Pharmacol. 2008;153:1105–1119. doi: 10.1038/sj.bjp.0707516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Casserly B, Klinger JR. Ambrisentan for the treatment of pulmonary arterial hypertension. Drug Des Devel Ther. 2009;2:265–280. doi: 10.2147/dddt.s3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shepard DR, Dreicer R. Zibotentan for the treatment of castrate-resistant prostate cancer. Expert Opin Investig Drugs. 19:899–908. doi: 10.1517/13543784.2010.491822. [DOI] [PubMed] [Google Scholar]
- 124.Wu-Wong JR. S-0139 (shionogi). Curr Opin Investig Drugs. 2002;3:1051–1056. [PubMed] [Google Scholar]