Abstract
Background and Purpose
Intracerebral hemorrhage (ICH) is swiftly followed by an inflammatory response. A key component of this response is the recruitment of leukocytes into the brain, which promote neurological injury in rodent models. However, the mechanisms by which leukocytes transmigrate across the endothelium into the injured brain are unclear. The present study examines leukocyte adhesion molecules (α4 integrin, L-selectin, and αLβ2 integrin) on four leukocyte subtypes to determine which are important for leukocyte recruitment after ICH.
Methods
We used the blood injection mouse model of ICH, whereby 25 μl of blood were injected into the striatum. Flow cytometry was used to quantify leukocyte populations and adhesion molecule expression in brain and blood. An α4 integrin blocking antibody was administered to evaluate the contribution of α4 in leukocyte migration and neurological injury.
Results
α4 integrin was elevated on all leukocyte populations in brain after ICH, whereas L-selectin was unchanged and αLβ2 was increased only on T cells. Antagonism of α4 resulted in decreased leukocyte transmigration and lessened neurobehavioral disability.
Conclusions
α4 integrin is an important cell adhesion molecule involved in neuroinflammation following ICH.
Keywords: intracerebral hemorrhage, monocytes, inflammation, adhesion molecules, integrins
Introduction
Intracerebral hemorrhage (ICH) initiates an inflammatory response that is characterized by leukocyte recruitment and elevated cytokine levels1. Specific leukocyte populations, including neutrophils, T cells, and inflammatory monocytes, promote secondary injury in models of ICH2–4. It is thought that these cells principally inflict damage through the release of reactive oxygen species, pro-inflammatory cytokines, and proteases3, 5, but the mechanisms used for migration into the CNS after ICH are unclear. While several studies have shown the importance of endothelial cell adhesion molecules, namely VAP-1 and ICAM-1, for leukocyte recruitment after ICH6, 7, no study has examined adhesion molecules on the surface of leukocytes. In the present study we examined changes in the levels of adhesion molecules on leukocytes in blood and brain. We also blocked α4 integrin function, which resulted in decreased leukocyte recruitment and improved motor function after ICH.
Methods
Protocols were approved by the UConn Health IACUC and were performed in accordance with NIH’s Guide for the Care and Use of Laboratory Animals. ICH was modeled8 using 25 μl autologous blood. Cells were analyzed using an LSRII cytometer (BD). For α4 integrin blocking, mice were injected with isotype control or anti-α4 (clone R1-2; 300 μg/mouse) 2–6 hours before ICH. Analysis was performed blinded to treatment. Detailed methods are in the Online Supplement.
Results
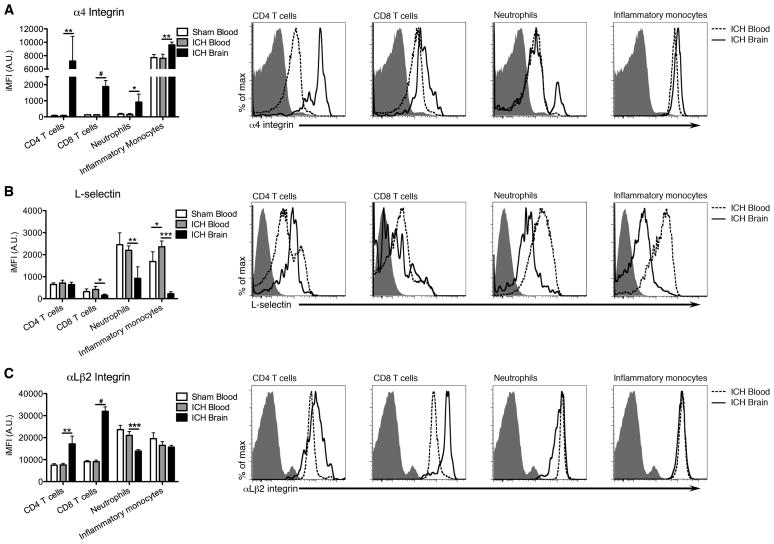
To determine how ICH affects leukocyte adhesion molecule expression, we performed flow cytometry on blood and brain 2 days following ICH. A mean of 11,128 ± 10,879 leukocytes were isolated from ICH brains versus 4,079 ± 305 cells in shams (n=4). The α4 integrin chain was elevated on all leukocyte populations in the ICH brain compared to blood (Figure 1A; Figure I Online Supplement). Inflammatory monocytes, which had the highest baseline α4, represented the largest population recruited to the ICH brain at day 2 (Figure II Online Supplement). Conversely, L-selectin was decreased on all leukocyte populations examined in the brain except for CD4 T cells, which were unchanged (Figure 1B). αLβ2 was higher on T cells in brain, while myeloid cells were unaffected (Figure 1C). Uniformly elevated α4 on all leukocyte populations suggests that it may mediate leukocyte recruitment after ICH.
Figure 1.
Adhesion molecule modulation following ICH. A, α4 integrin integrated mean fluorescence intensities (iMFIs) were elevated on leukocytes in brains at day 2. B, L-selectin iMFIs were not increased relative to blood. C, αLβ2 staining was increased on T cells, but decreased on neutrophils and unchanged on inflammatory monocytes. Shaded traces depict negative controls. N=4; *p<0.05, **p<0.01, ***p<0.001 by t test; #p<0.05 by U test; bars indicate mean ± SD.
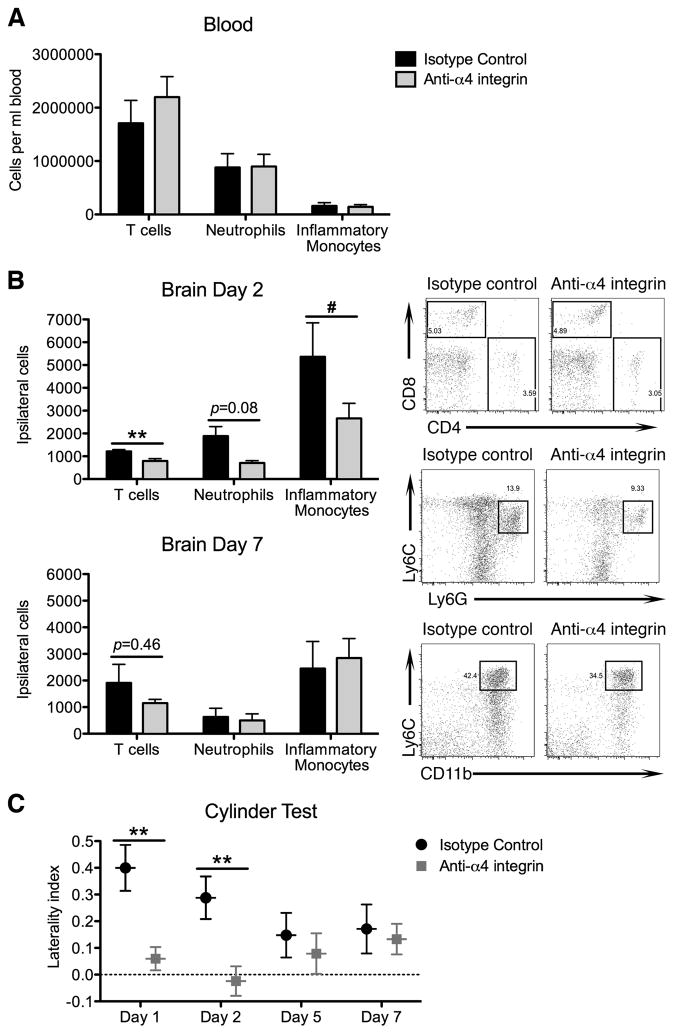
To determine if α4 is required for entry into the brain, we treated mice with an anti-α4 blocking antibody before ICH. Brain and blood samples were examined using flow cytometry 2 or 7 days later. Concentrations of T cells, neutrophils, and inflammatory monocytes were unchanged in blood by treatment (Figure 2A), as were physiological variables (Table I Online Supplement). However, recruitment of T cells and inflammatory monocytes was significantly diminished in day 2 anti-α4-treated brains, suggesting α4 integrin function is a fundamental mechanism by which leukocytes migrate into the hemorrhagic brain (Figure 2B). Leukocyte quantities isolated from isotype control-treated brains were similar to the untreated ICH brains in Figure 1. Importantly, anti-α4-treated mice displayed significantly improved left forelimb use by the cylinder test up to day 2 (Figure 2C). Together, these data demonstrate that α4 is an important cell adhesion molecule involved in acute leukocyte recruitment following ICH.
Figure 2.
α4 integrin blocking diminishes acute neuroinflammation. A, Concentrations of T cells, neutrophils, and inflammatory monocytes were unchanged in blood 2 days after antibody treatment. N=8–9. B, α4 blockade decreased T cell and inflammatory monocyte recruitment at day 2, but not day 7. Representative flow cytometry plots depict CD4 and CD8 T cells; Ly6G+ neutrophils; and Ly6Chi inflammatory monocytes at day 2. N=5–9. C, Anti-α4-treated mice displayed improved left forelimb use in the cylinder test. N=7–9. **p<0.01 by t test; #p<0.05 by U test; bars indicate mean ± SEM.
Discussion
The present study aimed to understand how adhesion molecules on leukocytes are involved in cell recruitment following ICH. All leukocyte populations examined displayed increased α4 integrin, whereas only T cells showed elevated αLβ2, and no population displayed increased L-selectin in brain. Interestingly, inflammatory monocytes, which were recently shown to worsen ICH injury3, represented the largest leukocyte population in brain and had the highest baseline α4 in blood. However, increases in adhesion molecules may not necessarily correlate with the influence of a particular molecule, as conformational changes influence ligand affinities9 and molecules may be downregulated after tissue entry. We therefore confirmed the role of α4 with an antagonist. Treatment with the α4 blocking antibody decreased leukocyte recruitment and reduced early motor deficits, indicating its importance in ICH. α4 heterodimerizes with β1 or β7 integrins. α4β1 is expressed on leukocytes and microglia, whereas α4β7 is found on gut-homing T cells and some vascular endothelium. Because the antibody recognizes the α4 subunit, we cannot attribute the observed benefit to a specific α4 heterodimer. Similarly, we cannot rule out the possibility that the antibody crosses a weakened blood brain barrier and binds microglial α4 in addition to that on leukocytes, or has systemic effects. Nonetheless, these results identify α4 integrin as an important cell adhesion molecule during acute sterile neuroinflammation.
Previous studies using α4 blocking antibodies in ischemic stroke models have shown benefits, both by reduced infarct volumes and improved neurobehavioral functions10–12. While these studies mainly attributed improvements to reduced T cell recruitment, they also showed decreased myeloperoxidase and Gr110, 11, markers common to inflammatory monocytes and neutrophils13, 14, indicating myeloid cells were also decreased by treatment. Using flow cytometry, the present study discriminates between inflammatory monocytes, neutrophils, and microglia and identifies monocytes as having the largest decrease during α4 blockade. Interestingly, many leukocytes entered the brain despite α4 blockade, yet there was little disability up to day 2 in treated mice. This suggests that treatment may also inhibit integrin signaling, contributing to altered phenotypes once in tissue. This requires further study.
The study was not intended to evaluate α4 integrin as a therapeutic target. However, our findings suggest that blocking α4 function may provide neurological benefits by reducing acute inflammation. The absence of differences at day 7 is likely due to the single treatment. While the half-life of the antibody is unknown, it is likely similar to the half-life of the isotype control (4–6 days)15. In addition, redundant migration mechanisms may compensate for blockade. Additional studies are needed to better characterize α4-mediated leukocyte recruitment using translationally-relevant dosing paradigms.
Supplementary Material
Acknowledgments
Sources of Funding: NIH F31NS083231 (MDH); K08NS078110 (LHS).
Footnotes
Disclosures: NIH and AHA grants (MDH; LHS) and AHA honoraria (MDH).
References
- 1.Dziedzic T, Bartus S, Klimkowicz A, Motyl M, Slowik A, Szczudlik A. Intracerebral hemorrhage triggers interleukin-6 and interleukin-10 release in blood. Stroke. 2002;33:2334–2335. doi: 10.1161/01.str.0000027211.73567.fa. [DOI] [PubMed] [Google Scholar]
- 2.Rolland WB, Lekic T, Krafft PR, Hasegawa Y, Altay O, Hartman R, et al. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp Neurol. 2013;241:45–55. doi: 10.1016/j.expneurol.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammond MD, Taylor RA, Mullen MT, Ai Y, Aguila HL, Mack M, et al. Ccr2+ly6chi inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci. 2014;34:3901–3909. doi: 10.1523/JNEUROSCI.4070-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sansing LH, Harris TH, Kasner SE, Hunter CA, Kariko K. Neutrophil depletion diminishes monocyte infiltration and improves functional outcome after experimental intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:173–178. doi: 10.1007/978-3-7091-0693-8_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen HX, O’Barr TJ, Anderson AJ. Polymorphonuclear leukocytes promote neurotoxicity through release of matrix metalloproteinases, reactive oxygen species, and tnf-alpha. J Neurochem. 2007;102:900–912. doi: 10.1111/j.1471-4159.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- 6.Ma Q, Manaenko A, Khatibi NH, Chen W, Zhang JH, Tang J. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J Cereb Blood Flow Metab. 2011;31:881–893. doi: 10.1038/jcbfm.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liesz A, Middelhoff M, Zhou W, Karcher S, Illanes S, Veltkamp R. Comparison of humoral neuroinflammation and adhesion molecule expression in two models of experimental intracerebral hemorrhage. Exp Transl Stroke Med. 2011;3:11. doi: 10.1186/2040-7378-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sansing LH, Kasner SE, McCullough L, Agarwal P, Welsh FA, Kariko K. Autologous blood injection to model spontaneous intracerebral hemorrhage in mice. J Vis Exp. 2011;54:e2618. doi: 10.3791/2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Relton JK, Sloan KE, Frew EM, Whalley ET, Adams SP, Lobb RR. Inhibition of alpha4 integrin protects against transient focal cerebral ischemia in normotensive and hypertensive rats. Stroke. 2001;32:199–205. doi: 10.1161/01.str.32.1.199. [DOI] [PubMed] [Google Scholar]
- 11.Liesz A, Zhou W, Mracsko E, Karcher S, Bauer H, Schwarting S, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134:704–720. doi: 10.1093/brain/awr008. [DOI] [PubMed] [Google Scholar]
- 12.Becker K, Kindrick D, Relton J, Harlan J, Winn R. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32:206–211. doi: 10.1161/01.str.32.1.206. [DOI] [PubMed] [Google Scholar]
- 13.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of ly6g-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 14.Swirski FK, Wildgruber M, Ueno T, Figueiredo JL, Panizzi P, Iwamoto Y, et al. Myeloperoxidase-rich ly-6c+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J Clin Invest. 2010;120:2627–2634. doi: 10.1172/JCI42304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988;18:313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.