Abstract
Tropomyosin regulates a wide variety of actin filament functions and is best known for the role that it plays together with troponin in controlling muscle activity. For effective performance on actin filaments, adjacent 42-nm-long tropomyosin molecules are joined together by a 9- to 10-residue head-to-tail overlapping domain to form a continuous cable that wraps around the F-actin helix. Yet, despite the apparent simplicity of tropomyosin’s coiled-coil structure and its well-known periodic association with successive actin subunits along F-actin, the structure of the tropomyosin cable on actin is uncertain. This is because the conformation of the overlap region that joins neighboring molecules is poorly understood, thus leaving a significant gap in our understanding of thin-filament structure and regulation. However, recent molecular-dynamics simulations of overlap segments defined their overall shape and provided unique and sufficient cues to model the whole actin-tropomyosin filament assembly in atomic detail. In this study, we show that these MD structures merge seamlessly onto the ends of tropomyosin coiled-coils. Adjacent tropomyosin molecules can then be joined together to provide a comprehensive model of the tropomyosin cable running continuously on F-actin. The resulting complete model presented here describes for the first time (to our knowledge) an atomic-level structure of αα-striated muscle tropomyosin bound to an actin filament that includes the critical overlap domain. Thus, the model provides a structural correlate to evaluate thin-filament mechanics, self-assembly mechanisms, and the effect of disease-causing mutations.
Introduction
By assembling in a head-to-tail fashion, tropomyosin coiled-coils form uninterrupted cables that run along the surface of actin filaments in virtually all eukaryotic cells (1,2). Tropomyosin serves as an actin regulatory protein and participates in the cooperative activation and inhibition of myosin cross-bridge–thin-filament interactions. In turn, this process results in cooperative transitions in actomyosin ATPase and consequently contractile activity. In skeletal and cardiac muscle fibers, the influence of tropomyosin on actomyosin formation is modulated by steric interactions with troponin and Ca2+ (3). In smooth muscle and other cells, in addition to influencing actomyosin-based motility, tropomyosin also acts as a thin-filament gatekeeper to control access of nonmotor actin-binding proteins while increasing the filament stability and limiting thin-filament remodeling (4–6). Thus, tropomyosin’s role in regulating muscle contraction (be it in striated or smooth muscle) may have evolved from more diverse cytoskeleton functions present in all cells.
Tropomyosin’s ability to perform regulatory and gatekeeping functions is defined by periodic interactions with successive actin subunits along the thin filament (1–3,6). Indeed, 3D electron microscopy reconstruction and computational approaches have revealed the axial and azimuthal positions of tropomyosin on actin at residue-specific resolution (7–9). However, these methods have not yet defined head-to-tail connections between adjacent tropomyosin molecules on actin, which represent only a fraction of the molecules’ total length (∼6–7%) but are necessary for a strong actin–tropomyosin interaction. The corresponding inability to generate complete atomic models of the tropomyosin cable on thin filaments thus limits interpretation of in vitro and in situ functional data. Hence, determining the conformation of the relatively short overlapping domain that is responsible for polymerization of otherwise canonically coiled-coil tropomyosin on actin is critical for understanding thin-filament mechanics and self-assembly mechanisms, as well as the effects of disease-causing mutations (1–6). In fact, deleting this 9- to 10-residue-long segment greatly limits the binding of tropomyosin to successive actin subunits (seven in muscles) along thin filaments (6) and, by precluding tropomyosin cable formation, prevents effective thin-filament cooperative behavior.
In a recent study (10), molecular-dynamics (MD) simulations of the tropomyosin overlap domain suggested a likely head-to-tail conformation of adjacent tropomyosins. That work was based on prior NMR and crystallographic structures of smooth- and striated-muscle tropomyosin fragments (11,12). It described the MD on 10-residue-long smooth- and-striated muscle overlap complexes (actin-free) set within ∼30-residue-long native sequences extending from the respective N- and C-terminal ends of the overlap, and thus redefined overlap structures in the absence of actin (10). Here, we analyze these tropomyosin segments in the context of a complete actin-tropomyosin complex. We expand on the earlier work by defining the curvature of the overlap domain, a parameter that is necessary to allow realistic binding of tropomyosin to F-actin within our model, which previously was neither apparent nor explicitly determined (10–12). All of these studies indicate that head-to-tail association between molecules leads to a compact N-terminal end of one tropomyosin inserting into an orthogonally oriented and splayed C-terminal end of a second tropomyosin. The resulting four-helix overlap nexus is held together primarily by core hydrophobic interactions. However, the MD additionally shows that formation of the four-stranded intermolecular connection involves a localized rotation of both C- and N-termini that occurs gradually over a ∼20-residue length on both sides of the nexus as the component helices twist away from the canonical coiled-coil symmetry (10). Beyond this region, i.e., distal to the 20 residues displaying noncanonical chain rotation, the canonical coiled-coil twist that characterizes the rest of the full-length tropomyosin is again apparent (1,10).
The curvature of the previously reported NMR and crystal structures, particularly the bending reported for the striated muscle overlap segment, appears to be considerably greater than what would be strictly compatible with linking adjacent helically arranged tropomyosin molecules on F-actin (11,12). Thus, unless these previous structures are very plastic, they will not be able to readily serve their structural function, viz., binding to actin. However, we recently showed that during MD, these variously bent smooth and striated overlap structures converged on a common average structure, with a curvature closely matching the gentle bending of superhelical tropomyosin on F-actin (10,13). Here, in a model based on these MD-determined structures, we demonstrate that no further deformation or distortions are required to superpose distal sides of the overlap structure directly onto canonical tropomyosin molecules lying in tandem on actin (Fig. 1 a). Thus, in a very simple and direct way, superhelically shaped tropomyosin coiled-coil models can be joined together on actin by the recently described overlap modules without deviating from symmetry constraints to fit properly on the F-actin helix (Fig. 1 a). Given the remarkable geometrical agreement between these models, each arrived at independently, we merged the different pieces of the thin-filament puzzle together and refined this composite structure to generate the first (to our knowledge) comprehensive model of actin-tropomyosin filaments in molecular detail. Our approach is discussed below and outlined in Fig. 2.
Figure 1.

The tropomyosin head-to-tail overlap structure on F-actin. (a) Two adjacent canonical tropomyosin coiled-coils on F-actin as described in Li et al. (7) and Orzechowski et al. (9), but here linked together by the refined overlap structure based on Li et al. (10) (gold arrow). The more tightly coiled N-terminus of one tropomyosin inserts into a splayed C-terminus of the other (yellow and green arrows). (b) Enlargement of the refined model of the end-to-end overlap structure, highlighting its hydrophobic (gray) and surrounding polar core residues (colored red, blue, and pink for acidic, basic, and neutral amino acids, respectively). (c) A view comparable to that in b, but now showing the tropomyosin overlap and adjacent pseudo-repeat domains contacting consensus actin-binding patches (red and blue spheres highlight interacting residues). Acidic tropomyosin residues (red arrows) associate with actin residues Lys-326, Lys-328, and Arg-147, whereas basic ones (blue arrows) interact with actin Asp-25. Interacting residues at the overlap are numbered. Figure 4c illustrates the refined model.
Figure 2.
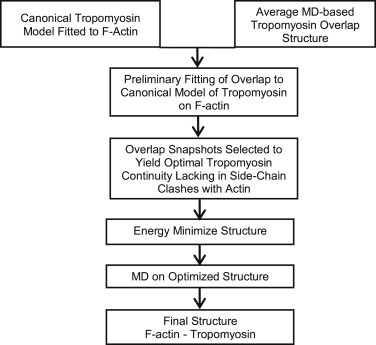
Flowchart outlining the protocol used to make the final model.
Materials and Methods
Building a preliminary model of the tropomyosin overlap domain on F-actin
Here, we focused our continued analysis on a 270 ns average MD structure of the striated muscle overlap structure (10) to be consistent with the vast majority of atomic models and structures that were built from the αα-striated muscle tropomyosin isoform (1,7,8,9,10,11,13). Moreover, despite the overall geometrical similarities of the striated- and smooth-muscle overlap domains (10), we did not consider the smooth-muscle MD structure further in this work because it is based on crystallography of an artificial chicken gizzard αα-overlap construct (12), which may not exist in nature since αβ-isoforms predominate in this tissue. Therefore, we superposed the average MD structure of the skeletal muscle αα-tropomyosin head-to-tail overlap segment (10) on the canonical model of tropomyosin (7,9) (Fig. 3). We used Cα atoms in residues 27 and 28 in the N-terminal part of the overlap segment and residue 252 in the C-terminal part of the overlap segment as alignment cues to link tandem tropomyosins on F-actin, using the program VMD (14). This superposition provided almost perfect continuity between the canonical model backbone and the distal ends of the overlap structure (Fig. 3).
Figure 3.

Building the preliminary model of the tropomyosin overlap domain on F-actin. (a) Canonical model of α-striated muscle tropomyosin fitted to F-actin, as characterized in Li et al. (7) and Orzechowski et al. (9). Note that a strictly canonical model yields a forbidden, completely parallel arrangement of N- and C-terminal coiled-coils in the overlap region that clash with each other (previously detailed in Lehman et al. (13)). (b) Native α-striated-muscle tropomyosin overlapping domain, averaged from MD, as described in Li et al. (10). Note that N- and C-terminal coiled-coil twisting generates an orthogonally oriented interface and no clashes. (c) Preliminary model of the tropomyosin cable on F-actin built from a and b (left: actin pointed end and tropomyosin C-terminal chains; right: N-terminal chains). Superposition of the MD average and the canonical model centered on residues 27, 28, and 252 (shown superposed), followed by deletion of residues 1–25 and 253–284 from the canonical model, yields the preliminary model. No clashes between any of the α-carbon chains are noted. To see this figure in color, go online.
Building a refined model of the tropomyosin overlap domain on F-actin
The preliminary actin-tropomyosin model described above was first used to align all snapshots contained in the MD trajectory of the overlap domain (10). To minimize any side-chain clashes between the superposed overlap and actin, we calculated the van der Waals interaction energies (15) for all of the snapshots aligned on F-actin. We then further examined 15 snapshots with the lowest energy in this series to determine which snapshots displayed optimal tropomyosin backbone continuity and no clashes after superposition. We used the best one to replace corresponding residues in the canonical structure by fusing it to residue 25 in the N-terminal part and to residue 253 in the C-terminal part in the canonical structure while leaving the rest of the canonical structure on F-actin (Fig. 4 a). As described below, we then carried out energy minimization and MD simulation on this model to refine the average structure of the overlap domain on F-actin, ensure its stability, and better define the curvature of the component Cα backbone, residue–residue interactions, and corresponding side-chain orientations.
Figure 4.
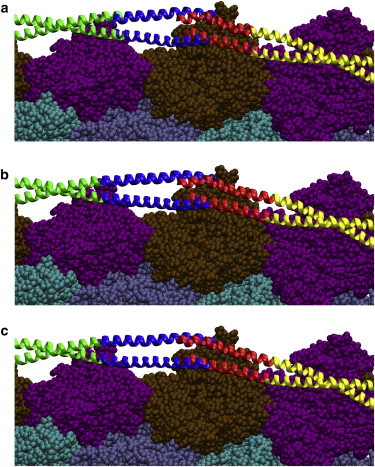
Refining the preliminary model of the tropomyosin overlap domain on F-actin. (a) Single snapshot from the MD trajectory of the α-striated muscle tropomyosin overlapping domain superposed on canonical tropomyosin (superposed overlap segment colored blue and red for C- and N-terminal parts). (b) Energy minimization of the actin-tropomyosin structure in a. Note the slight deviation between the upper C-terminal chain in the snapshot and the canonical structure in a, which is removed by minimization (b). (Although tropomyosin side chains are not shown, none clash with each other or with actin.) (c) Final refined model of actin-tropomyosin, averaged from the MD simulation that was initiated from the structure in (b) and then used for analysis. To see this figure in color, go online.
A series of preliminary energy-minimization calculations (15) was carried out on the preliminary structure in implicit solvent to relax any tropomyosin backbone strain. Minimization very subtly affected the disposition of four residues on either side of residues 25 and 253, and some side-chain orientations between tropomyosin and the surface of actin (Fig. 4 b). Then, three actin subunits of the relaxed structure containing tropomyosin N- and C-terminal residues 1–50 and 235–284 were solvated with water and counterions, and subjected to standard energy minimization and MD (15,16). The positions of the tropomyosin residues at the lateral ends of the structure (at residues 46–50 and 235–239) were fixed, as were residues on actin that do not interface with tropomyosin. MD simulation was carried out for >26 ns in explicit solvent containing 150 mM NaCl with an NPT ensemble at 310 K using NAMD version 2.6 and the CHARMM27 force field (15,16) as previously described (7,9,10). Backbone root mean-square deviation (RMSD) values as well as standard parameters (i.e., potential and kinetic energies, temperature, and volume) showed that the variance in the structure had stabilized by 6 ns of MD (the equilibration time taken for unbound tropomyosin (10); see Fig. S1 in the Supporting Material). The remainder of the MD trajectory was averaged to generate the refined actin-tropomyosin model (Fig. 4 c). (Although MD simulations carried out in implicit solvent can explore more computational space than, for example, simulations currently done in explicit water, the method is not ideal for characterizing the dynamics of interacting molecules such as tropomyosin and actin. Without a cushion of explicit water to separate them, the distance of tropomyosin from the surface of actin in implicit solvent would be expected to fall well below what is observed experimentally, while at the same time the conformation of tropomyosin would become distorted. Implicit water-based trials run on actin-tropomyosin confirmed that the protocol could not be used here, for the above reasons.)
Results and Discussion
The preliminary model of the tropomyosin overlap domain on F-actin
We constructed a preliminary model of the tropomyosin cable on F-actin by treating the average MD overlap structure described above as a rigid body and using it to replace and then connect the ends of tandem canonical α-tropomyosins lying on F-actin (coordinates taken from Li et al. (7,10) and Orzechowski et al. (9)). The alignment of α-carbon chains of the overlap and canonical models is seamless from residues 25–40 and 245–253, since here each model is canonical before the twist of the two diverges at the N- and C-termini. Superposition was best achieved by first aligning residues 27, 28, and 252 in the respective models (Figs. 1 a and 3 c) and then replacing tropomyosin residues 1–25 and 253–284 from the full-length tropomyosin model with the overlap structure. The few side-chain clashes between tropomyosin and actin in the new model were easily corrected by selecting conformers from the MD trajectory, which yielded optimal tropomyosin continuity but no clashes, and the corresponding actin-tropomyosin structure was then refined further.
Refining the model of tropomyosin on F-actin
To generate an optimized equilibrium structure and determine the average overlap structure on actin, we energy minimized the preliminary actin-tropomyosin structure. We then ran a new MD simulation for 26 ns on a segment of the preliminary model containing 50 N- and C-terminal tropomyosin residues (centered on the 10-residue overlap domain and 40 adjacent tropomyosin residues) linked to a three-actin-subunit-long section of the model (Fig. 4). We analyzed and averaged individual snapshots after discarding the first 6 ns of MD to ensure that equilibration had been reached, as described in previous studies (7) (Fig. S1). (Computation of an MD run on the two or more full-length head-to-tail linked tropomyosins on a longer F-actin filament would have been prohibitively expensive and would have provided information already available for the mid-piece of tropomyosin (7)).
An examination of the snapshots taken during the MD shows that the overlap nexus remains completely intact over the surface of F-actin throughout the simulation at an average 40.5 Å radius. No unfolding of individual helices or separation of the chains adjoining the overlap domain occurs. The entire structure retains the gently bent shape that closely matches the helical curvature of actin and, importantly, the curvature of canonical tropomyosin. The average twist angle (88.1° ± 1.7°) at the intersection of the N- and C-terminal chains stays very close to the initial orthogonal orientation, and the rotation of helices distal to the overlap still retains tropomyosin’s canonical coiled-coil twist (Figs. 1, b and c, and 4 c). These and earlier results (10) provide a solution that explains how the ends of the coiled-coils splay and gradually rotate to a 90° relative twist (as in NMR and crystal structures (11,12)) from a canonical (0°) orientation that defines the rest of the molecule (7,10,13).
The α-carbon backbones in the newly refined and preliminary structures are almost completely superposable and in each case connect seamlessly onto canonical tropomyosin, whereas the side chains in the refined model are reoriented to a small degree. This near equivalence confirms that tropomyosin can form an unbroken cable on actin characterized by canonical coiled-coils coupled together and stabilized by a twisted four-helix nexus. It follows that the tropomyosin cable will behave mechanically as one continuous unit and not as a series of jointed segments, and thus can maintain high torsional and flexural stiffness (13). Indeed, the side chains of very closely clustered hydrophobic residues found in the core of the four-helix nexus ensure its stability (Fig. 1 b), while polar, relatively large noncanonical core residues (e.g., Gln-263, Tyr-267, and Lys-15) flanking both sides of the nexus (Fig. 1 b) may act as molecular wedges that favor coiled-coil splaying and also provide the freedom for α-helices to rotate into the overlap complex (1,10,13,17).
Our previous data (18) and work by others (4) suggest that tropomyosin isoform sequence diversity and overlap stiffness variability may contribute to differences in muscle and nonmuscle cytoskeletal behaviors, as well as to differences between the regulatory responses of skeletal and smooth-muscle tropomyosins (19). We note that at least in striated muscle, the additional interaction of the troponin T tail domain (TnT1) over the overlap region of tropomyosin has an apparent stiffening effect (18) (to increase cooperativity in intact thin filaments). The effect of TnT tends to equalize the rigidity of the striated-muscle tropomyosin overlap and the rest of the coiled-coil (18), whereas such augmentation may be unnecessary for the smooth-muscle tropomyosin overlap, which inherently is very stiff (18).
Functional implications of the F-actin-tropomyosin cable
The organization of tropomyosin on F-actin presented here (see Movie S1 and the pdb file in the Supporting Material) builds on previous models indicating that the central 240 amino acids of the 284-residue-long tropomyosin molecule as a canonical coiled-coil make periodic electrostatic contacts with residues Lys-326, Lys-328, Arg-147, and Asp-25 on six successive actin subunits along F-actin (1,7,9). The new model now shows the disposition of the remainder of tropomyosin and how the overlapping segments interact with a seventh actin. Here, Asp-20 and Glu-23 on N-terminal chains in the overlap nexus lying closest to actin are likely to contact actin residues Lys-326, Lys-328, Arg-147, while Lys-7 of the same chain contacts actin residue Asp-25 (Fig. 1 c; Table S1; Movie S1). Plainly, one of the two N-terminal α-helical chains remains close to the canonical configuration and maintains contact with actin, while the splayed partners deviate from the canonical twist. According to this analysis, the relative twist of consecutive tropomyosins molecules will be in phase with each other, as previously predicted (13).
It is striking that despite its distinctive structure, the overlap domain region still interacts with the consensus binding patches on actin subunits. Indeed, previous energy-landscape measurements defined an interaction energy minimum dominated by electrostatic interactions that optimized the azimuthal and axial localization of the mid-piece of tropomyosin (residues 20–264) to these sites on F-actin (9). The additional electrostatic association of the overlap domain with consensus actin-binding residues noted here also contributes to the attractive interaction between tropomyosin and actin (see Table S1).
Interestingly, point mutations locating to tropomyosin residues 8, 21, 22, and 23 are associated with various cardiomyopathies (20), suggesting that these residues are part of a critical locus needed for proper actin–tropomyosin interaction, as suggested by our analysis (see Fig. 1 c). Thus, in addition to providing insights into thin-filament organization and function, the complete actin-tropomyosin model presented here also maps likely connections between mutations in myofibrillar proteins and the development of myopathies (21).
Moreover, our results on the structural mechanics of tropomyosin on F-actin offer a straightforward and plausible mechanism for the assembly of the tropomyosin cable along thin filaments. Whereas the MD structure of head-to-tail overlap discussed above is relatively static, our previous work (13) and that of others (reviewed in Brown and Cohen (1)) show that the last 20 residues at the free ends of unpolymerized tropomyosin exhibit considerable bending, twisting, and splaying dynamics. In that case, the N- and C-termini behave as if they are searching for binding partners to link into a head-to-tail nexus and to begin to form a continuous tropomyosin cable. It follows that as tropomyosin polymerizes and populates the filament, its local conformational plasticity will then diminish. Hence, our studies provide essential molecular correlates to evaluate thin-filament mechanics and self-assembly mechanisms. Although we have addressed the molecular-level architecture of the striated muscle tropomyosin chain on F-actin, it is clear that the structures of many of the 40 or so tropomyosin isoforms will also need to be resolved before the structure-function relationships of the tropomyosin superfamily can be truly understood.
Acknowledgments
We thank Drs. David Atkinson and Jeffrey Moore for insightful discussions.
This work was supported by a grant from the National Institutes of Health (R37-HL036153 to W.L.). The Massachusetts Green High Performance Computing Center provided computational resources.
Contributor Information
Stefan Fischer, Email: stefan.fischer@iwr.uni-heidelberg.de.
William Lehman, Email: wlehman@bu.edu.
Supporting Material
The movie begins with a rotation (± ∼180°) of two adjacent canonical tropomyosin coiled-coils on F-actin that are linked together by the overlap structure, as shown in Figure 1a in the main text. The movie then zooms in on the tropomyosin overlapping domain, first highlighting hydrophobic residues (gray) and surrounding polar core residues (colored red, blue, pink, for acidic, basic and neutral amino acids as in Figure 1b), and then illustrates tropomyosin residues making electrostatic contacts with Lys326, Lys328 and Arg147 on adjacent consensus actin-binding patches (with the same red/blue color convention for acidic and basic residues on tropomyosin and on actin as in Figure 1c).
References
- 1.Brown J.H., Cohen C. Regulation of muscle contraction by tropomyosin and troponin: how structure illuminates function. Adv. Protein Chem. 2005;71:121–159. doi: 10.1016/S0065-3233(04)71004-9. [DOI] [PubMed] [Google Scholar]
- 2.Holmes K.C., Lehman W. Gestalt-binding of tropomyosin to actin filaments. J. Muscle Res. Cell Motil. 2008;29:213–219. doi: 10.1007/s10974-008-9157-6. [DOI] [PubMed] [Google Scholar]
- 3.Gordon A.M., Homsher E., Regnier M. Regulation of contraction in striated muscle. Physiol. Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 4.Gunning P.W., Schevzov G., Hardeman E.C. Tropomyosin isoforms: divining rods for actin cytoskeleton function. Trends Cell Biol. 2005;15:333–341. doi: 10.1016/j.tcb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Wang C.L., Coluccio L.M. New insights into the regulation of the actin cytoskeleton by tropomyosin. Int. Rev. Cell Mol. Biol. 2010;281:91–128. doi: 10.1016/S1937-6448(10)81003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hitchcock-DeGregori S.E. Tropomyosin: function follows structure. Adv. Exp. Med. Biol. 2008;644:60–72. doi: 10.1007/978-0-387-85766-4_5. [DOI] [PubMed] [Google Scholar]
- 7.Li X.E., Tobacman L.S., Lehman W. Tropomyosin position on F-actin revealed by EM reconstruction and computational chemistry. Biophys. J. 2011;100:1005–1013. doi: 10.1016/j.bpj.2010.12.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barua B., Fagnant P.M., Hitchcock-DeGregori S.E. A periodic pattern of evolutionarily conserved basic and acidic residues constitutes the binding interface of actin-tropomyosin. J. Biol. Chem. 2013;288:9602–9609. doi: 10.1074/jbc.M113.451161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orzechowski M., Moore J.R., Lehman W. Tropomyosin movement on F-actin during muscle activation explained by energy landscapes. Arch. Biochem. Biophys. 2014;545:63–68. doi: 10.1016/j.abb.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X.E., Orzechowski M., Fischer S. Structure and flexibility of the tropomyosin overlap junction. Biochem. Biophys. Res. Commun. 2014;446:304–308. doi: 10.1016/j.bbrc.2014.02.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenfield N.J., Huang Y.J., Hitchcock-DeGregori S.E. Solution NMR structure of the junction between tropomyosin molecules: implications for actin binding and regulation. J. Mol. Biol. 2006;364:80–96. doi: 10.1016/j.jmb.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Frye J., Klenchin V.A., Rayment I. Structure of the tropomyosin overlap complex from chicken smooth muscle: insight into the diversity of N-terminal recognition. Biochemistry. 2010;49:4908–4920. doi: 10.1021/bi100349a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehman W., Li X.E., Fischer S. The structural dynamics of α-tropomyosin on F-actin shape the overlap complex between adjacent tropomyosin molecules. Arch. Biochem. Biophys. 2014;552-553:68–73. doi: 10.1016/j.abb.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. 27–28. [DOI] [PubMed] [Google Scholar]
- 15.Brooks B.R., Brooks C.L., 3rd, Karplus M. CHARMM: the biomolecular simulation program. J. Comput. Chem. 2009;30:1545–1614. doi: 10.1002/jcc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips J.C., Braun R., Schulten K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Mui S., Cohen C. The crystal structure of the C-terminal fragment of striated-muscle α-tropomyosin reveals a key troponin T recognition site. Proc. Natl. Acad. Sci. USA. 2002;99:7378–7383. doi: 10.1073/pnas.102179999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sousa D., Cammarato A., Lehman W. Electron microscopy and persistence length analysis of semi-rigid smooth muscle tropomyosin strands. Biophys. J. 2010;99:862–868. doi: 10.1016/j.bpj.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehrer S.S., Morris E.P. Comparison of the effects of smooth and skeletal tropomyosin on skeletal actomyosin subfragment 1 ATPase. J. Biol. Chem. 1984;259:2070–2072. [PubMed] [Google Scholar]
- 20.Redwood C., Robinson P. α-Tropomyosin mutations in inherited cardiomyopathies. J. Muscle Res. Cell Motil. 2013;34:285–294. doi: 10.1007/s10974-013-9358-5. [DOI] [PubMed] [Google Scholar]
- 21.Marston S., Memo M., Lehman W. Mutations in repeating structural motifs of tropomyosin cause gain of function in skeletal muscle myopathy patients. Hum. Mol. Genet. 2013;22:4978–4987. doi: 10.1093/hmg/ddt345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The movie begins with a rotation (± ∼180°) of two adjacent canonical tropomyosin coiled-coils on F-actin that are linked together by the overlap structure, as shown in Figure 1a in the main text. The movie then zooms in on the tropomyosin overlapping domain, first highlighting hydrophobic residues (gray) and surrounding polar core residues (colored red, blue, pink, for acidic, basic and neutral amino acids as in Figure 1b), and then illustrates tropomyosin residues making electrostatic contacts with Lys326, Lys328 and Arg147 on adjacent consensus actin-binding patches (with the same red/blue color convention for acidic and basic residues on tropomyosin and on actin as in Figure 1c).


