Abstract
A majority of cancer diagnoses and deaths occur in patients age ≥ 65 years. With the aging of the US population, the number of older adults with cancer will grow. Although the coming wave of older patients with cancer was anticipated in the early 1980s, when the need for more research on the cancer-aging interface was recognized, many knowledge gaps remain when it comes to treating older and/or frailer patients with cancer. Relatively little is known about the best way to balance the risks and benefits of existing cancer therapies in older patients; however, these patients continue to be underrepresented in clinical trials. Furthermore, the available clinical trials often do not include end points pertinent to the older adult population, such as preservation of function, cognition, and independence. As part of its ongoing effort to advance research in the field of geriatric oncology, the Cancer and Aging Research Group held a conference in November 2012 in collaboration with the National Cancer Institute, the National Institute on Aging, and the Alliance for Clinical Trials in Oncology. The goal was to develop recommendations and establish research guidelines for the design and implementation of therapeutic clinical trials for older and/or frail adults. The conference sought to identify knowledge gaps in cancer clinical trials for older adults and propose clinical trial designs to fill these gaps. The ultimate goal of this conference series is to develop research that will lead to evidence-based care for older and/or frail adults with cancer.
INTRODUCTION
Cancer is a disease of aging, with the majority of patients age > 65 years.1 Cancer incidence is expected to increase by 67% among individuals age ≥ 65 years from 2010 to 2030.2 Furthermore, because those diagnosed with cancer are also living longer, the proportion of cancer survivors age ≥ 65 years will increase by 42% between 2010 and 2020.3 This demographic wave of older patients with cancer was anticipated as early as the 1980s, leading to calls for greater attention to geriatric oncology and for increasing the interface between cancer and aging. B.J. Kennedy, MD, then president of the American Society of Clinical Oncology, predicted that cancer and aging would become a major problem in the United States.4 Yancik et al5 described the changing age structure of the nation's population and the discrepancy between chronologic and physiologic age. They identified the pressing need for increased research on cancer and aging.
Although much has been learned about aging and cancer since then, few clinical trials focus on the therapeutic decisions most directly facing older adults. Historically, older adults have been underrepresented in cancer clinical trials, and recent updated data suggest that this remains a significant concern.6–8 As a result, there is a significant lack of information on the safety and efficacy of cancer treatment for the growing numbers of older patients with cancer. This becomes even more important because the biology of certain cancers changes with aging, and therefore, specific studies of the efficacy of therapeutic approaches are needed across the age spectrum.9–11 Despite the increased incidence and prevalence of cancer among older adults, the literature reports that age-related differences in treatment patterns persist, with older adults often receiving less aggressive therapy,12–19 despite the fact that many older patients with cancer can tolerate and benefit from cancer-directed therapies. For example, patients age 70 to 79 years with acute myeloid leukemia fare better with chemotherapy than patients receiving palliative care.20 Conversely, a subset of older adults may be at increased vulnerability to treatment-related toxicities. There is increased understanding that chronologic age is a weak marker of physiologic age and that factors captured in a geriatric assessment (GA) can identify older adults at risk for cancer treatment toxicities.21–26
Compounding the overall problem is the underrepresentation of older adults in therapeutic clinical trials6,7,27,28 (Fig 1). Twenty-eight percent of individuals diagnosed with cancer are age ≥ 75 years1; however, < 10% of patients enrolled onto National Cancer Institute (NCI) Cooperative Group clinical trials are age ≥ 75 years.7 Although the overall number of patients enrolled onto clinical trials has declined over the past decade, the proportion of older adults in these trials remains the same. However, the portfolio of studies in the NCI Cooperative Group clinical trials was dominated by accrual from breast cancer trials, which were particularly slanted toward a group of patients younger than the general population of patients with the disease. In other disease types, such as prostate cancer, the age distribution of patients enrolled onto clinical trials was more reflective of the larger population with the disease.
Fig 1.
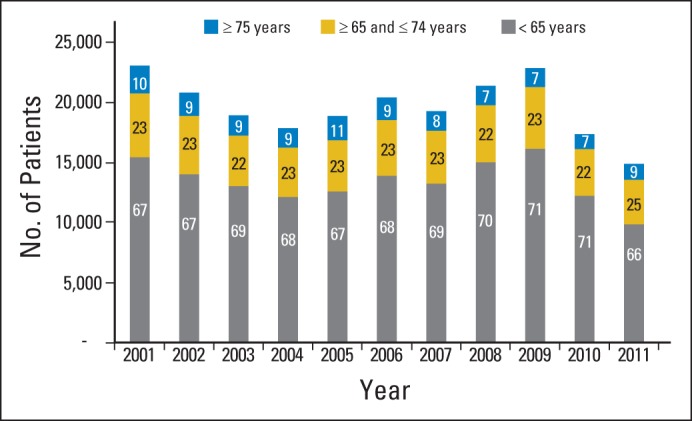
Age distribution for patients enrolled onto National Cancer Institute (NCI) adult cooperative group phase II and III treatment trials (all diseases) from 2001 to 2011. Percentage of patients enrolled in each age group is shown for each year, as reported by cooperative groups to the NCI Clinical Data Update System database (NCI Division of Cancer Treatment and Diagnosis) as of May 2012.
It is crucial to find ways to improve the accrual of healthy older adults to existing clinical trials and to develop research studies that address the knowledge gaps regarding older and/or frail adults who would not typically be enrolled onto standard trials. Some vital knowledge gaps may not be addressed by the larger phase III cancer trials for all ages. Those gaps affect the care of patients with physiologic decline and/or those with comorbid conditions that exclude them from certain clinical trials, placing them at increased risk for toxicity. Specific studies targeting those knowledge gaps are needed.
GOALS OF THE CONFERENCE ON OLDER AND/OR FRAIL PATIENTS IN THERAPEUTIC CLINICAL TRIALS
The Cancer and Aging Research Group (CARG), in collaboration with the National Institute on Aging (NIA) and the NCI, has been holding a conference series funded by a U13 grant to examine the level of evidence and areas of highest research priority in geriatric oncology, identify strengths in existing research methods, and foster multidisciplinary collaboration. The first of these conferences, held in September 2010, found that few therapeutic data exist for patients with cancer who are age ≥ 75 years or who have chronic health conditions.29–31 Conference participants further found that clinical, biologic, and physiologic markers of age are only rarely or inconsistently incorporated into clinical trials and that clinical trial infrastructure is often incompatible with the needs of older patients.
From November 17 to 18, 2012, CARG held the second conference in collaboration with the NIA, the NCI, and the Alliance of Clinical Trials in Oncology. The intent of this second conference was to focus on the design and implementation of therapeutic clinical trials for older and/or frail adults. The overall goal was to develop clinical trial designs specifically targeting questions that affect older and/or frailer adults with cancer, as well as to provide recommendations and create examples for others interested in geriatric oncology research. This article summarizes points raised at the conference and identifies common themes in geriatric oncology clinical trial design that emerged.
GERIATRICIZING TRIAL DESIGN
Defining the Study Population: Older and/or Frail Patients
The inclusion of older and/or frail patients in therapeutic clinical trials is hampered by difficulties in defining and recruiting this population. As one aspect of the research design considerations, an effort was made to define these groups for the purposes of cancer clinical trials.
Defining older.
Typically included in cancer clinical trials are the healthiest and most robust of older patients, with ready access to specialized cancer centers or clinical oncology programs.32 Those with second cancers or comorbidities including cognitive or functional impairments, cardiac disease, or organ dysfunction—all of which are more likely to occur among older patients—often are either explicitly excluded from or not actively enrolled onto clinical trials. Consequently, older patients typically seen by oncologists are less likely to be enrolled onto clinical trials, and those age > 75 years are especially unlikely to be included.6,7,27,28 Considering age alone, those age > 75 years are defined as older for purposes of recruitment and design efforts.
Defining frail.
Recognizing that a consensus has not yet been reached on a definition of frailty for oncology trials, one goal of this conference was to clarify the definition for older adults. A geriatric oncology definition of frailty is suggested: those older individuals who are at higher risk for cancer treatment toxicity because of age-associated conditions such as functional losses, cognitive impairment, or physiologic changes. This is distinct from, although may overlap with, the geriatrician's definition of frailty, which is a vulnerable health condition resulting in a decreased ability to respond to a stressor that is associated with a higher likelihood of functional decline, disability, hospitalization, and mortality.33,34 Two well-established ways of measuring frailty have been developed by Fried et al33 and Rockwood and Mitnitski.35 However, there are limitations in applying these definitions to geriatric oncology, because the definitions were developed for the general geriatric population and not specifically for older adults with cancer, whose physiologic stressors may be different. Often the stressors for a patient with cancer are surgery and/or chemotherapy, and tools are needed to identify older adults at risk for serious toxicity or functional loss resulting from these stressors. A way to apply this concept of frailty to older patients with cancer is beginning to emerge, with tools being developed to identify patients at high risk for chemotherapy toxicity.21,22
Study End Points
Therapeutic phase III clinical trials focus primarily on efficacy as measured by tumor response or overall and progression-free survival. However, these standard trial end points do not capture a key concept in geriatric medicine, which is maintenance of active life expectancy (ie, number of years an individual lives independently without significant disability). The effects of cancer therapies on physical or cognitive function could be just as important, if not more important, to older patients than response or survival.36 The inclusion of functional end points can aid in shared decision making by physicians and patients by identifying the most important areas for intervention.
Trial Designs: Opportunities, Strengths, and Weaknesses
Several study designs were proposed to help fill the gaps in knowledge regarding cancer therapy in older and/or frail adults. The advantages and limitations of these trial designs are summarized in Table 1.
Table 1.
Opportunities in Geriatric Oncology Clinical Trial Designs
| Design | Description and Characteristics | Potential Objectives and Outcomes | Advantages | Limitations and Vital Considerations | Clinical Trial Examples |
|---|---|---|---|---|---|
| RCT | RCTs are gold standard of clinical trial design; study participants are randomly assigned among different treatment arms | Determination of gold standard of treatment by comparing efficacy and tolerability of different outcomes | Excellent for direct comparison of different treatment regimens | Requires large sample size because of randomized design | CALGB 4990737 |
| Eligibility criteria considerations: Method 1: accrue only older patients Method 2: accrue patients of all ages, then stratify into age groups representative of general population with disease Trial design consideration: Adaptive (Bayesian) design; trial design is modified as study proceeds based on interim data analysis; randomization ratio can be altered by shifting patients to more effective treatment arm and eliminating underperforming arms |
Development of novel end points (including composite measures of tolerability and toxicity) | Method 1: Particularly important if there is age-related change in cancer biology Important for identifying treatment options among patients who are traditionally excluded from clinical trials because of chronic health conditions or concerns regarding toxicity and who would otherwise not enroll onto clinical trials for all age groups End points can be specifically tailored for geriatric oncology population Method 2: Allows for greater generalizability of results Would rectify current situation of older adults being under-represented on clinical trials |
Can be costly and time intensive Method 2: Could result in slower accrual due to the enrollment of specific age strata Lack of end points tailored specifically for geriatric oncology population |
PI, Hyman Muss; ClinicalTrials.gov ID, NCT00024102 Comparison of different adjuvant chemotherapy (standard treatment [AC or CMF] v new therapy [capecitabine]) treatments for older women with early-stage breast cancer |
|
| Prospective cohort study | Assessment of treatments currently on market to evaluate outcomes of interest in older patients | Identification of patterns of care | Enrollment of patients receiving standard-of-care treatment increases generalizability of findings | Treatment is not randomized; therefore, investigators unable to determine which treatment is most efficacious and least toxic for older patients | CALGB 36990138 |
| Cohort can be defined by host, tumor, or treatment factors | Understanding decision making | Can be used to understand patterns of care and decision making | Significant data management resources required to accurately capture drug dosing and toxicity data | PI, Jeanne Mandelblatt; ClinicalTrials.gov ID: NCT00068328 | |
| Observational No randomization Hypothesis driven |
Determination of toxicity and feasibility of delivering specific therapies | Patient preference as determinant of breast cancer adjuvant chemotherapy use in older women | |||
| Embedded study | Also known as correlative or ancillary study | Use of GA to describe cohort | Better baseline characterization of the geriatric oncology population that enters the study | The parent study may not be specifically targeted to older adults, thus limiting the sample size of older patients | CALGB 361006 |
| Additional measurements of interest to geriatric oncology research (such as GA measures) are placed within infrastructure of parent study | Use of GA in longitudinal follow-up to understand impact of therapy on function and other GA measures Identification of characteristics of specific group of patients who are at high risk for toxicity |
Ability to identify baseline predictors of treatment tolerance and/or longitudinal declines in function | If participation in embedded study is optional, then characteristics of patients who choose to enroll may not be representative of entire cohort and/or adequate sample size of older patients may not be accrued | PI, Heidi Klepin GA embedded in CALGB 1100139 PI, Geoffrey Uy; ClinicalTrials.gov ID for CALGB 11001, NCT01253070 Sorafenib tosylate and chemotherapy in treating older patients with AML |
|
| Single-arm trial | Current gold-standard design for phase II clinical trials | Evaluation of efficacy of drug for which there are limited data for older adults | Quantification of novel end points such as impact of therapy on functional status and QOL | No comparison of treatment under study with gold standard | CALGB 976240 |
| No randomization | Identification of predictors of toxicity based on GA variables or biomarkers | Fills gap in knowledge regarding efficacy, feasibility, and toxicity of drugs that have been understudied in older adults | PI, Stuart Lichtman; ClinicalTrials.gov ID, NCT00003092 | ||
| All patients receive treatment under study | Understanding of age-related changes in pharmacokinetics and pharmacodynamics of cancer therapeutics | Prospective evaluation of relationship of patient age and paclitaxel clinical pharmacology | |||
| Extended trial | Addition of cohort of older patients to treatment arm from RCT that was shown to be superior | Determination of tolerability of treatment in older adults | Trial infrastructure is already in place | Currently no precedent exists for reopening study several years after closure | No precedent |
| Accrual of older patients might be easier because efficacy of treatment has been previously demonstrated Additional data regarding tolerability in older patients will be obtained |
Accrual is only to superior arm to bolster data about tolerability in older adults; however, data regarding efficacy of treatment (compared with inferior arm) in older population will not be obtained |
Abbreviations: AC, doxorubicin and cyclophosphamide; AML, acute myeloid leukemia; CALGB, Cancer and Leukemia Group B; CMF, cyclophosphamide, methotrexate, and fluorouracil; GA, geriatric assessment; PI, principal investigators; QOL, quality of life; RCT, randomized controlled trial.
Randomized controlled trial.
The objective of this study design is to determine the gold standard of treatment using a randomized approach to ascertain the superiority (or lack of inferiority) of one treatment over another. Randomized controlled trials (RCTs) for older adults are particularly important if there are age-related changes in cancer biology that may affect treatment efficacy. Furthermore, they can include novel end points, such as composite measures of tolerability and treatment efficacy. However, RCTs can be costly and lengthy and require a large sample size.
Two approaches can be considered for the randomized design. First, the study could specifically focus on older adults and address questions that are most pertinent to the geriatric oncology population. An example is CALGB (Cancer and Leukemia Group B) 49907 (Alliance), which compared standard adjuvant polychemotherapy with monochemotherapy in adjuvant treatment for adults age ≥ 65 years with breast cancer. In this study, an adaptive Bayesian design was used,37 which allows for interim analysis of the accumulated data at specified time points. At these time points, if the treatment effect in one of the treatment arms satisfies a predefined futility boundary, accrual to that arm can be terminated while accrual to the other treatment arm(s) can be continued until the planned total sample size is reached. This study design is advantageous because of the potential for a smaller sample size requirement if the underperforming study arms are eliminated after interim data analysis.
The second approach is to accrue patients of all ages but purposefully stratify enrollment into age groups representative of the general population with the disease. An advantage of this approach is that the study results are more generalizable to the overall population with the disease. A disadvantage of this approach is that requiring enrollment of specific age strata may limit accrual speed. Furthermore, the study objectives and end points may not be tailored to the geriatric oncology population.
Prospective cohort study.
In a prospective cohort study, the cohort can be defined by the host, tumor, or treatment characteristics, depending on the research question. This design can be used to answer commonly posed questions in geriatric oncology regarding the feasibility, dosing, and toxicity of a selected regimen, particularly among patients receiving treatment as standard of care. A significant limitation is that this design does not identify the best treatment (ie, most efficacious and least toxic), because there is no randomized component. Furthermore, as with an RCT, significant data management resources are required to accurately capture and enter the dosing and toxicity data.
Another type of prospective cohort study is exemplified by CALGB 369901, which prospectively observed older women with nonmetastatic breast cancer receiving adjuvant treatment to understand treatment decision-making, quality-of-life, and survivorship issues.38 This study was open to accrual in parallel with CALGB 49907.37 Those patients who did not enroll onto 49907 were eligible for this prospective cohort study.
Embedded study.
An embedded study, also known as a correlative or ancillary study, is placed within the infrastructure of a parent study. An embedded study can be used to identify the characteristics of those patients at high risk for toxicity and to evaluate the toxicity profile of new drugs. An example is CALGB 361006, which embeds a comprehensive GA within the schema of CALGB 11001,39 a trial evaluating the efficacy of adding sorafenib tosylate to induction and postremission chemotherapy in patients age ≥ 60 years with FLT3-mutated acute myeloid leukemia. The goal of the companion substudy is to identify specific comprehensive GA measures that may predict overall survival and treatment-related mortality for older adults receiving this treatment. Several considerations in this study design are important. First, if participation in the embedded study is optional, a skewed sample may be accrued, limiting generalizability. Furthermore, the sample size of the embedded study should be determined a priori to reach the target accrual necessary to identify a vulnerable subgroup. A limitation of this design is that the parent study may not be specifically targeted to older adults. In such a case, there may be limited accrual of older adults to the embedded study.
Single-arm trial.
Single-arm trials can be used to assess the benefits and toxicities of specific drugs for which there are limited data in older adults. Additional advantages of a one-arm trial design are that novel end points such as the impact of therapy on function and quality of life can be assessed, and age-related changes in the pharmacology of cancer treatment can be evaluated. The addition of a younger cohort of patients can bolster the ability to identify age-related changes in pharmacokinetics across the age spectrum. The disadvantage of a single-arm trial is that it does not compare the study treatment with a gold standard.
An example of a single-arm trial is CALGB 9762,40 a prospective evaluation of the relationship between patient age and paclitaxel clinical pharmacology. This study sought to prospectively evaluate the association between patient age and the pharmacokinetics and toxicity profile of paclitaxel, as well as to understand the relationship between paclitaxel pharmacokinetics and toxicity.
Extended trial.
The extended trial design is a novel concept discussed at the conference, with no precedent to our knowledge. The goal of the extended trial design is to obtain data regarding a new gold standard within the older population. For example, once the results of a phase III study have been reported, the age distribution of the participants in the superior arm is examined. If the study failed to accrue an age distribution similar to the population of individuals at risk, the superior arm is reopened to accrue an adequate number of older adults. This study design aims to fill the knowledge gap regarding the tolerability of a new regimen in older cohorts. The limitation of this (hypothetic) design is that there is no precedent for reopening a study several years after the study has been closed. This would therefore require a shift in the present paradigm for the conduct of clinical trials. Furthermore, the extended trial design would not establish age-related differences in treatment efficacy between study arms included in the original randomized trial. Alternatively, if reopening a phase III study is considered too large a barrier to overcome, phase IV studies could potentially evaluate the tolerability of the new standard in populations with the disease that were underrepresented in the original study; however, there is no precedent for this approach either.
Considerations for Dosing Schema
The significant underrepresentation of older adults in US Food and Drug Administration registration trials8,28 has led to a dearth of information regarding the optimum dose and schedule of cancer therapeutics for the geriatric population. Differences in treatment patterns between older and younger adults have been noted.12–19 Concerns about the risk of toxicity may influence a health care provider's willingness to deliver the full chemotherapy dose with the first cycle of treatment, particularly if the treatment goal is palliation. In the geriatric literature, the adage “start low and go slow” may increase both the physician's and older patient's comfort with a new regimen, particularly when there are concerns about heightened toxicity risks. A way of applying this principle to geriatric oncology trials is to reduce the first dose, then escalate to standard dosage if the patient tolerates the treatment well. This approach was used in the FOCUS2 (Fluorouracil, Oxaliplatin, and CPT-11 [irinotecan]: Use and Sequencing 2) trial41 for older and/or frail adults with metastatic colorectal cancer. A potential downside of this approach is that patients would not receive a standard dose upfront, which could compromise efficacy. However, if dose escalation is performed rapidly, this is unlikely to have a major impact. Furthermore, it is not clear that the dose-reduced approach is associated with decreased toxicity. If this approach is used, it is favored in patients who are receiving therapy for metastatic disease, not for adjuvant treatment, where standard dosing should be used in those undergoing treatment with curative intent.
Trial Designs to Predict Treatment Tolerability
The general goal of studies to predict treatment tolerability is to develop risk-adapted strategies for treatment by identifying the profile (by toxicity risk, life expectancy, and/or tumor biology) of individuals who can or cannot tolerate a specific treatment. This optimizes the benefit-to-risk ratio. The aging process is heterogeneous, making chronologic age a relatively poor marker of overall physiologic and health status. Inclusion of a GA can help to deconstruct this heterogeneity by providing information regarding independent predictors of morbidity and mortality, such as functional status, comorbidities, nutritional status, psychologic state, social support, and cognitive function.42 These can be included as predictor and/or outcome variables. For example, the GA could be used at study entry as a predictor of treatment tolerability. Furthermore, the GA could be collected in longitudinal follow-up to understand the impact of treatment on GA variables (eg, function or cognition). Three potential trial designs were discussed.
All-comers design.
A key question in geriatric oncology is whether there is a subgroup of older patients who are at higher risk for toxicity. This trial design enrolls all comers with the goal of identifying the specific characteristics of patients who derive benefit from the treatment without significant toxicity, typically defined as grade ≥ 3, according to the NCI Common Terminology Criteria for Adverse Events, or grade 2, determined a priori to be of relevance.
Enrichment design.
If there is confidence that a specific group of individuals is at high risk for toxicity, an enrichment design allows the trial to accrue patients with those specific characteristics. To use an enrichment design, there must be agreement about risk factors for toxicity. However, a uniform definition of patients at high risk for toxicity has not yet been formally established within the geriatric oncology community. Recent research studies are starting to provide an evidence-based definition.21,22
Marker-by-treatment interaction design.
A marker-by-treatment design compares the risks and benefits of two treatment strategies for two groups of older patients: those predicted to be at low risk for toxicity versus those predicted to be at high risk for toxicity, based on a prespecified definition. At entry, eligible patients are stratified based on this toxicity risk and are subsequently randomly assigned to the treatment arms. In oncology clinical trials, a typical paradigm has been to add treatments to the gold standard to see if the efficacy can be improved. However, the cumulative addition of therapeutic agents can increase the risk of toxicity. If the toxicity exceeds a threshold, efficacy may be compromised because of the inability to deliver the therapy. The marker-by-treatment design can help weigh the risks and benefits of novel therapies (in comparison with the standard) between patients with different predicted risks of toxicity. A disadvantage of this approach is the requirement of a large sample to accomplish the study objectives.
Facilitating Enrollment of Older Adults
Older age alone should not be a contraindication to clinical trial enrollment; however, older adults are underrepresented in cancer clinical trials.6,7,27,28,43 One study found that older age was the sole reason why otherwise eligible patients were not offered clinical trial enrollment.44 Often a combination of patient-, provider-, study-, and system-related barriers may keep older patients with cancer from participating in therapeutic clinical trials.45–51 For example, patient nonparticipation has been attributed to wanting a different therapy,52 living too far from the cancer center,52,53 worrying about insurance reimbursements,52,53 or being ruled ineligible because of poor performance status, need for emergent therapy, or number of comorbid conditions.53 A lack of social support or a reluctance to travel to university centers where trials are most often conducted are additional deterrents to trial enrollment among older patients.54–56
Nevertheless, attitudes of older patients with cancer have not been shown to significantly result in lower enrollment. A majority of older patients report a positive attitude toward cancer clinical trials,57 and a survey of patients age > 70 years found that three quarters of these patients are willing to participate in clinical trials.58 Physician recommendations play an important role in patients' decisions regarding trials,58 and physician bias can be one of the main barriers to the enrollment of older patients.54
Overly restrictive eligibility criteria are also commonly cited as a reason for accrual difficulties, particularly for older and/or frail patients. Criteria that are too stringent jeopardize the generalizability of a study; however, criteria that are overly broad can jeopardize patient safety and generate an overly heterogeneous study population, which interferes with detecting a treatment effect. The reduction or elimination of irrelevant criteria that hinder enrollment and the better use of instruments that assess prognosis and risks for toxicity can improve inclusion criteria.
Difficulties in identifying appropriate clinical trials, as well as the complexity of the trials themselves, can impede recruitment of older adults. Reducing the complexity of study schemas as well as the number of correlative studies may increase study participation. The experience itself may also be enhanced by simply providing supportive settings that include such things as soundproof curtains, bedside hearing and visual assistance devices, nonskid floor surfaces that help prevent falls, natural lighting conditions to counteract sensory losses, safety measures geared for individuals with comorbidities, and resources and support infrastructure for caregivers.59,60 Culturally appropriate recruitment approaches and technology that allows remote data collection could also improve recruitment by eliminating the need for frequent travel to major medical centers. Collaboration between geriatricians and oncologists from the outset, as well as geriatric training for support staff, would facilitate the design and implementation of clinical trials to make them more amenable to the participation of older and/or frail patients.
CANCER SURVIVORS: TOPIC FOR THE NEXT U13 CONFERENCE
The number of cancer survivors increased from almost 4 million in 1977 to 13.7 million in 2012, and this number is expected to reach 18 million in the next 10 years.61 Approximately 60% of today's cancer survivors are age ≥ 65 years,3 and this number will steadily increase because of an overall rise in life expectancy and advances in early detection and cancer treatment. Approximately 16% of new diagnoses occur in individuals who already have a history of cancer, and this proportion is expected to increase.62 There is much to learn about caring for cancer survivors, accounting for both the risks of subsequent cancers as well as the immediate and longer-term effects of treatment.
Therapeutic clinical trials can address these issues by gathering pretreatment and follow-up data such as that captured in a geriatric assessment, along with information on socioeconomic status and access to health resources, social support (or more importantly, among the aging population, social isolation), and modifiable factors such as smoking history, nutrition status, signs of depression, and level of physical activity. The next U13 conference (scheduled for May 2015) will address these questions.
DISCUSSION
Cancer is associated with aging, and although a majority of cancer diagnoses occur in individuals age ≥ 65 years, these patients continue to be underrepresented in cancer research and clinical trials. In addition, the standard clinical trial design rarely addresses end points of particular interest to older adults (such as preservation of function). To increase the enrollment of older adults onto clinical trials, clinical trials must be developed specifically for those individuals who do not meet the eligibility criteria or are not fit enough for enrollment onto clinical trials focused on individuals of all ages.
We have presented the results of a recent U13 conference held by CARG in collaboration with the NIA, the NCI, and the Alliance of Clinical Trials in Oncology, including proposals for improved clinical trial designs and their advantages and disadvantages for the geriatric oncology population. These proposals can serve as a blueprint for individuals who are entering or engaged in the field of geriatric oncology research and help in the consideration of trial designs that are best suited to answer the research questions they are posing. Ultimately, there is hope that this ongoing conference series will contribute to substantial enhancement of the evidence base so critical for the adequate treatment of older and/or frail individuals with cancer.
Acknowledgment
We thank Basil Eldadah, MD, PhD (program officer, Geriatrics Branch, Division of Geriatrics and Clinical Gerontology, National Institute on Aging), for his participation in this U13 conference series. We thank Frances McFarland Horne and Carol Pearce for their assistance in preparing the manuscript.
Appendix
Attendees at the U13 Conference, Chicago, IL, November 17-18, 2012 (affiliation at time of conference): Andrew Artz (University of Chicago), Lodovico Balducci (H. Lee Moffitt Cancer Center), Karla Ballman (Mayo Clinic), Myra Barginear (Hofstra North Shore–LIJ School of Medicine), Beverly Canin (Breast Cancer Action, Breast Cancer Options), Ben Clark (American Society of Clinical Oncology), Harvey Cohen (U13 Oversight Board member; Duke University), William Dale (U13 Oversight Board member; University of Chicago), Efrat Dotan (Fox Chase Cancer Center), Basil Eldadah (U13 Oversight Board member; National Institutes of Health, National Institute on Aging), Susan Ellenberg (University of Pennsylvania), Martine Extermann (U13 Oversight Board member; H. Lee Moffitt Cancer Center), Betty Ferrell (U13 Oversight Board member; City of Hope National Medical Center), Gini Fleming (University of Chicago), David Flores (University of Texas), Ajeet Gajra (SUNY Upstate Medical University), Ilene Galinsky (Dana-Farber Cancer Institute), Richard Goldberg (North Carolina Cancer Hospital), Abdo Haddad (Cleveland Clinic), Paul Hamlin (Memorial Sloan-Kettering Cancer Center), Holly Holmes (MD Anderson Cancer Center), Joleen Hubbard (Mayo Clinic), Arti Hurria (U13 Oversight Board member; City of Hope National Medical Center), Aminah Jatoi (Mayo Clinic), Gretchen G. Kimmick (Duke University), Heidi Klepin (Wake Forest University), Marianna Koczywas (City of Hope National Medical Center), Ronald Maggiore (University of Chicago), Allison Magnuson (University of Rochester), Supriya Mohile (U13 Oversight Board member; University of Rochester), Margaret Mooney (National Institutes of Health, National Cancer Institute), Vicki A. Morrison (University of Minnesota), Ewa Mrozek (Ohio State University), Hyman Muss (U13 Oversight Board member; University of North Carolina Chapel Hill), Arash Naeim (University of California Los Angeles), Nitya Nathwani (City of Hope National Medical Center), Rebecca Olin (University of California San Francisco), Cynthia Owusu (Case Western Reserve University), Ira Parker (University of California San Diego), Carolyn Presley (Yale University), Erika Ramsdale (University of Chicago), Arati Rao (Duke University), Marilyn Raymond (American Society of Clinical Oncology), Ellen Ritchie (New York Presbyterian/Weill Cornell), Miriam Rodin (Saint Louis University), Julia Rowland (National Institutes of Health, National Cancer Institute), Saleha Sajid (University of Chicago), Richard Schilsky (U13 Oversight Board member; University of Chicago), Armin Shahrokni (University of California Los Angeles), Dale Shepard (Cleveland Clinic), Walter Stadler (University of Chicago), Richard Stone (Dana-Farber Cancer Institute), William Tew (Memorial Sloan-Kettering Cancer Center), Pamela Valera (Albert Einstein College of Medicine), Tanya Wildes (Washington University School of Medicine), and Elizabeth Won (Memorial Sloan-Kettering Cancer Center).
Footnotes
Written on behalf of the Cancer and Aging Research Group.
Supported by Grant No. U13 AG038151 from the National Institute on Aging and National Cancer Institute (NCI; National Institutes of Health); by the Alliance for Clinical Trials in Oncology; and by City of Hope and in part by Grants No. CA31946 from the NCI to the Alliance for Clinical Trials in Oncology and No. CA33601 to the Alliance Statistics and Data Center.
Presented at the 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013, and 2013 American Geriatrics Society Annual Meeting, Dallas, TX, May 2-5, 2013.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Arti Hurria, GTx (C), Seattle Genetics (C); Hyman B. Muss, Pfizer (C), Eisai (C) Stock Ownership: None Honoraria: None Research Funding: Arti Hurria, GlaxoSmithKline, Celgene; Martine Extermann, GTx Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Arti Hurria, William Dale, Karla V. Ballman, Harvey J. Cohen, Betty Ferrell, Kenneth E. Schmader, Supriya G. Mohile
Administrative support: Hyman B. Muss
Collection and assembly of data: Arti Hurria, William Dale, Margaret Mooney, Supriya G. Mohile
Data analysis and interpretation: Arti Hurria, William Dale, Margaret Mooney, Julia H. Rowland, Harvey J. Cohen, Hyman B. Muss, Richard L. Schilsky, Martine Extermann, Kenneth E. Schmader, Supriya G. Mohile
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Howlander N, Noone NA, Krapcho M, et al., editors. Cancer Statistics Review, 1975-2010. http://seer.cancer.gov/csr/1975_2010/
- 2.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 3.Parry C, Kent EE, Mariotto AB, et al. Cancer survivors: A booming population. Cancer Epidemiol Biomarkers Prev. 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy BJ. Aging and cancer. J Clin Oncol. 1988;6:1903–1911. doi: 10.1200/JCO.1988.6.12.1903. [DOI] [PubMed] [Google Scholar]
- 5.Yancik R, Carbone PP, editors. New York, NY: Raven Press; 1983. Perspectives on Prevention and Treatment of Cancer in the Elderly. [Google Scholar]
- 6.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 7.Mooney MM. Older patients in NCI-sponsored clinical treatment trials. http://meetinglibrary.asco.org/content/68859?media=vm.
- 8.Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: Known problem, little progress. J Clin Oncol. 2012;30:2036–2038. doi: 10.1200/JCO.2012.41.6727. [DOI] [PubMed] [Google Scholar]
- 9.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fröhling S, Schlenk RF, Kayser S, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: Results from AMLSG trial. AML HD98-B. Blood. 2006;108:3280–3288. doi: 10.1182/blood-2006-04-014324. [DOI] [PubMed] [Google Scholar]
- 11.Leith CP, Kopecky KJ, Chen IM, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: A Southwest Oncology Group Study. Blood. 1999;94:1086–1099. [PubMed] [Google Scholar]
- 12.DeMichele A, Putt M, Zhang Y, et al. Older age predicts a decline in adjuvant chemotherapy recommendations for patients with breast carcinoma: Evidence from a tertiary care cohort of chemotherapy-eligible patients. Cancer. 2003;97:2150–2159. doi: 10.1002/cncr.11338. [DOI] [PubMed] [Google Scholar]
- 13.Bouchardy C, Rapiti E, Fioretta G, et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol. 2003;21:3580–3587. doi: 10.1200/JCO.2003.02.046. [DOI] [PubMed] [Google Scholar]
- 14.Smith TJ, Penberthy L, Desch CE, et al. Differences in initial treatment patterns and outcomes of lung cancer in the elderly. Lung Cancer. 1995;13:235–252. doi: 10.1016/0169-5002(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 15.Houterman S, Janssen-Heijnen ML, Verheij CD, et al. Greater influence of age than co-morbidity on primary treatment and complications of prostate cancer patients: An in-depth population-based study. Prostate Cancer Prostatic Dis. 2006;9:179–184. doi: 10.1038/sj.pcan.4500868. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz KL, Alibhai SM, Tomlinson G, et al. Continued undertreatment of older men with localized prostate cancer. Urology. 2003;62:860–865. doi: 10.1016/s0090-4295(03)00690-3. [DOI] [PubMed] [Google Scholar]
- 17.Hurria A, Wong FL, Villaluna D, et al. Role of age and health in treatment recommendations for older adults with breast cancer: The perspective of oncologists and primary care providers. J Clin Oncol. 2008;26:5386–5392. doi: 10.1200/JCO.2008.17.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrag D, Cramer LD, Bach PB, et al. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93:850–857. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 19.Berry MF, Worni M, Pietrobon R, et al. Variability in the treatment of elderly patients with stage IIIA (N2) non-small-cell lung cancer. J Thorac Oncol. 2013;8:744–752. doi: 10.1097/JTO.0b013e31828916aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 21.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 23.Aparicio T, Jouve JL, Teillet L, et al. Geriatric factors predict chemotherapy feasibility: Ancillary results of FFCD 2001-02 phase III study in first-line chemotherapy for metastatic colorectal cancer in elderly patients. J Clin Oncol. 2013;31:1464–1470. doi: 10.1200/JCO.2012.42.9894. [DOI] [PubMed] [Google Scholar]
- 24.Wildes TM, Ruwe AP, Fournier C, et al. Geriatric assessment is associated with completion of chemotherapy, toxicity, and survival in older adults with cancer. J Geriatr Oncol. 2013;4:227–234. doi: 10.1016/j.jgo.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramjaun A, Nassif MO, Krotneva S, et al. Improved targeting of cancer care for older patients: A systematic review of the utility of comprehensive geriatric assessment. J Geriatr Oncol. 2013;4:271–281. doi: 10.1016/j.jgo.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Extermann M, Wedding U. Comorbidity and geriatric assessment for older patients with hematologic malignancies: A review of the evidence. J Geriatr Oncol. 2012;3:49–57. [Google Scholar]
- 27.Trimble EL, Carter CL, Cain D, et al. Representation of older patients in cancer treatment trials. Cancer. 1994;74(suppl):2208–2214. doi: 10.1002/1097-0142(19941001)74:7+<2208::aid-cncr2820741737>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 29.Dale W, Mohile SG, Eldadah BA, et al. Biological, clinical, and psychosocial correlates at the interface of cancer and aging research. J Natl Cancer Inst. 2012;104:581–589. doi: 10.1093/jnci/djs145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurria A, Mohile SG, Dale W. Research priorities in geriatric oncology: Addressing the needs of an aging population. J Natl Compr Canc Netw. 2012;10:286–288. doi: 10.6004/jnccn.2012.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohile S, Dale W, Hurria A. Geriatric oncology research to improve clinical care. Nat Rev Clin Oncol. 2012;9:571–578. doi: 10.1038/nrclinonc.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross CP, Herrin J, Wong N, et al. Enrolling older persons in cancer trials: The effect of sociodemographic, protocol, and recruitment center characteristics. J Clin Oncol. 2005;23:4755–4763. doi: 10.1200/JCO.2005.14.365. [DOI] [PubMed] [Google Scholar]
- 33.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 34.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology—Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 35.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 36.Fried TR, Bradley EH, Towle VR, et al. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 37.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandelblatt JS, Sheppard VB, Hurria A, et al. Breast cancer adjuvant chemotherapy decisions in older women: The role of patient preference and interactions with physicians. J Clin Oncol. 2010;28:3146–3153. doi: 10.1200/JCO.2009.24.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Cancer Institute. Sorafenib tosylate and chemotherapy in treating older patients with acute myeloid leukemia. http://clinicaltrials.gov/ct2/show/study/NCT01253070.
- 40.Lichtman SM, Hollis D, Miller AA, et al. Prospective evaluation of the relationship of patient age and paclitaxel clinical pharmacology: Cancer and Leukemia Group B (CALGB 9762) J Clin Oncol. 2006;24:1846–1851. doi: 10.1200/JCO.2005.03.9289. [DOI] [PubMed] [Google Scholar]
- 41.Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): An open-label, randomised factorial trial. Lancet. 2011;377:1749–1759. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puts MT, Hardt J, Monette J, et al. Use of geriatric assessment for older adults in the oncology setting: A systematic review. J Natl Cancer Inst. 2012;104:1133–1163. doi: 10.1093/jnci/djs285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yee KW, Pater JL, Pho L, et al. Enrollment of older patients in cancer treatment trials in Canada: Why is age a barrier? J Clin Oncol. 2003;21:1618–1623. doi: 10.1200/JCO.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 44.Kemeny MM, Peterson BL, Kornblith AB, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003;21:2268–2275. doi: 10.1200/JCO.2003.09.124. [DOI] [PubMed] [Google Scholar]
- 45.Horn L, Keedy VL, Campbell N, et al. Identifying barriers associated with enrollment of patients with lung cancer into clinical trials. Clin Lung Cancer. 2013;14:14–18. doi: 10.1016/j.cllc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Eads J. Identification of barriers to clinical trials: The impact of education level. J Clin Oncol. 2011;29(suppl):384s. abstr CRA6003. [Google Scholar]
- 47.Spaar A, Frey M, Turk A, et al. Recruitment barriers in a randomized controlled trial from the physicians' perspective: A postal survey. BMC Med Res Methodol. 2009;9:14. doi: 10.1186/1471-2288-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howerton MW, Gibbons MC, Baffi CR, et al. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer. 2007;109:465–476. doi: 10.1002/cncr.22436. [DOI] [PubMed] [Google Scholar]
- 49.Gross CP, Murthy V, Li Y, et al. Cancer trial enrollment after state-mandated reimbursement. J Natl Cancer Inst. 2004;96:1063–1069. doi: 10.1093/jnci/djh193. [DOI] [PubMed] [Google Scholar]
- 50.Gross CP, Wong N, Dubin JA, et al. Enrollment of older persons in cancer trials after the medicare reimbursement policy change. Arch Intern Med. 2005;165:1514–1520. doi: 10.1001/archinte.165.13.1514. [DOI] [PubMed] [Google Scholar]
- 51.Cooley ME, Sarna L, Brown JK, et al. Challenges of recruitment and retention in multisite clinical research. Cancer Nurs. 2003;26:376–384. doi: 10.1097/00002820-200310000-00006. quiz 385-386. [DOI] [PubMed] [Google Scholar]
- 52.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 53.Baggstrom MQ, Waqar SN, Sezhiyan AK, et al. Barriers to enrollment in non-small cell lung cancer therapeutic clinical trials. J Thorac Oncol. 2011;6:98–102. doi: 10.1097/JTO.0b013e3181fb50d8. [DOI] [PubMed] [Google Scholar]
- 54.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23:3112–3124. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 55.Penberthy L, Brown R, Wilson-Genderson M, et al. Barriers to therapeutic clinical trials enrollment: Differences between African-American and White cancer patients identified at the time of eligibility assessment. Clin Trials. 2012;9:788–797. doi: 10.1177/1740774512458992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basche M, Barón AE, Eckhardt SG, et al. Barriers to enrollment of elderly adults in early-phase cancer clinical trials. J Oncol Pract. 2008;4:162–168. doi: 10.1200/JOP.0842001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Comis RL, Miller JD, Aldigé CR, et al. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21:830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 58.Townsley CA, Chan KK, Pond GR, et al. Understanding the attitudes of the elderly towards enrolment into cancer clinical trials. BMC Cancer. 2006;6:34. doi: 10.1186/1471-2407-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartocollis A. For the elderly, emergency rooms of their own. New York Times. 2012 Apr 10;:A1. [Google Scholar]
- 60.Hwang U, Morrison RS. The geriatric emergency department. J Am Geriatr Soc. 2007;55:1873–1876. doi: 10.1111/j.1532-5415.2007.01400.x. [DOI] [PubMed] [Google Scholar]
- 61.de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: Prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22:561–570. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mariotto AB, Rowland JH, Ries LA, et al. Multiple cancer prevalence: A growing challenge in long-term survivorship. Cancer Epidemiol Biomarkers Prev. 2007;16:566–571. doi: 10.1158/1055-9965.EPI-06-0782. [DOI] [PubMed] [Google Scholar]


