SUMMARY
Soluble Aβ oligomers contribute importantly to synaptotoxicity in Alzheimer's disease, but their dynamics in vivo remain unclear. Here, we found that soluble Aβ oligomers were sequestered from brain interstitial fluid onto brain membranes much more rapidly than non-toxic monomers and were recovered in part as bound to GM1 ganglioside on membranes. Aβ oligomers bound strongly to GM1 ganglioside, and blocking the sialic acid residue on GM1 decreased oligomer-mediated LTP impairment in mouse hippocampal slices. In a hAPP transgenic mouse model, substantial levels of GM1-bound Aβ42 were recovered from brain membrane fractions. We also detected GM1-bound Aβ in human CSF, and its levels correlated with Aβ42, suggesting its potential as a biomarker of Aβ-related membrane dysfunction. Together, these findings highlight a novel mechanism whereby hydrophobic Aβ oligomers become sequestered onto GM1 ganglioside and presumably other lipids on neuronal membranes, where they may induce progressive functional and structural changes.
INTRODUCTION
The most prevalent neurodegenerative disorder, Alzheimer's disease (AD) impairs episodic declarative memory and executive function early in its clinical phase. Enhanced synapse loss, particularly in the temporal and frontal cortices, helps distinguish AD from normal brain aging (West et al., 1994) and serves as a strong correlate of cognitive decline (Terry et al., 1991). Research in many laboratories has provided extensive evidence that AD is initially a disorder of selected synapses in which soluble, low molecular weight (LMW) oligomers of amyloid β-protein (Aβ) can act as prime synaptotoxic agents (reviewed in Mucke and Selkoe, 2012). In one example, mice expressing an APP mutation that causes AD in humans underwent rapid formation and stabilization of Aβ oligomers accompanied by profound synaptic and neuronal loss in the absence of fibrillar amyloid plaques in the cortex (Tomiyama et al., 2010). In light of numerous such studies implicating a pathogenic role of soluble Aβ oligomers and the evidence that lowered Aβ42 monomer levels in CSF represents the earliest biomarker for AD (Bateman et al., 2012; Craig-Schapiro et al., 2009; Fagan et al., 2009; Golde et al., 2011; Morris and Selkoe, 2011), the search for such oligomers in biological fluids, primarily in human cerebrospinal fluid (CSF), has intensified in recent years (Benilova et al., 2012). The recent report that a Phase 3 trial of the Aβ-specific monoclonal antibody solanezumab produced a small but significant cognitive benefit in patients with mild AD (Doody, 2012) has made it even more critical to understand the earliest changes in the economy of synaptotoxic Aβ oligomers in the brain and biological fluids. A few reports of the detection of apparent Aβ oligomers in CSF and plasma have appeared (Fukumoto et al., 2010; Gao et al., 2010; Klyubin et al., 2008; Villemagne et al., 2010); however, the interpretation of these reports has been clouded by failure to define the precise oligomeric unit the assays are detecting, an inability to exclude definitively the detection of Aβ monomers, and in some cases, the lack of validating the assays on natural oligomers in biological samples.
In this context, we have systematically examined the steady-state levels of LMW Aβ oligomers in aqueous compartments of the central nervous system (CNS), i.e., the brain interstitial fluid (ISF) and CSF of a well-characterized mouse model of AD, the J20 hAPP transgenic (tg) line (Mucke et al., 2000). While we readily detected monomers, we failed to detect LMW (<35 kDa) Aβ oligomers in the CNS fluid compartment using several biochemical methods. We then hypothesized that due to their increased hydrophobicity, Aβ oligomers may bind to cell membranes, pre-existing Aβ aggregates (in plaque-containing brains) or other hydrophobic surfaces much more rapidly than monomers do. If so, this could help clarify potential mechanisms behind observations that Aβ oligomers, but not monomers, can exert synaptotoxicity (Gong et al., 2003; Klyubin et al., 2005; Lacor et al., 2004; Lambert et al., 1998; Lesné et al., 2006; Shankar et al., 2007; 2008; Walsh et al., 2002), alter tau and other cytoskeletal proteins (Gotz et al., 2001; Jin et al., 2011; Lewis et al., 2001; Oddo et al., 2004; Zempel et al., 2010), and induce other cytotoxic effects. To address this concept, we quantified the in vivo half-life of microinjected Aβ dimers vs. monomers in the ISF of healthy, wild-type (wt) mice using microdialysis and ISF injections of synthetic Aβ dimers and natural oligomers isolated from human (AD) brain tissue. We followed the fate of the microinjected Aβ and found that most of it became tightly bound to endogenous GM1 ganglioside on membrane extracts of the brains. In vitro binding assays indicated that Aβ dimers bind to GM1 ganglioside much more strongly than monomers. Masking the sialic acid residue on GM1 decreased Aβ oligomer-mediated LTP inhibition in wt mouse hippocampal slices. Next, we examined brain membrane extracts of J20 tg mice prior to plaque formation and recovered a significantly higher level of endogenous Aβ42 than Aβ40 complexed with GM1. The levels of GM1-Aβ complexes obtained from brain membrane extracts were inversely proportional to the levels of free ISF Aβ with age, suggesting that the formation of the GM1-Aβ complexes is a dynamic equilibrium. Finally, using a sensitive immunoprecipitation (IP) technique, we discovered low levels of GM1-bound Aβ directly in human CSF, and its levels correlated significantly with the CSF levels of Aβ42. The implications of these new findings for understanding the dynamic economy of soluble Aβ oligomers in the brain are discussed.
RESULTS
Steady-state levels of LMW Aβ oligomers in CNS fluids are very low or undetectable
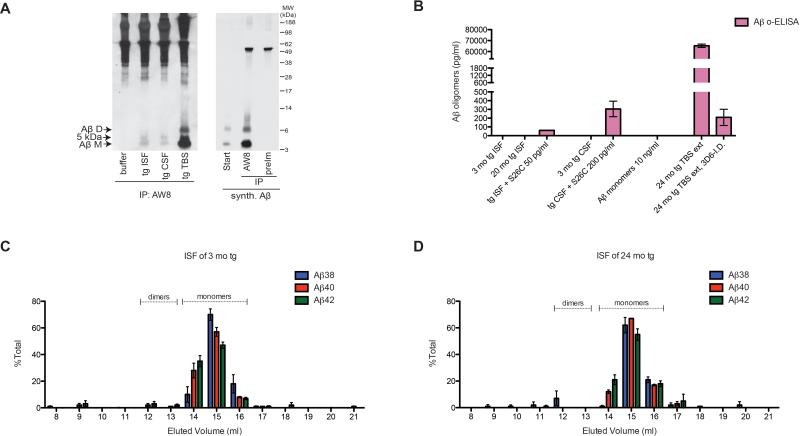
We initially assessed the levels of soluble Aβ oligomers in the hippocampal ISF and CSF. We define Aβ oligomers here as soluble LMW oligomers (dimers, trimers, tetramers, etc.) that remain in the supernatant after high speed ultracentrifugation, as opposed to high molecular weight, prefibrillar Aβ aggregates that can often be pelleted by ultracentrifugation. To obtain hippocampal ISF, we performed in vivo microdialysis in wake, behaving J20 tg mice using microdialysis rates (0.2-0.4 μl/min) low enough to allow ready recovery of soluble Aβ (Cirrito et al., 2003; Hong et al., 2011). CSF was collected from the cisterna magna of anesthetized J20 mice (DeMattos et al., 2002), a method which, in contrast to microdialysis, does not limit the recovery of soluble molecules according to their size. Anti-Aβ immunoprecipitates of hippocampal ISF and CSF yielded the 4 kDa monomer and a 5 kDa Aβ-immunoreactive band migrating just above it, but no SDS-stable Aβ dimers (which run at 6.5 kDa in these gels) (Figures 1A and S1A). This apparent lack of Aβ oligomers in both tg ISF and CSF was not due to an inability of our two polyclonal Aβ antisera (AW8 (Hong et al., 2011) or R1282 (Podlisny et al., 1998)) to immunoprecipitate oligomers, as each effectively pulled down both the endogenous Aβ dimers present in Tris-buffered saline (TBS) extracts of 20 mo tg mouse brains and the synthetic wt Aβ and disulfide-crosslinked (Aβ1-40 S26C)2 dimers (Shankar et al., 2008) (Figures 1A, S1A and S1B).
Figure 1. Aβ oligomers are not detected in J20 hAPP tg brain ISF or CSF.
(A) IP/WB of buffer (1.5% BSA in aCSF), 3 mo tg hippocampal ISF and CSF, 20 mo tg TBS extract, and synthetic Aβ. IP: AW8 anti-Aβ sera (1:100) or pre-immune AW8 sera (preIm, 1:100); WB: 3D6+2G3+21F12. D=dimer, M=monomer, MW=Molecular weight. Representative of 10 experiments. (B) ISF of 3 mo tg (N=4 mice) and 20 mo tg (N=7 mice) and CSF of 3 mo tg (N=7 mice) were assayed using Aβ o-ELISA. Sensitivity and specificity of o-ELISA were tested using tg ISF and CSF spiked with low levels of synthetic Aβ dimers or monomers and 20 mo tg TBS extract and its corresponding 3D6 Aβ-immunodepleted sample. (C-D) 250 μl hippocampal ISF from 3 mo tg (C) and 20 mo tg (D) were loaded onto Superdex 75 SEC column and resultant fractions were subjected to 6E10 Aβ triplex ELISA. Value=normalized mean ± SEM; N=3 mice each. Dotted lines indicate where synthetic Aβ dimers and monomers were eluted using the same SEC column. See also Figure S1.
Next, we used a recently developed Aβ oligomer-ELISA (o-ELISA) that specifically recognizes natural Aβ oligomers of a wide range of sizes (dimers up to prefibrillar soluble oligomers), but not Aβ monomers, and detects small amounts (sensitivity ≤6 pg/ml) of both synthetic and natural Aβ oligomers isolated from AD cortex (Yang et al., 2013). However, the o-ELISA failed to detect any Aβ oligomer signal in 3 mo and 20 mo tg ISF or 3 mo tg CSF (Figure 1B). Using a conventional ELISA that recognizes all Aβ1-x species including monomers, we confirmed that the tg mouse ISF and CSF indeed contained ample amounts of Aβ (Figure S1C). We excluded the possibility that molecules in ISF or CSF interfere with the o-ELISA technique, as spiking these CNS fluids with low amounts of pure S26C Aβ dimers gave positive signals in the expected range (Figure 1B). We next eluted ISF of 3 mo and 20 mo tg mice off a Superdex 75 size exclusion chromatography (SEC) column and subjected the resultant SEC fractions to a triplex ELISA that can detect low-femtogram amounts of endogenous Aβ38, Aβ40 and Aβ42 (detection antibody: 6E10). Whereas SEC fractions #14-16 in which Aβ monomers elute (i.e., the ~4 kDa fractions) gave abundant signals for all three Aβ peptides, fractions #12 and 13 in which dimers elute (i.e., the ~8 kDa fractions (Figure S1D) yielded no detectable signal above background from ISF at either age (Figures 1C and D). We confirmed that the Aβ triplex ELISA is capable of detecting SEC fractions of synthetic dimers and monomers to commensurate degrees (Figures S1D and S1E). Collectively, these results from multiple sensitive biochemical assays – IP/WB, o-ELISA, and SEC followed by ultrasensitive Aβ triplex ELISA – suggest that steady-state levels of LMW Aβ oligomers in brain ISF or CSF of J20 tg mice are very low and thus below the detection limits of the methods used here (Figure S1C).
Aβ oligomers are rapidly sequestered away from hippocampal ISF in vivo
In light of this failure to detect significant amounts of LMW oligomers in brain ISF and CSF, we asked whether the half-life of Aβ oligomers in ISF is much shorter than that of monomers. We acutely administered exogenous Aβ dimers or monomers into hippocampal ISF of behaving wt mice using a cannula attached to the microdialysis probe. To distinguish the acutely administered Aβ from endogenous Aβ, we injected Aβ of human sequence into ISF of wt mice and quantified the recovery of injected human Aβ in ISF microdialysates using Aβ1-x ELISA, which recognizes human but not mouse Aβ. As our monomer source, we used wt Aβ1-40 monomers, and as the Aβ dimer-rich source, we used the S26C dimer, which was almost all dimeric and stayed as such throughout the time of our in vivo microdialysis (Figures S2A and S2B). We first empirically determined the concentrations of monomers and dimers that would allow equal percentages of these species to flow across the microdialysis membrane in vitro; the concentrations turned out to be 8 nM for monomers and 40 nM for dimers (see Supplemental Experimental Procedures; Figure S2C). In stark contrast to these in vitro test-tube experiments where the recoveries of Aβ dimers and monomers were equivalent (Figure 2A), the in vivo recovery of the injected dimers in the ISF dialysate was <10% of that of the injected monomers (Figure 2B). This large difference in the relative recoveries of Aβ dimers vs. monomers between in vitro (102 ± 11.4%) and in vivo (8.9 ± 2.8%) microdialysis was highly significant(p<0.0001) (Figure 2C). Importantly, the disappearance of the injected Aβ dimers from wt ISF occurred rapidly (Figure 2D). The low recovery of injected Aβ dimers vs. monomers in the ISF was not due to a general limit on in vivo diffusion, nor did we obtain evidence of acute perturbations of several non-Aβ analytes in the respective microdialysates (Figures S2D-F). The rapid disappearance of Aβ oligomers from brain ISF was confirmed using microinjection of a second source of synthetic Aβ dimers, namely, non-crosslinked wt dimers (Figures S2H and S2I).
Figure 2. Aβ dimers are rapidly sequestered away from the hippocampal ISF in vivo to bind membranes.
(A) (Aβ1-40 S26C)2 dimers (40 nM) or Aβ1-40 monomers (8 nM) in test tubes were microdialyzed at 0.1, 0.4 and 1.0 μl/min and the resulting microdialysates were assessed using Aβ1-x ELISA. N=3 per group. (B) Aβ dimers or monomers were administered to living wt hippocampal ISF via a microdialysis cannula and post-injection ISF was collected at 0.4 μl/min for 1 h and analyzed for Aβ1-x. N=6 mice and N=5 mice for monomer- and dimer-injected. *: P=0.0126 by two-tailed Student's t test. (C) In vivo recovery of injected dimers was 8.85 ± 2.81% of that of injected monomers (N=5 pairs), in contrast to the in vitro paradigm (102 ± 11.4% (N=3 pairs)). ***: P<0.0001 by two-tailed Student's t test. (D) Representative hourly monitoring of injected Aβ monomers (blue triangle) or S26C Aβ dimers (red circle) in ISF of wt mice before and after injection. Inset shows values using a finer y-scale for t=2-5 h. (E) 320 pg Aβ monomers or S26C dimers were injected into hippocampi of wt mice and their brain homogenates were immediately fractionated for R1282 Aβ IP. WB: 3D6+6E10. Both monomers and dimers were recovered as monomers in the TBS and TBS-Tx. Representative of 4 experiments. (F) IP/WB quantification by ImageJ of N=8 mice injected side by side with monomers or dimers. Proportion of Aβ recovered in TBS-Tx vs TBS extracts post Aβ dimer injection was 23 ± 6.4 fold than that of Aβ monomer injection. ***: P<0.001 by 2-way ANOVA, followed by Bonferonni post-test. Value=mean ± SEM. See also Figure S2.
Aβ dimers injected into hippocampal ISF in vivo are principally recovered in the membrane fraction as monomers bound to GM1 ganglioside
To follow the fate of Aβ oligomers released into ISF, we injected Aβ dimers or monomers in low, endogenous amounts (320 pg) into the hippocampal ISF of wt mice and immediately (<1 min) sacrificed the mice for brain fractionation. The cytosolic/extracellular (TBS) and membrane-associated (TBS with 1% Triton-X100 (TBS-Tx)) fractions were promptly immunoprecipitated for human Aβ. A much higher percentage of the injected Aβ was immunoprecipitated from brain membrane fractions of the dimer-injected mice than those of the monomer-injected mice (Figures 2E and 2F). Surface biotinylation experiments in intact primary hippocampal neurons demonstrated the surface retention of Aβ dimers, but not monomers, on the neuronal plasma membrane; this surface binding occurred prior to lysing the biotinylated cells (Figure S3A). The latter finding, together with the rapid sequestration of Aβ dimers away from ISF and to the brain membrane fraction (Figure 2), suggests that Aβ dimers bind cellular membranes more avidly than monomers do, and may explain their very low or undetectable levels in CNS fluids (ISF and CSF) at steady state.
In contrast to the in vitro microdialysis experiments where Aβ dimers remained stable as dimers for 24-36 h (Figure S2B), the Aβ S26C dimers we injected into ISF in vivo were recovered as reduced monomers in both the ISF microdialysates (1 h post injection) (Figure S2G) and the brain TBS and TBS-Tx fractions (Figures 2E and 3A: see S26C 320 pg in vivo), except when a much higher concentration of Aβ S26C dimers was injected (Figure 3A: see S26C 320 ng in vivo). The conversion of the disulfide crosslinked synthetic dimers to monomers when injected in vivo could be explained by the brain providing a reducing environment. We therefore decided to isolate natural Aβ dimers directly from TBS cortical extracts of typical AD brains using SEC (Shankar et al., 2008) and injected these human Aβ dimers into the ISF of wt mice. Even this largely dimeric material was recovered by IP/WB from the brain homogenates as 4- and 5-kDa Aβ monomers (Figure 3A: see huAD TBS dimers in vivo). Almost all of the injected Aβ dimers (whether synthetic or from human brain) were recovered in the TBS- and TBS-Tx-soluble extracts (Fig. 3A), and none was recovered in the TBS-Tx-insoluble pellets (data not shown); the latter suggests that the injected dimers did not accumulate into large insoluble aggregates during the short time course of our experiment.
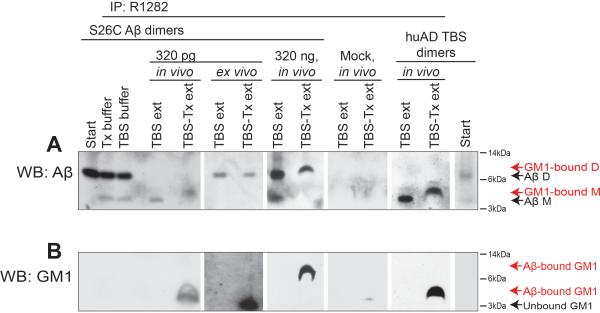
Figure 3. Aβ injected to wt hippocampus in vivo is recovered from the membrane-associated fraction as bound to GM1 ganglioside.
320 pg or 320 ng synthetic S26C Aβ dimers, huAD TBS dimers or buffer (mock) were injected to anesthetized wt mice, then the brains were immediately harvested for brain fractionation. The in vivo injected brain fractions (TBS and TBS-Tx extracts) or S26C Aβ spiked ex vivo to extracts or to buffer alone were immunoprecipitated for Aβ using R1282. WB: 3D6+6E10 for Aβ (A) or CTβ for GM1 (B). Representative blots of N=5, 3, 5 and 5 mice for 320 pg S26C, 320 ng S26C, mock or huAD TBS dimer injections, respectively. See also Figure S3.
Intriguingly, whereas the injected Aβ immunoprecipitated from TBS extract ran at the size of monomers (4 kDa), the injected Aβ immunoprecipitated from TBS-Tx extract ran slightly higher as a 5-kDa species (Figure 3A: see S26C 320 pg in vivo, and huAD TBS dimers in vivo). Based on a prior report of a GM1 ganglioside-bound form of human Aβ monomer occurring as a 5-kDa species in diffuse plaque-rich cortex of early AD or Down syndrome (DS) cases (Yanagisawa et al., 1995), we probed the microinjected, immunoprecipitated Aβ species with the β-subunit of cholera toxin (CTβ), which specifically binds GM1 ganglioside (van Heyningen, 1974). The immunoprecipitated 5-kDa Aβ species recovered from the TBS-Tx extract was reactive with CTβ, indicating that the Aβ in the membrane fraction was bound to GM1 ganglioside (Figure 3B: 1st and 5th panels). High-resolution imaging of the hippocampal CA1 region of such wt mouse that received in vivo Aβ injection demonstrated that most of the retained Aβ was co-localized with endogenous GM1 ganglioside (Figure S3B). This result on brain sections suggests that the association of GM1 with Aβ occurs in vivo, that is, it can be seen without tissue homogenization. Likewise, the above-mentioned surface biotinylation of Aβ on intact neurons (Figure S3A) supports this conclusion. Importantly, we failed to recover the 5 kDa Aβ species when we performed the acute Aβ injections into mice lacking gangliotetraose gangliosides (i.e., with a disrupted gene for GM2/GD2 synthase (Sheikh et al., 1999)), thus providing confirmatory evidence that the 5 kDa Aβ species is GM1 ganglioside-bound Aβ (Figure S3C). Interestingly, we did not observe a 5 kDa GM1-bound Aβ species when we spiked the Aβ S26C dimers ex vivo into postmortem wt mouse brain homogenates (Figure 3: see 320 pg ex vivo), suggesting that the binding of Aβ to endogenous GM1 on membranes required the intact environment of the living brain. Furthermore, when we injected a high level of Aβ S26C in vivo, we recovered from the TBS-Tx fraction the Aβ S26C as intact dimers bound to GM1, namely, a ~10 kDa CTβ-positive Aβ species (Figures 3A and B: see 320 ng in vivo).
In light of the reported co-localization of PrPc to GM1 ganglioside on membranes (Sanghera et al., 2008; Théry et al., 2009) and recent reports that PrPc acts as a key binding receptor for Aβ oligomers (Chen et al., 2010; Laurén et al., 2009), we asked whether PrPc is required for the observed binding of the acutely injected Aβ to endogenous GM1 ganglioside. After similar acute injection of a high amount (320 ng) of Aβ S26C dimer into the hippocampus of PrnP−/− (null) mice, the animals were sacrificed immediately for brain homogenization. We recovered Aβ S26C as bound to endogenous GM1 in PrnP null mice (Figure S3D) in a manner indistinguishable from the injection results in wt mice (Figure 3: 320 ng in vivo). Therefore, PrPc is not required for the binding of Aβ to GM1 ganglioside on brain membranes.
Aβ oligomers preferentially bind to GM1 ganglioside
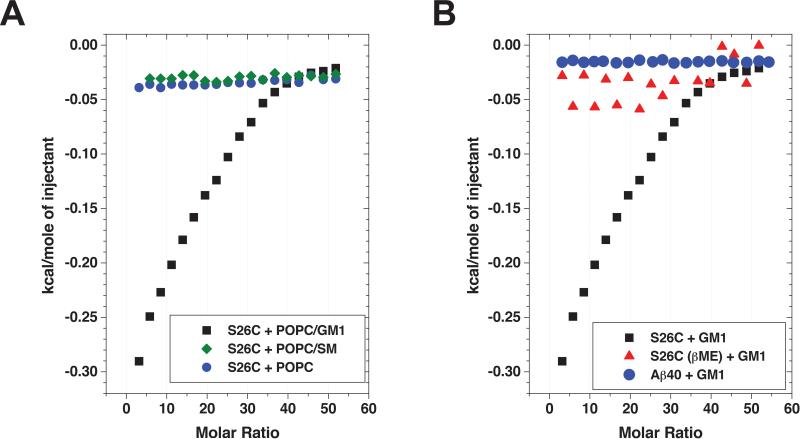
We next performed isothermal titration calorimetry (ITC), measuring Aβ binding to or incorporation into lipid membranes by detection of concomitant heat release (Seelig, 2004). To use ITC to quantitatively assess membrane binding in vitro, we titrated suspensions of small unilamellar vesicles composed of POPC/GM1 (9:1) into freshly prepared Aβ S26C dimer solutions and measured the observed heat release via ITC. Analysis of the partition equilibrium revealed an apparent binding constant Kp of the Aβ S26C interaction with GM1 of ~10000 M−1 per lipid molecule bound (Figure 4A). As a control for the specificity of the interaction of Aβ S26C with GM1, we repeated the ITC experiments titrating suspensions of either pure POPC or POPC/SM (9:1) into Aβ S26C dimer solutions. These experiments yielded no detectable heat release during the titration, indicating a lack of interaction between Aβ S26C and POPC alone or POPC plus sphingomyelin (Figure 4A). Similar negative results were obtained upon titrating POPC/GM1 into solutions of either wt Aβ40 monomer or Aβ S26C solutions with reducing agent (1% βME) present (i.e., monomers) (Figure 4B). These ITC experiments suggest that the dimeric state of the Aβ protein binds GM1 much more avidly than the monomeric state, and that the monosialotetrahexosyl head group of GM1 may be necessary for efficient Aβ binding to lipid membranes, at least in vitro.
Figure 4. Aβ dimers bind to GM1 ganglioside much more avidly than Aβ monomers.
(A) Suspensions of POPC/GM1 (9:1), POPC/sphingomyelin (SM) (9:1) or pure POPC small unilamellar vesicles at 40 mM were titrated into freshly prepared Aβ S26C dimer solutions (15 μM) and subsequent heat release was measured. The hyperbolic shape of the titration curve in case of POPC/GM1 (9:1) indicates a partition equilibrium of Aβ S26C dimers into the lipid membrane, while titrations of POPC/sphingomyelin (SM) (9:1) or pure POPC vesicles yielded no detectable heat release. (B) Titrating suspensions of POPC/GM1 (9:1) into solutions containing wt Aβ40 monomers or Aβ S26C with 1% βME present (i.e., reduced monomers) yielded no detectable heat release during the titration when compared to Aβ S26C dimers. Representative of 3 experiments each using two different preparations of Aβ S26C and vesicles.
Blocking the GM1 sialic acid moiety prevents Aβ oligomer-mediated LTP impairment
We asked whether the binding of Aβ dimers to GM1 ganglioside has functional consequences, that is whether it plays a role in the known ability of the dimers to potently inhibit long-term potentiation (LTP) (Li et al., 2011; Shankar et al., 2008). The sialic acid of GM1 has been shown to be required for the in vitro binding of Aβ to GM1 (Ariga et al., 2001; Choo-Smith et al., 1997; McLaurin and Chakrabartty, 1996). We therefore asked whether masking the sialic acid with CTβ, which binds to the two terminal sugars of GM1 including sialic acid (Merritt et al., 1994), may interfere with the binding of Aβ oligomers to GM1 in hippocampal slices and thus reveal any functional consequences of the binding. At a dose (0.4 μM) at which CTβ by itself caused a slight decrease of LTP magnitude in wt mouse hippocampal slices (140 ± 6%, n=8; Figure 5A, blue circles), pre-treatment with CTβ fully prevented the impairment of hippocampal LTP by soluble S26C Aβ dimers (153 ± 3%, n=9, green triangles) (Figure 5). These data suggest that the binding of Aβ oligomers to GM1 ganglioside plays a required role in mediating Aβ-induced synaptic dysfunction.
Figure 5. Pre-treatment of hippocampal slices with CTβ blocks Aβ oligomer-mediated LTP inhibition.
(A) S26C dimers (10 nM) alone blocked LTP induction (129 ± 6%, n=12, red inverted triangles) compared to vehicle alone (aCSF) (159 ± 6%, n=12, black squares). Whereas CTβ (0.4 μM) alone slightly decreased LTP (140 ± 6%, n=8, blue circles), it fully restored the S26C dimer-impaired hippocampal LTP (153 ± 4%, n=9, green triangles). n=slices. (B) The effect of S26C alone vs. S26C on slices pretreated with CTβ is compared. Value=mean ± SEM at 55 min of LTP induction. ***: P<0.0001 by two-tailed Student's t test.
Endogenous Aβ, particularly Aβ42, is complexed to GM1 ganglioside on membranes of young hAPP transgenic mice
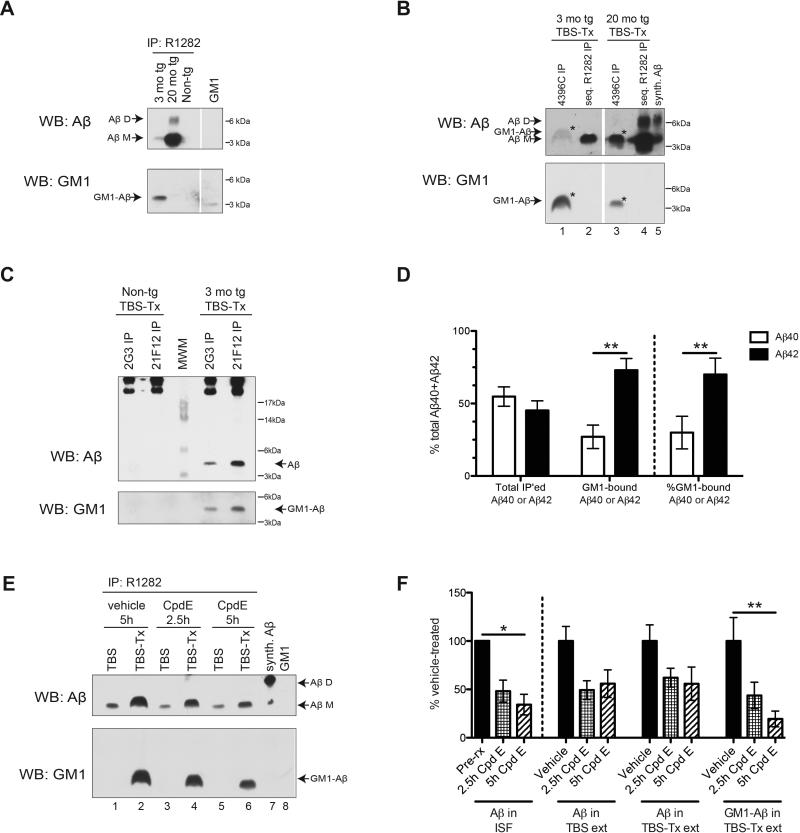
We next asked whether endogenous Aβ occurs in a complex with GM1 ganglioside on brain membranes. Using an Aβ antiserum (R1282), we immunoprecipitated the brain TBS-Tx extracts of 3 mo J20 tg, 20 mo J20 tg, or non-tg littermate mice and found that the TBS-Tx extracts of the plaque-free 3 mo tg mice contained CTβ-positive GM1-bound Aβ (Figure 6A). In contrast, the plaque-rich 20 mo tg contained very little GM1-bound Aβ in the membrane extract despite having far more total Aβ (Figure 6A). We failed to detect other brain lipids such as galactocerebroside (GCB) or phosphatidylethanolamine (PE) in the Aβ immunoprecipitates of 3 mo tg TBS-Tx (Figure S4B). Moreover, thin-layer chromatography of the R1282 Aβ immunoprecipitates showed lack of enrichment in the major brain lipids detectable by the lipid dye primulin (Figure S4C), whereas the Aβ immunoprecipitate of the 3 mo tg TBS-Tx (but not that of the non-tg) yielded a CTβ-positive spot (Figure S4D). Next, we took advantage of a monoclonal antibody (4396C) specifically raised against GM1-bound Aβ isolated from membrane fractions of human brains rich in diffuse plaques (Hayashi et al., 2004; Yanagisawa et al., 1997). We confirmed that 4396C immunoprecipitates neither Aβ nor GM1 alone (Figure S4A), but that it immunoprecipitates the GM1-bound Aβ complex from 3 mo tg TBS-Tx, leaving behind in the supernatant an Aβ species that is not CTβ-positive, as recovered by a subsequent R1282 IP (Figures 6B and S4A). The 4396C immunoprecipitate from 3 mo tg TBS-Tx contained a CTβ-positive 5 kDa Aβ species (Figure 6B, lane 1) with a characteristic cap-like shape akin to that of the 5 kDa Aβ from wt TBS-Tx that received in vivo Aβ injection (Figures 2E and 3). As in the R1282 Aβ immunoprecipitates of 20 mo tg TBS-Tx (Figure 6A), 4396C brought down very low levels of CTβ-positive 5-kDa Aβ from these old, plaque-rich animals (Figures 6B). Using C-terminal specific Aβ antibodies for IP, we found substantially more Aβ42 than Aβ40 to be GM1 ganglioside-bound, despite there being comparable levels of total Aβ40 and Aβ42 recovered from the membrane extracts of 3 mo tg (Figures 6C and D). Collectively, these various findings in J20 tg mice support the hypothesis that more hydrophobic Aβ species (oligomers>monomers; Aβ42>Aβ40) are sequestered onto neuronal membranes, primarily onto GM1 ganglioside.
Figure 6. Aβ in membrane-bound fraction of pre-plaque J20 tg mice is associated with GM1 ganglioside.
(A, B, C and E) IP: As indicated, WB: 3D6+6E10+266+2G3+21F12 for Aβ or CTβ for GM1. (A) R1282 Aβ IP of 3 mo tg, 20 mo tg or non-tg littermate control TBS-Tx extracts. (B) 3 mo tg and 20 mo tg TBS-Tx were first subjected to 4396C IP, then the immunodepleted supernatants were subjected to R1282 IP. Asterisk=GM1-Aβ. Representative of 3 experiments. (C, D) 2G3 or 21F12 IP of 3 mo tg and littermate control TBS-Tx extracts. (D) Quantification of total, GM1-bound or % GM1-bound Aβ in 3 mo tg TBS-Tx pulled down by 2G3 (Aβ40) or 21F12 (Aβ42). N=6 mice. (E, F) 3 mo tg were injected with Compound E or vehicle; 2.5 h or 5 h later, their brains were harvested and their TBS and TBS-Tx extracts were subjected to R1282 IP. (F) Quantification of total or GM1-bound Aβ recovered from TBS and TBS-Tx with or without treatment. N=4, 3 and 4 mice for vehicle-, 2.5 h, and 5 h Compound E-treated, respectively. (D, F) Value=mean ± SEM. *: P<0.05 or **: P<0.01 by 2-way ANOVA, followed by Bonferonni post-test. See also Figure S4.
In contrast to the Aβ acute microinjection experiments in 3 mo wt mice, where most of the exogenous Aβ was recovered as bound to GM1 ganglioside in the membrane extracts (Figure 3), a smaller portion of endogenous Aβ (~5-30%) was recovered as bound to GM1 ganglioside in the 3 mo J20 tg brains (Figures 6B and S4D). Furthermore, the higher recovery of endogenous GM1-bound Aβ in 3 mo tg brain (pre-plaque, high ISF Aβ42) vs. 20 mo tg brain (plaque-rich, low ISF Aβ42) may be associated with the levels of available ISF Aβ at these two ages (Hong et al., 2011). To assess whether it is predominantly the endogenous Aβ which is actively released into the ISF that binds GM1, we treated the 3 mo tg mice with Compound E, a strong inhibitor of γ-secretase that induces a rapid decline in brain ISF Aβ (t1/2 of ~2 h) (Hong et al., 2011). Compound E decreased the levels of GM1-bound Aβ in the 3 mo tg TBS-Tx in a manner comparable to its reduction of ISF Aβ levels (Figures 6E and F), suggesting that there is a rapid equilibrium between soluble (ISF) and membrane-bound (TBS-Tx) pools of Aβ. We confirmed that Compound E had no effect on total GM1 levels in the brain (data not shown). Collectively, all of the results in this section suggest that newly generated Aβ, and especially Aβ42, upon being released into the extracellular fluid (i.e., ISF), readily binds to GM1 ganglioside on adjacent membranes, and this can apparently occur early in the AD pathogenic process, i.e., before plaques form. Finally, whereas we recover dimers in the 20 mo tg TBS-Tx, they do not seem to be associated with GM1, as evidenced by the negative CTβ blotting in the R1282 Aβ immunoprecipitates (Figure 6A). The dimers we observe in the TBS-Tx extract of plaque-rich 20 mo tg may not necessarily be associated with membranes per se but may represent Aβ oligomers that are extracted from plaques during tissue homogenization in TBS-Tx (Hong et al., 2011).
GM1-bound Aβ is detectable in human CSF and correlates with CSF Aβ42 levels
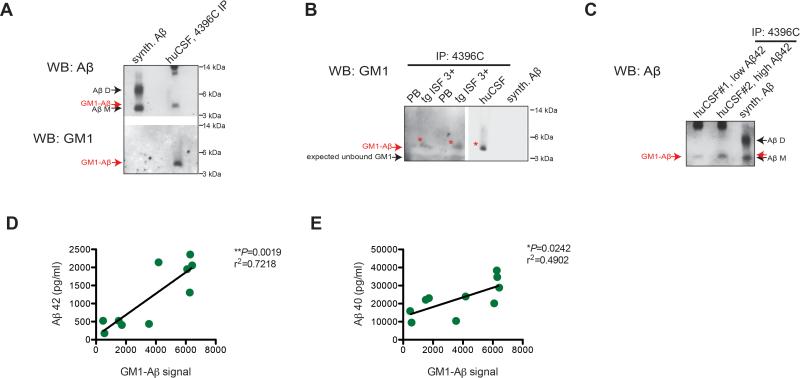
Using the 4396C antibody that is specific for the GM1-Aβ complex (Figure S4A), we recovered a soluble GM1-bound Aβ species in normal human CSF (n=10 samples), as evidenced by positive reaction for Aβ and GM1 (CTβ+) at the ~4.5 - 5 kDa position (Figure 7A: 4396C IP/Aβ WB first, then stripped and blotted with CTβ; Figure 7B: 4396C IP directly blotted with CTβ). The levels of GM1-bound Aβ in human CSF showed a significant direct correlation (r2=0.72) with levels of Aβ42 in the same CSF samples (Figures 7C and 7D) and to a lesser but still significant degree (r2=0.49) with levels of Aβ40 (Figure 7E).
Figure 7. Low levels of GM1-bound Aβ are recovered from hAPP tg mouse ISF and human CSF.
(A-C) IP: 4396C, WB: CTβ for GM1 ganglioside or WB: 3D6+6E10+266+2G3+21F12 for Aβ. (A, B) 4396C pull-down of human CSF probed for Aβ then stripped and reprobed for GM1 (A) or GM1 alone on a naïve IP blot (B; Red asterisk=GM1-Aβ). (C) CSF from 2 humans with differing levels of Aβ42 was immunoprecipitated for GM1-bound Aβ. Representative blots of at least 3 experiments. (D, E) Levels of GM1-bound Aβ recovered using 4396C in ten human CSF samples showed a strong correlation to their levels of Aβ42 (D; 21F12/3D6B ELISA) and less but still significant to levels of Aβ40 (E; 2G3/3D6B ELISA).
DISCUSSION
Despite the extensive genetic and biochemical evidence for a pathogenic role of soluble Aβ oligomers in the most common cognitive syndrome of late life, our understanding of the dynamics of Aβ oligomers in the CNS in vivo is limited. To address key mechanistic and biomarker-related questions of whether Aβ oligomers are present in biological fluids and the nature of their dynamics, we first searched for oligomers in the two CNS aqueous compartments, ISF and CSF, of tg mice that express substantial levels of hAPP and undergo progressive AD-like cerebral deposition of human Aβ. Despite combining three sensitive biochemical methods, IP-WB, o-ELISA, and SEC followed by triplex ELISA, we failed to detect soluble LMW (<35 kDa) Aβ oligomers in hippocampal ISF or CSF of this AD mouse model. These new results are consistent with two previous ELISA reports that failed to detect Aβ oligomers in human CSF (Esparza et al., 2013; Yang et al., 2013), one of which used two highly specific Aβ o-ELISAs in samples of CSF from 90 humans (including elderly subjects with either presymptomatic or early clinical AD) (Yang et al., 2013). Based on these studies and the current work, we conclude that the steady-state levels of LMW Aβ oligomers in the brain's aqueous compartments are very low (<0.02% of total CSF Aβ levels) and thus below the detection limits of the specific methods used here.
To explore this dearth of soluble oligomers mechanistically, we performed in vivo Aβ microinjection experiments into the hippocampal ISF of wt (plaque-free) mice and observed far lower recovery of injected dimers than of injected monomers, raising the hypothesis that soluble Aβ dimers released into the extracellular space are unlikely to remain very long in aqueous fluids. In accord, their relative hydrophobicity appeared to drive the sequestration of the dimers away from the extracellular fluid and onto local membranes. A much higher proportion of the injected dimers was recovered in the membrane (TBS-Tx) extract than in the soluble (TBS) fraction of the brain. Surface biotinylation experiments on intact hippocampal neurons and a quantitative in vitro binding assay using isothermal calorimetry likewise demonstrated that Aβ oligomers bind membranes much more avidly than Aβ monomers do. We therefore conclude that any newly generated and released Aβ oligomers may be rapidly sequestered from the extracellular fluid (ISF) onto plasma membranes of surrounding cells in the brain parenchyma.
In mimicking newly formed Aβ oligomers in the brain, the in vivo injected dimers rapidly became membrane-bound, in contrast to the monomers. Intriguingly, both synthetic dimers and natural dimers isolated from human AD cortex that were injected at low levels into wt mouse ISF were recovered in membrane extracts as monomers bound to GM1 ganglioside, thus shifting their electrophoretic migration to ~5 kDa. This, along with the ITC binding assays and the microinjection experiments in GM1 KO mice, suggests a relative propensity for Aβ dimers to bind to GM1-containing lipids on membranes. This realization led us to use pan-Aβ, C-terminal specific, and GM1-Aβ-specific antibodies to probe Aβ species in a hAPP tg mouse model, and we observed substantial levels of endogenous GM1-bound Aβ, in particular the Aβ42 peptide, in the brain membrane fraction at an early age (3 mo), before plaques form in this model. This result is reminiscent of an earlier study showing that Aβ42 can be found associated with GM1 ganglioside in the brains of AD and DS subjects who had abundant diffuse plaques but little to no mature plaques or neurofibrillary tangles (Yanagisawa et al., 1995). The disassociation of dimers to monomers (unless given at high ng levels) seems to occur after the dimers’ bind to membranes, as suggested by both the surface biotinylation experiment in primary hippocampal neurons and the ITC. The use of a covalently crosslinked form of Aβ oligomers would be insightful to further understand this process. In addition, it would be interesting to measure the half-life and fate of oligomers in brains already having very abundant Aβ plaques, as we previously did with the monomers (Hong et al., 2011).
To date, we have failed to detect an enrichment of other brain lipids bound to Aβ in our assays, including certain phospholipids, ceramides and phosphosphingolipids that we probed for and that may be present at higher levels than GM1 in the CNS. Moreover, preliminary data using GM2/GD2 synthase KO mice suggested a possible gene-dosage effect of GM1 in mediating the sequestration of Aβ dimers onto membranes: the less GM1 the brain had, the lower the percent of injected Aβ dimers that we recovered in the membrane fraction (data not shown). However, caution is needed in using this mouse model, as these mice now have robustly increased levels of GM3 and GD3 in the CNS (Wu et al., 2001); GM3 has also been shown in vitro to bind to and facilitate the aggregation of Aβ (Ariga et al., 2001; Grimm et al., 2012; Yahi et al., 2010; Yamamoto et al., 2005). Given these confounding factors, we do not regard this GM1 KO model as ideal for the study of Aβ effects. It is important to emphasize that although our data using an array of techniques uncover a preferential binding of Aβ oligomers to GM1 gangliosides and that this Aβ-GM1 binding survives denaturation by SDS and heat, there may well be other lipid targets to which Aβ binds. Lipidomic mass spectrometry is currently being pursued to examine various lipid candidates. The key finding that we can recover a GM1-Aβ complex in fresh human CSF samples suggests that the GM1-Aβ complex exists in vivo in the human brain (i.e., in the absence of tissue homogenization) and that it reaches the CSF in small amounts. Likewise, immunohistochemistry of mouse brain cryostat sections post-in vivo Aβ injection showed that the remaining injected Aβ was principally co-localized with endogenous GM1 ganglioside, again eliminating tissue homogenization as an artifactual reason for this association. Finally, the spiking of Aβ oligomers into postmortem brain membrane extracts ex vivo failed to induce any binding of the exogenous Aβ to endogenous GM1 gangliosides, suggesting that the formation of the GM1-Aβ complex that we recover both in wt in vivo microinjection studies and in hAPP tg mice requires the environment of the living brain.
Individual laboratories have reported various potential protein receptors for Aβ oligomers, including α7 nicotinic receptors, NMDA, mGluR and AMPA receptors, insulin receptors, PrPc and LilrB2/PirB (De Felice et al., 2007; 2009; Kim et al., 2013; Laurén et al., 2009; Wang et al., 2000; Zhao et al., 2010). Although it is possible that Aβ oligomers bind to specific cell-surface proteins such as these and that this stabilizes their presence on the plasma membrane, the amphipathic hydrophobicity of the self-aggregating Aβ42 peptide suggests a biochemically more plausible scenario: that the hydrophobic Aβ42 oligomers released into the extracellular fluid (ISF) become rapidly sequestered onto hydrophobic surfaces of adjacent lipid membranes. Our data here suggest that GM1 ganglioside could be a principal lipid target of Aβ42 oligomers early in the course of AD-type β-amyloidosis. Gangliosides are particularly enriched in the nervous system compared to other tissues, and the subcellular localization of GM1 has been mapped out to be mostly in “raft-like domains” enriched in cholesterol and sphingomyelin on plasma membranes of neuronal processes, including pre- and postsynaptic membranes, and on certain normal lipid vesicles such as exosomes (Hansson et al., 1977; Pernber et al., 2012; Sonnino et al., 2007; Svennerholm and Gottfries, 1994; Théry et al., 2009; Yamamoto et al., 2008). Concentrations of GM1 and to a lesser extent GM2 have been reported to be increased in detergent-resistant membranes from frontal cortices of early AD brains (Molander-Melin et al., 2005) and the Aβ-GM1 complexes were found in the cortical neuropil and not on amyloid plaques per se in early AD and DS brains (Hayashi et al., 2004). Both our in vivo ISF studies and in vitro ITC binding data indicate that Aβ dimers can rapidly and avidly associate with GM1-containing lipid membranes. We note that hydrophobicity alone may not be the key factor for the Aβ oligomers’ preferential binding to GM1, in particular given our current ITC and previous in vitro data that the negatively charged sialic acid of gangliosides may be a prime binding site (Ariga et al., 2001; Choo-Smith et al., 1997; McLaurin and Chakrabartty, 1996).
Collectively, our results elucidate the complex economy of newly secreted Aβ oligomers as regards their membrane association and progressive loss of solubility over time. Our findings here help explain the apparently very low levels of Aβ oligomers as soluble, freely diffusible species in ISF and CSF at steady state in vivo, and they support the hypothesis that due to their increased hydrophobicity, Aβ42 oligomers primarily bind to GM1 ganglioside (and potentially other surface lipids) on neuronal membranes. Blocking the specific GM1 receptor site using CTβ rescues the well-documented impairment of hippocampal LTP by Aβ oligomers, suggesting that the binding of Aβ to GM1 may be a step which leads to downstream synaptotoxic effects of Aβ. Thus, Aβ, upon binding to GM1 ganglioside, may then mediate subtle but progressively detrimental biophysical alterations of neuronal membranes—for e.g., by disrupting membrane fluidity or ion permeability—and thus lead secondarily to aberrant function and turnover of a variety of transmembrane receptors.
Finally, we show that a GM1-bound Aβ species can be detected, albeit in low amounts, in human CSF and that its levels are significantly correlated with the levels of Aβ42 (and less so of Aβ40). Reduced CSF levels of Aβ42 in subjects with presymptomatic or early AD are a widely validated biomarker of the disease process, and these levels appear to relate inversely to amyloid plaque burden, degree of brain atrophy and severity of cognitive deficits in humans (Bateman et al., 2012; Fagan et al., 2009; Motter et al., 1995; Shaw et al., 2009). Interestingly, there has been a report of increased GM1 gangliosides in the CSF of AD patients vs. healthy controls (Blennow et al., 1991); however, the level of total gangliosides may not necessarily reflect the portion of Aβ-bound GM1. Accordingly, it will now be important to examine whether the GM1-bound Aβ complexes in CSF we describe could serve as an early biomarker in presymptomatic subjects, especially in light of the challenges of quantifying Aβ oligomers in CSF.
EXPERIMENTAL PROCEDURES
Animals
All animal procedures were approved by the Harvard Medical School Institutional Animal Care and Use Committee. See Supplemental Experimental Procedures for details.
In vivo microdialysis
Microdialysis was performed in the hippocampus of awake and normally behaving mice housed in a “Raturn” cage system (Bioanalytical Systems) as previously described (Cirrito et al., 2003; Hong et al., 2011). 1.5% bovine serum albumin in artificial CSF (in mM: 1.3 CaCl2, 1.2 MgSO4, 3 KCl, 0.4 KH2PO4, 25 NaHCO3, and 122 NaCl, pH 7.35) was perfused using BR-2 probes with 35-kDa MWCO membranes (Bioanalytical Systems) at 0.2 or 0.4 μl/min using an infusion syringe pump (Stoelting).
CSF collection
CSF was collected from cisterna magna of deeply anesthetized mice (DeMattos et al., 2002). Midline incision was made from top of the skull to dorsal thorax, followed by excision of muscles and excess tissue from base of the skull to first vertebrae. A small hole was made to arachnoid membrane covering the cistern using tip of 29 G ½” needle and CSF exiting compartment was collected using a gel-loading pipet.
Half-life studies of Aβ in ISF in vivo
3 μl of 8 nM Aβ monomers or 40 nM dimers was injected into the hippocampus of adult C57BL6xDBA2 mice through a combination infusion cannula and microdialysis probe (IBR-2, Bioanalytical Systems) at 0.2 μl/min. ISF was collected hourly at 0.4 μl/min, paused during the injection, then restarted 1 min after injection. ISF was analyzed for levels of Aβ by Aβ1-x ELISA or o-ELISA or for levels of lactate, pyruvate, urea and glucose (Yale Center for Clinical Investigation).
Intracortical injection of Aβ
Synthetic Aβ or natural Aβ isolated from AD cortex (1-2 μl vol.) was injected at 0.5 μl/min via a Hamilton syringe into left hippocampus of anesthetized mice (Hong et al., 2011). Mice were sacrificed <1 min post end of injection for biochemical analysis.
Mouse brain sample preparation for biochemical analyses
Brains were homogenized using a mechanical Dounce homogenizer with 20 strokes at 4000 rpm in ice-cold TBS (20 mM Tris-HCl, 150 mM NaCl, pH 7.4) and protease inhibitors at 4:1 TBS volume:brain wet weight. Homogenate was centrifuged for 30 min at 175,000 g in a 4 °C TLA100.2 rotor on Beckman TL100 (resulting supernatant=“TBS extract”). Pellet was homogenized in ice-cold TBS with 1% Triton-X (TBS-Tx) and protease inhibitors at 4:1 TBS-Tx volume:brain wet weight and centrifuged for 30 min at 175,000 g in a 4°C TLA100.2 rotor (resulting supernatant=“TBS-Tx extract”). TBS-Tx pellet was incubated with 88% formic acid at RT for 2 h and lyophilized (“FA extract”).
Immunoprecipitation and Western blot for Aβ and GM1 ganglioside
IP/WB was performed as previously described with minor modifications (Walsh et al., 2000). See Supplemental Experimental Procedures for details.
Aβ ELISA
Sandwich ELISAs for Aβ were performed using the MULTI-ARRAY® 96-well Plate platform (MesoScale Discovery) as described previously (Hong et al., 2011; Yang et al., 2013). See Supplemental Experimental Procedures for details.
Size-exclusion chromatography
ISF sampled at 0.4 μl/min (250 μl) or synthetic Aβ (2 ng) were eluted at 0.5 ml/min from a Superdex 75 10/300GL column (GE Healthcare) with 50 mM ammonium acetate, pH 8.5. Resulting 1-ml fractions were lyophilized then reconstituted in 60 μl perfusion buffer and analyzed using 6E10 Aβ Triplex ELISA. For WB analysis, samples were subjected to WB using 3D6 and ECL Plus WB Detection Reagent.
Preparation of Aβ isolated from AD cortex
Aβ from TBS extract of human AD cortical tissue was prepared as previously described (Yang et al., 2013). Briefly, frozen temporal or frontal cortex samples were dissected then homogenized as described above for mouse brain sample preparation. TBS extract was then subjected to IP with 3D6 (Elan, plc) and the Aβ immunoprecipitate was eluted from a Superdex 75 SEC column in 50 mM ammonium acetate, pH 8.5. The SEC elution profile for Aβ was verified by SDS-PAGE. The corresponding fractions where Aβ dimers were eluted were used for the injection studies.
Isothermal titration calorimetry
For ITC, a Microcal ITC-200 instrument with a motor driven syringe was used. Volume of the cell was 204 μl. Small volumes of small unilamellar vesicles, obtained by sanitation (40 mM total lipid) were injected into the protein solution (15 μM) of calorimetry cell at 20 °C. Buffer used was 25 mM ammonium bicarbonate, pH 8.4, with 1 vol% β-mercapto ethanol added in case of the S26C monomer titrations. Monomeric or dimeric forms of Aβ used for experiments were freshly eluted off a Superdex 75 SEC column and the corresponding fractions were kept on ice before use. For the experiments, 19 injections of 2 μl were used, after a pre-injection of 0.4 μl to account for diffusion of titrant from the syringe. Integration of the heat signals and correction of the base line was done in Microcal Origin 5.0. Control titrations of lipid suspension into buffer alone were subtracted from the experimental data. The apparent partition constant was calculated from the initial heat signals where the equilibrium seems to be independent from electrostatic interaction between the protein molecules (Seelig, 1997).
Electrophysiology on acute hippocampal slices
Hippocampal slices were prepared as previously described (Li et al., 2013). Briefly, 350 μm thick transverse slices from hippocampus of 6-8 wk old C57BL/6x129 were incubated for >90 min in aCSF containing (in mM): 124 NaCl, 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 2.5 CaCl2, 26 NaHCO3, 10 D-glucose, pH 7.4, 310 mOsm, then were submerged beneath continuously perfused aCSF saturated with 95% O2 and 5% CO2 for stimulation at RT (~24 °C). For electrophysiology, standard field excitatory postsynaptic potentials (fEPSP) were recorded in the CA1 region of hippocampus as previously described (Li et al., 2013). Briefly, stimulating electrode was placed in Schaffer collaterals to deliver test and conditioning stimuli, and borosilicate glass recording electrode filled with aCSF was positioned 200-300 μm from the stimulating electrode in stratum radiatum of CA1. To induce LTP, two consecutive trains (1 s) of stimuli at 100 Hz separated by 20 s, a protocol that induces LTP lasting ~1.5 h in wt mice of this genetic background, were applied to the slices. The field potentials were amplified 100x using Axon Instruments 200B amplifer and digitized with Digidata 1322A. The data were sampled at 10 kHz and filtered at 2 kHz. Traces were obtained by pClamp 9.2 and analyzed using the Clampfit 9.2.
Statistical analysis
Data was analyzed using PRISM (Graphpad Software) for one-way or two-way analysis of variance (ANOVA) followed by Bonferroni post-hoc test if means were significantly different by ANOVA, or by two-tailed Student's t test, as appropriate.
Supplementary Material
HIGHLIGHTS.
Aβ oligomers are rapidly sequestered away from hippocampal ISF in vivo
Aβ oligomers bind to GM1 on neuronal membranes (oligomer>monomer; Aβ42> Aβ40)
Blocking the GM1 sialic acid moiety decreases Aβ oligomer-mediated LTP inhibition
GM1-bound Aβ can be recovered in human CSF and correlates with CSF Aβ42 levels
Acknowledgements
We are very grateful to R. Ledeen (NJMS) for GM2/GD2 synthase KO mice, D. Harris (BU) for PrnP KO mice, R. Sperling (BWH) for human CSF samples, L. Mucke (UCSF) for J20 mice, DM Walsh (BWH) for AW8 antibody, and Elan, plc (South San Francisco, CA) for 3D6, 2G3 and 21F12 antibodies. We thank DR Podlisny and WT Cavanaugh for technical assistance, MJ LaVoie and TL Young-Pearse for critical reading of manuscript, and members of the Selkoe laboratory for helpful comments. Supported by NIH grant AG027443 (DJS), PPG P01 AG036694 (Sperling/DJS), a Harvard NeuroDiscovery Center Pre-doctoral Training Fellowship (SH) and a Jerome L. Rappaport Fellowship at HMS (SH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests Statement. DJS is a consultant to Elan and a director of Prothena.
REFERENCES
- Ariga T, Kobayashi K, Hasegawa A, Kiso M, Ishida H, Miyatake T. Characterization of High-Affinity Binding between Gangliosides and Amyloid β-Protein. Arch Biochem Biophys. 2001;388:225–230. doi: 10.1006/abbi.2001.2304. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM. Clinical and biomarker changes in dominantly inherited alzheimer's disease. New England Journal of Medicine. 2012 doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- Blennow K, Davidsson P, Wallin A, Fredman P, Gottfries CG, Karlsson I, Mansson JE, Svennerholm L. Gangliosides in Cerebrospinal Fluid in 'Probable Alzheimer`s Disease’. Archives of Neurology. 1991;48:1032–1035. doi: 10.1001/archneur.1991.00530220048018. [DOI] [PubMed] [Google Scholar]
- Chen S, Yadav SP, Surewicz WK. Interaction between Human Prion Protein and Amyloid-(A) Oligomers: ROLE OF N-TERMINAL RESIDUES. Journal of Biological Chemistry. 2010;285:26377–26383. doi: 10.1074/jbc.M110.145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo-Smith LP, Garzon-Rodriguez W, Glabe CG, Surewicz WK. Acceleration of amyloid fibril formation by specific binding of Abeta-(1-40) peptide to ganglioside-containing membrane vesicles. J Biol Chem. 1997;272:22987–22990. doi: 10.1074/jbc.272.37.22987. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig-Schapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer's disease. Neurobiol Dis. 2009;35:128–140. doi: 10.1016/j.nbd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta Oligomers Induce Neuronal Oxidative Stress through an N-Methyl-D-aspartate Receptor-dependent Mechanism That Is Blocked by the Alzheimer Drug Memantine. Journal of Biological Chemistry. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Vieira MNN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao W-Q, Ferreira ST, Klein WL. Protection of synapses against Alzheimer's-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci USA. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Parsadanian M, O'Dell MA, Foss EM, Paul SM, Holtzman DM. Plaque-associated disruption of CSF and plasma amyloid-beta (Abeta) equilibrium in a mouse model of Alzheimer's disease. J Neurochem. 2002;81:229–236. doi: 10.1046/j.1471-4159.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- Doody RS. Phase 3 Studies of Solanezumab for Mild to Moderate Alzheimer's Disease. 137th Annual Meeting of the American Neurological Association (Boston) 2012 [Google Scholar]
- Esparza TJ, Zhao H, Cirrito JR, Cairns NJ, Bateman RJ, Holtzman DM, Brody DL. Amyloid-beta oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol. 2013;73:104–119. doi: 10.1002/ana.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H, Tokuda T, Kasai T, Ishigami N, Hidaka H, Kondo M, Allsop D, Nakagawa M. High-molecular-weight beta-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. The FASEB Journal. 2010;24:2716–2726. doi: 10.1096/fj.09-150359. [DOI] [PubMed] [Google Scholar]
- Gao CM, Yam AY, Wang X, Magdangal E, Salisbury C, Peretz D, Zuckermann RN, Connolly MD, Hansson O, Minthon L, et al. Aβ40 Oligomers Identified as a Potential Biomarker for the Diagnosis of Alzheimer's Disease. PLoS ONE. 2010;5:e15725. doi: 10.1371/journal.pone.0015725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE, Schneider LS, Koo EH. Anti-aβ therapeutics in Alzheimer's disease: the need for a paradigm shift. Neuron. 2011;69:203–213. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Chang L, Viola K, Lacor P, Lambert M, Finch C, Krafft G, Klein W. Alzheimer's disease-affected brain: presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci USA. 2003;100:10417. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Grimm MOW, Zinser EG, Grösgen S, Hundsdörfer B, Rothhaar TL, Burg VK, Kaestner L, Bayer TA, Lipp P, Müller U, et al. Amyloid Precursor Protein (APP) Mediated Regulation of Ganglioside Homeostasis Linking Alzheimer's Disease Pathology with Ganglioside Metabolism. PLoS ONE. 2012;7:e34095. doi: 10.1371/journal.pone.0034095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson HA, Holmgren J, Svennerholm L. Ultrastructural localization of cell membrane GM1 ganglioside by cholera toxin. Proc Natl Acad Sci USA. 1977;74:3782–3786. doi: 10.1073/pnas.74.9.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Kimura N, Yamaguchi H, Hasegawa K, Yokoseki T, Shibata M, Yamamoto N, Michikawa M, Yoshikawa Y, Terao K, et al. A seed for Alzheimer amyloid in the brain. J Neurosci. 2004;24:4894–4902. doi: 10.1523/JNEUROSCI.0861-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Quintero-Monzon O, Ostaszewski BL, Podlisny DR, Cavanaugh WT, Yang T, Holtzman DM, Cirrito JR, Selkoe DJ. Dynamic Analysis of Amyloid -Protein in Behaving Mice Reveals Opposing Changes in ISF versus Parenchymal A during Age-Related Plaque Formation. Journal of Neuroscience. 2011;31:15861–15869. doi: 10.1523/JNEUROSCI.3272-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci USA. 2011;108:5819–5824. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Vidal GS, Djurisic M, William CM, Birnbaum ME, Garcia KC, Hyman BT, Shatz CJ. Human LilrB2 Is a -Amyloid Receptor and Its Murine Homolog PirB Regulates Synaptic Plasticity in an Alzheimer's Model. Science. 2013;341:1399–1404. doi: 10.1126/science.1242077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyubin I, Betts V, Welzel AT, Blennow K, Zetterberg H, Wallin A, Lemere CA, Cullen WK, Peng Y, Wisniewski T, et al. Amyloid beta protein dimer-containing human CSF disrupts synaptic plasticity: prevention by systemic passive immunization. Journal of Neuroscience. 2008;28:4231–4237. doi: 10.1523/JNEUROSCI.5161-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, Spooner ET, Jiang L, Anwyl R, Selkoe DJ, et al. Amyloid |[beta]| protein immunotherapy neutralizes A|[beta]| oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, et al. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. Journal of Neuroscience. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. Journal of Neuroscience. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jin M, Zhang D, Yang T, Koeglsperger T, Fu H, Selkoe DJ. Environmental Novelty Activates β2-Adrenergic Signaling to Prevent the Impairment of Hippocampal LTP by Aβ Oligomers. Neuron. 2013;77:929–941. doi: 10.1016/j.neuron.2012.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin J, Chakrabartty A. Membrane disruption by Alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J Biol Chem. 1996;271:26482–26489. doi: 10.1074/jbc.271.43.26482. [DOI] [PubMed] [Google Scholar]
- Merritt EA, Sarfaty S, van den Akker F, L'Hoir C, Martial JA, Hol WG. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Sci. 1994;3:166–175. doi: 10.1002/pro.5560030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander-Melin M, Blennow K, Bogdanovic N, Dellheden B, Mansson J-E, Fredman P. Structural membrane alterations in Alzheimer brains found to be associated with regional disease development; increased density of gangliosides GM1 and GM2 and loss of cholesterol in detergent-resistant membrane domains. J Neurochem. 2005;92:171–182. doi: 10.1111/j.1471-4159.2004.02849.x. [DOI] [PubMed] [Google Scholar]
- Morris JC, Selkoe DJ. Recommendations for the incorporation of biomarkers into Alzheimer clinical trials: an overview. Neurobiology of Aging. 2011;32:S1. doi: 10.1016/j.neurobiolaging.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, Chang L, Miller B, Clark C, Green R. Reduction of β-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- Mucke L, Selkoe DJ. Neurotoxicity of Amyloid β-Protein: Synaptic and Network Dysfunction. Cold Spring Harbor Perspectives in Medicine. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu G-Q, Mallory M, Rockenstein E, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, Mcconlogue L. High-Level Neuronal Expression of Abeta 1-42 in Wild-Type Human Amyloid Protein Precursor Transgenic Mice: Synaptotoxicity without Plaque Formation. Journal of Neuroscience. 2000;20:4050. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, Laferla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Pernber Z, Blennow K, Bogdanovic N, Mansson JE, Blomqvist M. Altered distribution of the gangliosides GM1 and GM2 in Alzheimer's disease. Dement Geriatr Cogn Disord. 2012;33:174–188. doi: 10.1159/000338181. [DOI] [PubMed] [Google Scholar]
- Podlisny MB, Walsh DM, Amarante P, Ostaszewski BL, Stimson ER, Maggio JE, Teplow DB, Selkoe DJ. Oligomerization of endogenous and synthetic amyloid beta-protein at nanomolar levels in cell culture and stabilization of monomer by Congo red. Biochemistry. 1998;37:3602–3611. doi: 10.1021/bi972029u. [DOI] [PubMed] [Google Scholar]
- Sanghera N, Wall M, Vénien-Bryan C, Pinheiro TJT. Globular and pre-fibrillar prion aggregates are toxic to neuronal cells and perturb their electrophysiology. PLoS ONE. 2008;1784:873–881. doi: 10.1016/j.bbapap.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Seelig J. Titration calorimetry of lipid–peptide interactions. Biochimica Et Biophysica Acta (BBA) - Reviews on Biomembranes. 1997;1331:103–116. doi: 10.1016/s0304-4157(97)00002-6. [DOI] [PubMed] [Google Scholar]
- Seelig J. Thermodynamics of lipid–peptide interactions. Biochimica Et Biophysica Acta (BBA) - Biomembranes. 2004;1666:40–50. doi: 10.1016/j.bbamem.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural Oligomers of the Alzheimer Amyloid-Protein Induce Reversible Synapse Loss by Modulating an NMDA-Type Glutamate Receptor-Dependent Signaling Pathway. Journal of Neuroscience. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh KA, Sun J, Liu Y, Kawai H, Crawford TO, Proia RL, Griffin JW, Schnaar RL. Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc Natl Acad Sci USA. 1999;96:7532–7537. doi: 10.1073/pnas.96.13.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnino S, Mauri L, Chigorno V, Prinetti A. Gangliosides as components of lipid membrane domains. Glycobiology. 2007;17:1R–13R. doi: 10.1093/glycob/cwl052. [DOI] [PubMed] [Google Scholar]
- Svennerholm L, Gottfries CG. Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II). J Neurochem. 1994;62:1039–1047. doi: 10.1046/j.1471-4159.1994.62031039.x. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Tomiyama T, Matsuyama S, Iso H, Umeda T, Takuma H, Ohnishi K, Ishibashi K, Teraoka R, Sakama N, Yamashita T. A Mouse Model of Amyloid {beta} Oligomers: Their Contribution to Synaptic Alteration, Abnormal Tau Phosphorylation, Glial Activation, and Neuronal Loss In Vivo. Journal of Neuroscience. 2010;30:4845. doi: 10.1523/JNEUROSCI.5825-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heyningen S. Cholera toxin: interaction of subunits with ganglioside GM1. Science. 1974;183:656. doi: 10.1126/science.183.4125.656. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Perez KA, Pike KE, Kok WM, Rowe CC, White AR, Bourgeat P, Salvado O, Bedo J, Hutton CA, et al. Blood-Borne Amyloid-Dimer Correlates with Clinical Markers of Alzheimer's Disease. Journal of Neuroscience. 2010;30:6315–6322. doi: 10.1523/JNEUROSCI.5180-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39:10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang HY, Lee DH, D'Andrea MR, Peterson PA, Shank RP, Reitz AB. beta-Amyloid(1-42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer's disease pathology. J Biol Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Wu G, Xie X, Lu Z-H, Ledeen RW. Cerebellar neurons lacking complex gangliosides degenerate in the presence of depolarizing levels of potassium. Proc Natl Acad Sci USA. 2001;98:307–312. doi: 10.1073/pnas.011523698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahi N, Aulas A, Fantini J. How Cholesterol Constrains Glycolipid Conformation for Optimal Recognition of Alzheimer's β Amyloid Peptide (Aβ1-40). PLoS ONE. 2010;5:e9079. doi: 10.1371/journal.pone.0009079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Hirabayashi Y, Amari M, Yamaguchi H, Romanov G, Van Nostrand WE, Yanagisawa K. Assembly of hereditary amyloid β-protein variants in the presence of favorable gangliosides. FEBS Lett. 2005;579:2185–2190. doi: 10.1016/j.febslet.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Matsubara T, Sato T, Yanagisawa K. Age-dependent high-density clustering of GM1 ganglioside at presynaptic neuritic terminals promotes amyloid beta-protein fibrillogenesis. Biochim Biophys Acta. 2008;1778:2717–2726. doi: 10.1016/j.bbamem.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, McLaurin J, Michikawa M, Chakrabartty A, Ihara Y. Amyloid beta-protein (A beta) associated with lipid molecules: immunoreactivity distinct from that of soluble A beta. FEBS Lett. 1997;420:43–46. doi: 10.1016/s0014-5793(97)01484-1. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Odaka A, Suzuki N, Ihara Y. GM1 ganglioside-bound amyloid beta-protein (A beta): a possible form of preamyloid in Alzheimer's disease. Nat Med. 1995;1:1062–1066. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]
- Yang T, Hong S, O'Maley T, Sperling RA, Walsh DM, Selkoe DJ. New ELISAs with high specificity for soluble oligomers of amyloid β-protein detect natural Aβ oligomers in human brain but not CSF. Alzheimer's and Dementia. 2013 doi: 10.1016/j.jalz.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempel H, Thies E, Mandelkow E, Mandelkow E-M. Aβ oligomers cause localized Ca2+ elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W-Q, Santini F, Breese R, Ross D, Zhang XD, Stone DJ, Ferrer M, Townsend M, Wolfe AL, Seager MA, et al. Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. Journal of Biological Chemistry. 2010;285:7619–7632. doi: 10.1074/jbc.M109.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.