Summary
Background
Davunetide (AL-108, NAP) is an eightamino acid peptide that promotes microtubule stability and decreases tau phosphorylation in pre-clinical studies. Since PSP is tightly linked to tau pathology, davunetide could be an effective treatment for PSP.The goals of this study were to evaluate the efficacy and safety of davunetide in PSP.
Methods
A phase 2/3 double-blind, parallel group, clinical trial of davunetide 30 mg or placebo (randomized 1:1) administered intranasally twice daily for 52 weeks was conducted at 48centers. Participants met modifiedNNIPPS criteria for possible or probable PSP. Co-primary endpointswere the change from baseline in PSP Rating Scale (PSPRS) and Schwab and England ADL(SEADL) scale at up to 52 weeks. Data from all individuals who received at least one dose of medication and had a post-baseline efficacy assessment were compared using a rank-based method.Secondary outcomes included the Clinical Global Impression of Change (CGIC) and the change in regional brain volumeon MRI. Clinicaltrials.gov identifier: NCT01110720.
Findings
360 participants were screened, 313 were randomized and 243 (77.6%) completed the study. There were no group differences in PSPRS (mean difference: 0.49 [95% CI: −1.5, 2.5], p = 0.72) or SEADL (1% [−2, 4%], p = 0.76) change from baseline (CFB) and mean 52 week CFB PSPRS scores were similar between the davunetide (11.3 [9.8,12.8]) and placebo groups (10.9 [9.1, 13.0]). There wereno differences in any of the secondary or exploratory endpoints. There were 11deaths in the davunetide group and tenin the placebo group. There were more nasal adverse events in the davunetide group.
Interpretation
Davunetide is well tolerated but is not an effective treatment for PSP. Clinical trials of disease modifying therapy are feasible in PSP and should be pursued with other promising tau-directed therapies.
Funding
Allon Therapeutics
Introduction
Progressive supranuclear palsy (PSP) is a neurodegenerative cause of atypical Parkinsonism for which there are no approved or effective therapies.1 At autopsy, insoluble aggregates of the microtubule associated protein tau are found in neurons and glia throughout the brain, most prominently in the brainstem, deep cerebellar nuclei and basal ganglia, with variable involvement of neocortical regions.2,3 The most common clinical presentation of PSP, termed Richardson’s syndrome, has a prevalence of approximately 6.5 cases per 100,000 individuals,4 and characteristically involves early and severe gait instability with falls, a slowing of vertical greater than horizontal saccadic eye movements progressing to a supranuclear restriction of gaze, slowed movement, rigidity of the axial musculature, dysphagia and pseudobulbar affect, along with variable neuropsychiatric abnormalities and dementia. Genetically, PSP is strongly linked to the H1 tau gene (MAPT) haplotype and other single nucleotide polymorphisms within the MAPT gene,5 and MAPTmutations that lead to increased inclusion of the alternatively spliced exon 10,containing one of the four potential microtubule binding domains, can lead to an autosomal dominant familial PSP syndrome.6 A diagnosis of Richardson’s syndrome is highly predictive of underlying PSP or related four repeat tau pathologies,7 and therefore PSP has been suggested to be an ideal population for testing tau or microtubule-directed therapeutics for neurodegenerative disease.8
Davunetide (AL-108, NAP) is an eight amino acid peptide (single-letter amino acid code: NAPVSIPQ) derived from Activity Dependent Neuroprotective Protein (ADNP), a growth factor released from glial cells in response to exposure to vasoactive intestinal peptide. In cell culture models, davunetide has potent neuroprotective effects on cell death and microtubule disruption from a variety of toxic insults,and in transgenic mice carrying one or more human MAPT mutations that typically lead to severe autosomal dominant disease, davunetide ameliorates deposition of hyperphosphorylated, insoluble forms of tau and improves performance on behavioral tests such as the Morris water maze.9 A twelve week Phase 2 randomized placebo-controlled clinical trial of davunetide administered intranasallly in 144 individuals with amnestic mild cognitive impairment suggested a potential treatment benefit on attention and working memory.10 Since executive function deficits, often involving working memory and attention,are common in PSP,11 and based on davunetide’s proposed mechanism of action involving stabilization of microtubules and decreased tau pathology, we hypothesized that davunetide would be an effective therapy for PSP. To test this hypothesis we carried out a multicenter, randomized, parallel group, double-blind, placebo-controlled trial of davunetide for PSP. The primary objectives of the study were to determine the safety of davunetide, and its efficacy in reducing the rate of progression of clinical features of PSP.
Methods
Participants
Patients were recruited from 48 study centers in Australia, Canada, France, Germany, the United Kingdom andthe United States(full list of sites given at end of text). Study visits occurred between September 30, 2010 and November 1, 2012. Ethics board approval was obtained at each site and all participants gave written informed consent as per local regulations.
All participants met the following criteria for PSP (Richardson’s syndrome), which were modified from the PSP criteria from the National Neuroprotection in Parkinson’s Plus (NNIPPS) study:12 at least a 12-month history of postural instability or falls,occurring during the first 3 years that symptoms werepresent; decreased downward saccade velocity or supranuclear ophthalmoplegia;and an akinetic-rigid syndrome with prominent axial rigidity. In addition, at screening, individuals had to be between 41 to 85 years old;have a mini-mental state examination (MMSE)score ≥ 15; live outside a skilled nursing facility or dementia care facility; be able to ambulate independently or to take at least 5 steps with minimal assistance; have PSP symptoms for less than 5 years or symptoms for more than 5 years with a Progressive Supranuclear Palsy Rating Scale (PSPRS)13 of score ≥ 40; and be able to undergoa MRI scan during screening. Concomitant medications: Participants were allowed to take levodopa and other Parkinson’s medications, with the exception of rasagiline, if the dose had been stable for 60 days prior to screening. Participants were permitted to take rasagiline or co-enzyme Q10 if the dosewas stable for at least 90 days prior to screening. Exclusion criteriaincluded a clear and robust benefit from levodopa at the time of screening, evidence of motor neuron disease, use of acetylcholinesterase inhibitors, antipsychotics (other than quetiapine), memantine, lithium, methylene blue or other putative disease modifying drugs for PSP.
Clinicaltrials.gov identifier: NCT01110720.
Randomization, dose, and blinding
Participants were randomized to davunetide (the acetate salt of NAPVSIPQ)30 mg intranasally (two 0.1 ml puffs per nostril) twice dailyfrom a multi-dose, metered nasal spray, or matching placebo nasal spray (lacking davunetide, in identical bottles with similar nasal sensation when administered), for 52 weeks of treatment. The dose was the maximal dose that could be feasibly delivered by this route and corresponded to approximately twice that found to be effective in animal models.9 All participants and study personnel were blinded to treatment assignment. Randomization was 1:1 using permuted blocks. Randomization was stratified centrally by baseline PSPRS (< 40, ≥ 40), coQ10 use (yes, no), and age (< 70, ≥ 70) using an Interactive Web Response System (IWRS) that was incorporated into the electronic data capture system,and managed by the contract research organization that supported data collection (Research Pharmaceutical Services, Fort Washington, PA, USA).
Sample size determination
The target sample size was 300. This trial was designedto achieve a 2-sided alpha level of 0.05 at 90% power if the treatment effect was 4.13 PSPRS points (i.e., a 37.5% difference relative to an expected placebo change from baseline of 11.0,standard deviation of 11.0), based on publishednatural history data.13
Study procedures
Each participant participated in eight study visits over approximately 59 weeks. After the screening visit, a randomization/baseline visit occurred within 42 days, during which initial study medication was dispensed. Participants returned at weeks three, six, 13, 26, 39 and 52 weeks. After the week 52 visit, the study medication was stopped, and individuals were contacted by telephone at week 53 to assess adverse events and clinical status. Participantsfor whom an early termination visit was not possible due to disease progression or other factors, were assessed by telephone using a telephone questionnaire. Study medication compliance was assessed by comparing the weights of the nasal spray bottles at dispensing versus when individuals returned at follow up visits. Participants were deemed compliant if the calculated weight differences were within 75% to125% of expected.The primary efficacy measures were assessed at baseline and weekssix, 13, 26, 39 and 52 and safety measures at all visits. Adverse events were grouped by Medical Dictionary for Regulatory Activities (MedRA) system organ class (www.meddramsso.com). Serious Adverse Events were defined as those leading to hospitalization or death.
Outcome measures
The primary outcomes were the PSP Rating Scale (PSPRS)13 and the Schwab and England Activities of Daily Living Scale (SEADL).14 The PSPRS assesses 28 signs and symptoms of PSP in 6 categories: daily activities, behavior, bulbar, ocular motor, limb motor and gait/midline. Scores on the PSPRS range from a low of 0 (normal) to a high of 100 (most disability). The SEADL is an 11-point (0%, 10%, 20%...100%; 100% is normal) ordinal scale, which measures overall disability based on a patient and informant interview.Secondary outcomes included the Clinical Global Impression of Disease Severity (CGIds)15 or Change (CGIC)16 and brain ventricular volume as measured by boundary shift integral analysis of T1-weighted MRI images.17 For the MRI substudy, MRI scans were performed on 1.5 or 3T scanners and all sites were qualified by the Mayo Clinic, Mayo Aging and Dementia Imaging Research (ADIR) laboratory. Midbrain and superior cerebellar peduncle volumes were derived by label propagation in SPM5and treated as exploratory outcomes (Supplemental Methods).Exploratory neuropsychological outcomes measured included the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS),18 and three additional neuropsychological assessments of executive function (the color trails, phonemic fluency [F, A or S words per minute] and letter-number sequencing) were also included.11 Mood was assessed using the Geriatric Depression Scale.19 Safety and tolerability of davunetide was assessed by treatment emergent adverse events, electrocardiograms, nasal examination and clinical laboratory measures.
A subset of patients participated in an exploratory fluid biomarker study, undergoing lumbar punctures at baseline and 52 weeksto measure CSF amyloid beta1-42, tau, phosphorylated tau and neurofilament light chain (NfL) and plasma phosphorylated neurofilament heavy chain (pNFH);20 a genotyping substudy that determined the tau haplotype (H1 vs. H2) in each individual using single nucleotide polymorphism markers from a recent PSP genome wide association studyas previously described, 5 or a quantitative ocular motor study that measured vertical and horizontal saccades using infrared oculography (Supplemental Methods).
Statistical analysis
Primary and secondary outcomes were analyzed using an intent-to-treat (ITT) approach that included all participants who received at least one dose of medication and had a post-baseline efficacy assessment. For the primary analyses, a rank-based method was used to incorporate deaths and loss to follow-up as well as to allow for possibly skewed data. We assumed patients lost to follow up behaved like placebo participants. This method is similar to the rank based Wilcoxon test, but participantswhodie are treated as if they have the worst changes from baseline for the purpose of assigning ranks.21 Participantswhodiscontinued follow-up would be expected to continue to decline with respect to the PSPRS and SEADL if they could be followed. For participantswhodiscontinued follow-up for reasons other than death, the last observation recorded was used to assign the rank after subtracting the mean change from that time point to the end of the treatment period experienced by the placebo group. Using these ranks, a stratified rank test was performed through the use of a permutation test with strata formed by the randomization strata as described. 22 For the CSF analyses, 52 week change from baseline values were compared between treatment groups using a Wilcoxon test. Since no treatment-related differences were identified, data were combined for Spearman correlations with clinical and imaging variables.
Role of the funding source
The study was sponsored by Allon Therapeutics, Inc. (Vancouver, BC, CAN), a company that was purchased in August, 2013, by Paladin Laboratories. The study was designed by an academic steering committee in collaboration with the sponsor. The sponsor funded data collection, the planned analyses and an initial interpretation of the data. All authors had full access to the data, no medical writer or editor was employed, and the decision to submit the manuscript was made by the authors without input from any corporate entity.
Results
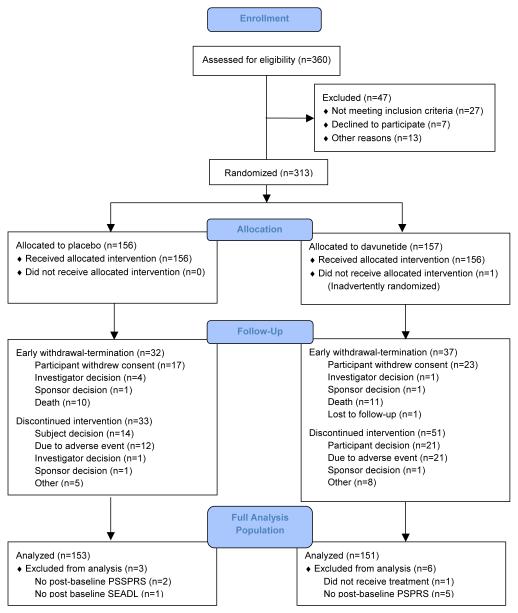
360 individuals were assessed for eligibility and 313 were randomized to davunetide (n = 157) or placebo (n = 156; Figure 1). There were no differences in baseline characteristics between the davunetide and placebo groups (Table 1). Medication compliance was similar between the two groups (mean for both = 94%).
Figure 1.
Atudy Profile
Table 1.
Baseline Characteristics of Study Population
| Davunetide | Placebo | Total | ||
|---|---|---|---|---|
| Number | 151 | 153 | 304 | |
| Age (years) | 68 (67,69) | 67 (66,68) | 68 (67,69) | |
| Sex - n (%) | female (%) | 71 (47) | 71 (47) | 142 (47) |
| Disease duration | n (%) > 5 years |
15 (10) | 12 (8) | 27 (9) |
| Weight (kg [95% CI]) | 77.9 (75.5,80.3) | 77.3 (74.5,80) | 77.6 (75.8,79.4) | |
| Race - n (%) | White | 132 (87.4) | 134 (87.6) | 266 (87.5) |
| Region –n (%) | Australia | 9 (6.0) | 5 (3.3) | 14 (4.6) |
| Europe | 45 (29.8) | 47 (30.7) | 92 (30.3) | |
| N. America | 97 (64.2) | 101 (66.0) | 198 (65.1) | |
| Tau Haplotype – n (% genotyped participants) |
H1/H1 | 119 (95.9) | 110 (93.2) | 229 (94.6) |
| H1/H2 | 5 (4.0) | 8 (6.8) | 13 (5.3) | |
| H2/H2 | 0 | 0 | 0 | |
| Missing | 27 (17.9) | 35 (22.9) | 62 (20.4) | |
| MMSE | 26 (25.4,26.6) | 26 (25.5,26.5) | 26 (25.6,26.4) | |
|
Concomitant medication
used during study |
||||
| CoQ10 use | n (%) | 30 (19.9) | 30 (19.6) | 60 (19.7) |
| Levodopa use | n (%) | 61 (39.1) | 70 (44.9) | 131 (42.0) |
| Primary outcomes | ||||
| PSPRS | 40 (38,42) | 39 (37,41) | 40 (39,41) | |
| SEADL | 0.5 (0.47,0.53) | 0.5 (0.47,0.52) | 0.5 (0.48,0.52) | |
|
Secondary/exploratory
outcomes |
||||
| GDS | 12 (11,14) | 13 (12,14) | 13 (12,14) | |
| CGIds | 3.9 (3.8,4.0) | 3.9 (3.7,4.1) | 3.9 (3.8,4.0) | |
| RBANS | (raw[95% CI]) | 141 (135,146) | 142 (137,147) | 141 (138,145) |
| RBANS | (scaled) | 73 (71,76) | 72.8 (71,75) | 73.1 (72,75) |
| Fluency | (words/min) | 11 (10,12) | 11.1 (11,12) | 11. (10,12) |
| Letter number seq. | (score) | 6.8 (6.4,7.2) | 7.1 (6.6,7.6) | 7.0 (6.7,7.3) |
| Color Trails 1 | 167.6 (156, 179) | 166.1 (154, 178) | 167.5 (159, 176) | |
| Color Trails 2 | 239 (228,250) | 241.9 (231,253) | 240 (232,249) | |
| MR Imaging | ||||
| Number | 145 | 146 | 291 | |
| Ventricular volume | (× 104 mm3) | 5.0 (4.7,5.4) | 4.9 (4.5,5.3) | 5.0 (4.7,5.2) |
| Whole brain volume | (× 104 mm3) | 127.8 (125.7,129.9) | 127.2 (125,129) | 127.5 (126,129) |
| Midbrain volume | (× 104 mm3) | 69 (0.68,0.70) | 0.69 (0.68,0.70) | 0.69 (0.68,0.70) |
| SCP volume | (× 104 mm3) | 0.039 (0.037,0.041) | 0.039 (0.037,0.041) | 0.039 (0.037,0.04) |
| CSF Analytes | ||||
| Number | 25 | 23 | 48 | |
| Beta amyloid (1-42) | (pg/ml) | 385 (348, 422) | 383 (334, 432) | 384 (354, 414) |
| Total tau | (pg/ml) | 60 (51.1,68.7) | 59.2 (48.7,69.6) | 59.5 (52.8,66.3) |
| Phosphorylated tau | (pg/ml) | 24.4 (21.9,26.9) | 24.7 (22.0,27.3) | 24.5 (22.7,26.4) |
| Neurofilament Light Chain | (pg/ml) | 4693 (3888, 5499) | 5719 (4233, 7205) | 5185 (4355, 6015) |
| Plasma analyte | ||||
| Number | 26 | 25 | 51 | |
| Phosphorylated Neurofiament Heavy Chain |
(pg/ml) | 800 (590,1010) | 720 (550, 890) | 760 (660,920) |
| Ocular motor | ||||
| Number | 13 | 6 | 19 | |
| Horiz. Saccade Latency | (msec) | 240 (217, 262) | 205 (169, 241) | 228 (209, 249) |
| Number | 7 | 5 | 12 | |
| Vertical First Saccade Gain | (degree) | 1.8 (0.6,3.0) | 1.8 (0.04,3.6) | 1.8 (0.8, 2.2) |
Abbreviations: MMSE: Mini-mental State Examination; PSPRS: Progressive Supranuclear Palsy Rating Scale; SEADL: Schwaab and England Activities of Daily Living Scale; GDS: Geriatric Depression Scale; CGIds: Clinical Global Impression of Disease Severity; RBANS: Repeatable Battery for the Assessment of Neuropsychological Disease Severity; SCP = superior cerebellar peduncle.
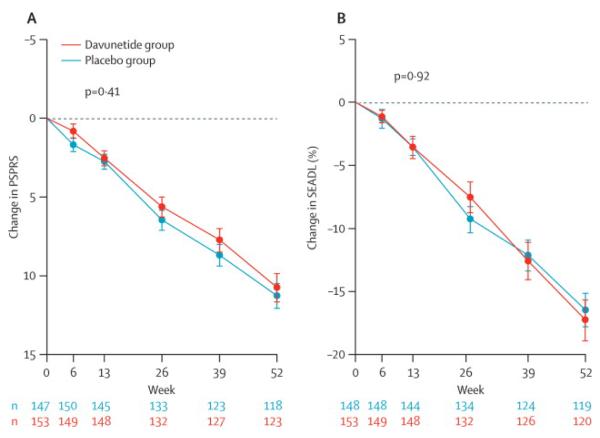
There were no differences in the efficacy primary endpoints, the 52 week change from baseline in the PSPRS and SEADL, between the davunetide and placebo group in either the primary ITT analysis(Table 2; Figure 2)or an analysis of participants who completed all study visits (Table 3). Likewise, sensitivity analyses to account for number and time of deaths in each group, as well as effects of demographic variables and concomitant medications, failed to reveal any differences between treatment groups. There were no group differences on the secondary efficacy measurements, the CGIC and the change in ventricular volume on MRI scans. The exploratory clinical endpoints, including the RBANS, executive function tests, CGIds, GDS also did not demonstrate a treatment effect.
Table 2.
Mean Difference in Change in Outcome Measures from Intent to Treat Analysis
| Median rank, value (95%CI) | Mean difference in values (95%CI) |
p value | ||
|---|---|---|---|---|
| Davunetide | Placebo | |||
| n = 151 | n = 153 | |||
| Primary Outcomes | ||||
| PSPRS | 152.5, 11.8 (10.5, 13.0) | 152.5, 11.8 (10.5, 13.0) | 0 (−2.2, 1.8) | 0.41 |
| SEADL | 153, −0.20 (−0.20, −0.17) | 153, −0.20 (−0.22, −0.17) | 0 (−0.02, 0) | 0.92 |
| Secondary Outcomes | ||||
| CGIC | 168.5, 6.0 (5.9, 6.1) | 168.5, 6.0 (5.5, 6.1) | 0 (0, 0) | 0.34 |
| MRI Ventricular vol.(× 104 mm3) | 121.0, 0.38 (0.33, 0.45) | 112.0, 0.38 (0.32, 0.45) | 0.005 (−0.07, 0.08) | 0.72 |
N and P values are from full analysis population (Figure 1).Abbreviations: PSPRS: Progressive Supranuclear Palsy Rating Scale; SEADL: Schwaab and England Activities of Daily Living Scale; CGIC: Clinical Global Impression of Change
Figure 2. Change from baseline in primary outcome measures, the PSPRS and SEADL.
(A) Least squares mean ± SEM change from baseline in PSPRS from primary ITT analysis. P = 0.41. An increase in PSPRS indicates worse disease.
(B) Least squares mean ± SEM change from baseine in SEADL from primary ITT analysis. P = 0.92. A decrease in SEADL indicates worse performance.
Table 3.
Mean Difference in Change in Outcome Measures from Baseline to 52 Week (Observed Cases)
| Mean 52 wk change (95%CI) | Mean difference (95%CI) |
p value | |||
|---|---|---|---|---|---|
| Davunetide | Placebo | Total | |||
| Clinical | |||||
| n = 118 | n = 123 | n = 241 | |||
| PSPRSa | 11.3 (9.8,12.8) | 10.9 (9.1,13.0) | 11.1 (9.9,12.3) | 0.49 (−1.5, 2.5) | 0.72 |
| SEADLa | −0.16 (−0.19,0.13) | −0.17 (−0.20,−0.14) | −0.17 (−0.19,−0.15) | 0.01 (−0.02,0.04) | 0.76 |
| CGICb | 5.0 (4.8,5.2) | 5.0 (4.8,5.2) | 5.0 (4.9,5.1) | 0.0 (−0.14,0.34) | 0.99 |
| CGIdsc | 0.91 (0.78,1.0) | 0.87 (0.70,1.0) | 0.89 (0.78,1.0) | 0.03 (−0.19,0.25) | 0.73 |
| GDSc | 0.32 (−0.67,1.3) | 0.77 (−0.11,1.6) | 0.55 (−0.11,1.2) | −0.45 (−1.8,0.91) | 0.52 |
| Neuropsychological | n = 106 | n = 111 | n = 217 | ||
| RBANS(Total Scaled)c | −5.6 (−7.3,−3.9) | −6.4 (−7.9,−4.9) | 6.4 (5.3,7.5) | 0.74 (−1.7,3.1) | 0.55 |
| RBANS(Total Raw) | −20.1 (−24.3,−15.8) | −23.8 (−33.8,−13.9) | −22.0 (−24.8,−19.3) | 3.8 (−2.8,10.3) | 0.35 |
| Phonemic Fluency(words/min)c | −2.0 (−1.2,−2.8) | −2.3 (−3.1,−1.5) | −2.1 (−2.7,−1.5) | 0.32 (−0.64,2.0) | 0.63 |
| Letter number seq.c | −1.0 (−1.45,−0.55) | −1.1 (−1.6,−0.62) | −1.1 (−1.4,−0.72) | 0.11 (−0.58,0.80) | 0.73 |
| Color Trails 2c | 36.0 (25.8,46.3) | 31.5 (21.6,41.4) | 33.8 (26.6,40.9) | 4.5 (−10.3,19.3) | 0.56 |
| MRI | |||||
| Absolute volume change | n = 111 | n = 108 | n = 219 | ||
| Ventricular vol.(× 104 mm3)b | 0.43 (0.36,0.50) | 0.42 (0.30,0.44) | 0.43 (0.31,0.41) | 0.01 (−10.6,10.6) | 0.97 |
| Whole brain vol.(× 104 mm3)c | −1.0 (−1.3,−0.79) | −1.2 (−1.4,−0.92) | −1.1 (−1.27,−0.93) | 0.15 (−0.09,0.37) | 0.31 |
| Midbrain volume(× 104 mm3)c | −0.02 (−0.03,−0.02) | −0.02 (−0.03,−0.02) | −0.02 (−0.03,−0.02) | −0.00 (−0.003,0.003) | 0.61 |
| SCP volume(× 104 mm3)c | −0.003(−0.003,−0.002) | −0.003 (−0.004,−0.002) | −0.003 (−0.003,−0.002) | 0.0005 (−0.0002,0.001) | 0.38 |
| Percent volume change | |||||
| Ventricular volume | 9.2% (7.8%, 11%) | 9.6% (7.7%, 10%) | 9.4% (8.6%, 10%) | 0.38% (−1.0%, 1.7%) | 0.64 |
| Whole brain volume | −0.79% (−0.61%,−0.98%) | −0.78% (−1.1%,−0.74%) | −0.86% (−0.86%,−0.85%) | −0.13% (−0.4%,0.2%) | 0.25 |
| Midbrain volume | −3.6% (−4.0%,−3.0%) | −3.4% (−3.9%,−2.9%) | −3.5% (−3.9%,−3.1%) | 0.1% (−0.6%, 0.8%) | 0.76 |
| SCP volume | −6.6% (−9.6%,−6%) | −7.9% (−0.27%,−11%) | −7.3% (−8.5%,−6.0%) | −1.3% (−3.8%, 1.2%) | 0.36 |
| CSF | |||||
| n = 10 | n = 12 | n = 22 | |||
| Aβ 1-42(pg/ml)c | 6.2 (−63.3,75.7) | 37.7 (−17.3,92.8) | 23.6 (−19.3,66.4) | −31.5 (−93.6,57.2) | 0.89 |
| NfL(pg/ml)c | 494 (−197,1186) | 922 (349,1496) | 755 (309,1201) | −498 (−587,−410) | 0.43 |
| Total tau(pg/ml)c | −4.6 (−20.1,10.9) | 9.7 (−1.5,21.0) | 2.9 (−6.7,12.5) | −14.3 (−33.4,4.8) | 0.42 |
| pTau(pg/ml)c | −1.2 (−5.1,2.7) | −0.18 (−6.2,5.9) | −0.67 (−2.3,3.0) | −1.0 (−8.2,6.2) | 0.38 |
| Plasma | |||||
| n=11 | n=12 | n=23 | |||
| pNFH (pg/ml) c | −80 (−0.380, 220) | −160 (−650,330) | −120 (−410, 170) | 80 (−490,650) | 0.93 |
| Ocular motor | |||||
| Horizontal Saccade Latency | n=13 | n=6 | n=19 | ||
| Absolute Change (msec) c | 51.1 (20.6, 81.7) | 20.9 (−3.5,45.3) | 41.6 (18.8, 64.4) | 30.2 (−8.8, 69.3) | 0.24 |
| Percent Change | 21.5% (9.7%, 33.4%) | 8.7% (−3.3%, 20.7%) | 17.5% (8.3%, 26.7%) | 12.8% (−4.1%, 29.7%) | 0.32 |
| Vertical First Saccade Gain | n=7 | n=5 | n=12 | ||
| Absolute Change(degrees) c | −0.86 (−1.4, −0.27) | −0.60 (−1.3,0.06) | −0.74 (−1.2, −0.31) | −0.26 (−1.1, 0.63) | 0.74 |
| Percent Change | −48.3% (−68.1%, −28.5%) | −43.7% (−59.5%, −27.8%) | −46.4% (−59.2%, −33.5%) | −4.6% (−30.0%, 20.7%) | 0.99 |
N and P values are from participants with complete 52 week data.
Primary endpoints;
Secondary endpoints;
Exploratory endpoints. Abbreviations: PSPRS: Progressive Supranuclear Palsy Rating Scale; SEADL: Schwaab and England Activities of Daily Living Scale; GDS: Geriatric Depression Scale; CGIds: Clinical Global Impression of Disease Severity; RBANS: Repeatable Battery for the Assessment of Neuropsychological Disease Severity; SCP = superior cerebellar peduncle; NfL = Neurofilament Light Chain; pTau= phosphorylated tau (residue 181).
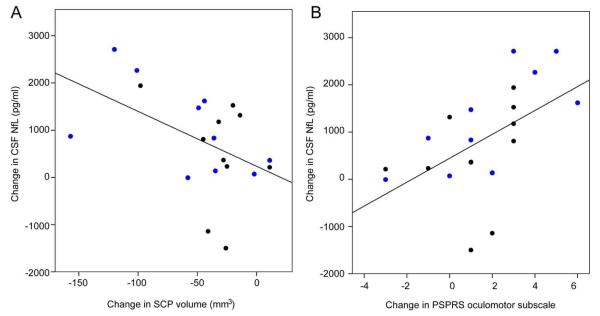
For the exploratory imaging, ocular motor, plasma and CSF biomarker endpoints, there were no differences in rates of change between treatment groups (Table 3). All MRI measurements, including total ventricular, total brain, midbrain and superior cerebellar peduncle (SCP)volume demonstrated progressive atrophy over one year based on examination of 95% confidence intervals in the combined study population (Table 3). Horizontal saccade latency increased and vertical first saccade gain decreased over one year (no changes in other measures; data not shown). Of the five fluid analytes, only the CSF NfL concentrations changed over time. Interestingly, in a post hoc analysis, the one year change in NfL levels were correlated with the change in SCP volume (Spearman’s rho = −0.450, p = 0.045; Figure 3) as well as the oculomotor subscale of the PSPRS (rho = 0.609; p = 0.003).
Figure 3. Longitudinal change in CSF Nf-L correlated with imaging and clinical variables.
Change in CSF neurofilament light chain concentration is correlated with (A) change in superior cerebellar peduncle volume measured on MRI (Spearman’s rho = −0.450, p = 0.045;n = 19) and (B) change in PSPRS oculomotor subscale (rho = 0.609; p = 0.003, n = 20). Blue circles: davunetide; black circles: placebo.
Davunetide was well tolerated. Comparable numbers of individuals experienced treatment emergent adverse events (Table 4). There were 11 deaths in the davunetide group and 10on placebo. There were 54 Serious Adverse Events in each of the treatment groups. Most adverse events were mild to moderate in intensity and were evenly distributed between treatment groups. There were more participants in the davunetide-treated group (n=21) than in the placebo-treated group (n= 12) who had AEs leading to study drug discontinuation, with most related to epistaxis or nasal congestion. There were no differences in the ECG or safety laboratory values between treatment groups (data not shown).
Table 4.
Adverse Events Occurring in Greater than 5% of Study Participants
| System Organ Class Preferred Term |
Davunetide (N=156) n (%) |
Placebo (N=156) n (%) |
Total (N=312) n (%) |
|---|---|---|---|
| Participants with at Least One Event | 145 (92.9) | 148 (94.9) | 293 (93.9) |
| Eye disorders | 30 (19.2) | 20 (12.8) | 50 (16.0) |
| Gastrointestinal disorders | 37 (23.7) | 48 (30.8) | 85 (27.2) |
| Constipation | 12 (7.7) | 12 (7.7) | 24 (7.7) |
| Dysphagia | 8 (5.1) | 10 (6.4) | 18 (5.8) |
| General disorders and administration site conditions | 24 (15.4) | 27 (17.3) | 51 (16.3) |
| Infections and infestations | 61 (39.1) | 68 (43.6) | 129 (41.3) |
| Urinary tract infection | 18 (11.5) | 31 (19.9) | 49 (15.7) |
| Nasopharyngitis | 7 (4.5) | 11 (7.1) | 18 (5.8) |
| Injury, poisoning and procedural complications | 84 (53.8) | 86 (55.1) | 170 (54.5) |
| Fall | 58 (37.2) | 56 (35.9) | 114 (36.5) |
| Skin laceration | 26 (16.7) | 28 (17.9) | 54 (17.3) |
| Contusion | 6 (3.8) | 16 (10.3) | 22 (7.1) |
| Investigations | 31 (19.9) | 22 (14.1) | 53 (17.0) |
| Metabolism and nutrition disorders | 14 (9.0) | 7 (4.5) | 21 (6.7) |
| Musculoskeletal and connective tissue disorders | 35 (22.4) | 43 (27.6) | 78 (25.0) |
| Nervous system disorders | 40 (25.6) | 62 (39.7) | 102 (32.7) |
| Dizziness | 6 (3.8) | 12 (7.7) | 18 (5.8) |
| Progressive supranuclear palsy, worsening | 6 (3.8) | 11 (7.1) | 17 (5.4) |
| Psychiatric disorders | 31 (19.9) | 47 (30.1) | 78 (25.0) |
| Depression | 9 (5.8) | 12 (7.7) | 21 (6.7) |
| Insomnia | 6 (3.8) | 11 (7.1) | 17 (5.4) |
| Renal and urinary disorders | 18 (11.5) | 20 (12.8) | 38 (12.2) |
| Respiratory, thoracic and mediastinal disorders | 77 (49.4) | 61 (39.1) | 138 (44.2) |
| Epistaxis | 18 (11.5) | 13 (8.3) | 31 (9.9) |
| Cough | 12 (7.7) | 12 (7.7) | 24 (7.7) |
| Rhinorrhea | 15 (9.6) | 8 (5.1) | 23 (7.4) |
| Nasal congestion | 18 (11.5) | 3 (1.9) | 21 (6.7) |
| Nasal mucosal disorder | 9 (5.8) | 8 (5.1) | 17 (5.4) |
| Nasal discomfort | 15 (9.6) | 1 (0.6) | 16 (5.1) |
| Skin and subcutaneous tissue disorders | 18 (11.5) | 21 (13.5) | 39 (12.5) |
| Vascular disorders | 10 (6.4) | 10 (6.4) | 20 (6.4) |
Adverse events leading to study medication discontinuation in greater than two individuals are shown in bold.
Discussion
This phase 2/3 clinical trial found no effect of 52 weeks of twice daily 30 mg intranasally-administered davunetide treatment in PSP other than small differences in the rate of nasal adverse events. The groups were well-matched at baseline, and the primary outcome measures, the PSPRS and the SEADL, demonstrated the expected annual rates of decline in both treatment groups, suggesting that the study was adequately powered to detect a treatment effect of intranasal davunetide if one had been present. No effects of davunetide treatment were observed on the secondary outcome measures, the CGIC and the annual rate of brain atrophy as measured by total ventricular volume on volumetric MRI scans. In addition, no effects of davunetide treatment were observed on the rate of change in a variety of exploratory cognitive assessments, volumetric MRI measurements or, in a small subset of individuals, CSF neurodegenerative disease biomarkers. Because pharmacokinetic measurements of davunetide were not performed as part of this study, and there are no known pharmacodynamic biomarkers available for davunetide, we cannot be certain that sufficient concentrations of davunetide entered the CNS to exert an effect, we had the correct dose or that the drug engaged its target. However, our results definitively show that davunetide as administered is not an effective treatment for PSP, despite preclinical data suggesting benefits related to ameliorating tau pathology. Nonetheless, this study is one of the largest clinical trials ever conducted in PSP, and it provides a wealth of new information about disease progression, longitudinal imaging and fluid biomarker changes that will be very useful in designing future PSP studies.
This is the first pivotal (intended for registration) clinical trial of a tau-directed therapeutic agent for PSP. It is also the largestmulticenter clinical trial to employ the PSPRS. 13 A recent, negative, year-long, multicenter trial of the glycogen synthase kinase 3 (GSK-3) inhibitor, tideglusib, also used the PSPRS and identified a similar rate of decline.23 Previous, shorter duration, single center clinical trials used the PSPRS, with one showing a small benefit of co-enzyme Q10 therapy24 and another showing no effect of donepezil treatment on this scale.25 Importantly, we found a mean annual rate of decline on the PSPRS of 11.0 (9.9 – 12.3) points which is nearly identical to the single center validation study for which only one rater examined all the individuals. 13 The survival rate of approximately 93.1% over 53 weeks of follow-up was slightly higher than predicted from the PSPRS validation study (86.9%) based on the mean baseline PSPRS score of 40 in our patient population, but similar to the tideglusib trial.13 For the co-primary endpoint, the SEADL, we determined an annual rate of decline of 17 (15 -19)% which is nearly identical to that reported for NNIPPS clinical trial of riluzole for PSP and the tideglusib trial,in patients displaying a similar baseline degree of impairment. 12, 15 Together, these results suggest that the diagnostic criteria for PSP we used are reliable for recruiting mild-moderately impaired PSP patients into multicenter trials, and that once enrolled, such individuals follow a highly predictable rate of clinical decline.
This is the first study to report longitudinal CSF biomarker analyses in PSP. With the caveat that we had limited numbers of samples to analyze,we found that the standard (INNOBIA AlzBio3) total tau, phosphorylated tau and beta amyloid levels commonly studied in Alzheimer’s Disease (AD) did not change over one year in PSP. In contrast, CSF NfL levels increased over 52 weeks in the PSP participants and the longitudinal changes in CSF NfL levels were correlated with changes in clinical ocular motor ratings on the PSPRS and changes in superior cerebellar peduncle (SCP) volume on MRI scans. Of note, CSF NfL levels have previously been shown to be elevated in PSP and correlated with disease severity as measured by Hoehn and Yahr scores,26 and are correlated with disease severity in other forms of frontotemporal lobar degeneration, but not AD. 20 NfL levels decrease in response to disease modifying therapy in multiple sclerosis.27 Our findings suggest that CSF NfL is likely to be a useful biomarker of disease progression in PSP. Although we observed no longitudinal changes in CSF tau or phosphorylated tau, both measurements have identified treatment effects of anti-amyloid therapies in AD clinical trials where no longitudinal increase in levels was identified in the placebo group28 or comparable natural history studies.29 Thus it remains possible that these measurements may be useful in future PSP trials, particularly those involving tau-directed therapeutic agents.
This is the largest multicenter study to measure longitudinal brain volume changes in PSP. We found no treatment-related difference in brain atrophy rates, with a mean annual brain atrophy rate similar to the approximately 1% atrophy rate reported previously in single center natural history studies.17, 30, 31 The recent tideglusib trial included a smaller MRI substudy (n = 37) that showedreducedwhole brain and parietal-occipital atrophy rates in treated individuals.32 Of note, the mean annual brain atrophy rate in the tideglusib study’s placebo group was 3.1%, which is higher than what we observed(0.86%).Since we used similar volumetric methods to previous PSP MRI natural history studies and obtained similar results, the differences in brain atrophy rates between our study and the tideglusib MRI substudy may have arisen from the different volumetric analysis methods they used or differences in clinical characteristics between the MRI study populations. While our mean annual ventricular volume expansion rate of approximately 9.4% was similar to previous studies, midbrain atrophy rates have been more variable, and ouridentified rate of 3.6% fell in the lower range of previously reported values (2.2 – 10.5%). Our 7.3% annual rate of SCP atrophy was higher than the 3.5% rate reported in a previous study.30 It is likely that the subtle differences in regional atrophy rates arose from the different volumetric imaging methods employed here.
This study clearly demonstrates the feasibility of conducting multicenter clinical trials in PSP, and the striking reproducibility of rates of change on standard outcome measures such as the PSPRS. It also demonstrates the potential utility of volumetric brain imaging and CSF NfL as biomarkers of disease progression in PSP. Similar to a recent clinical trial of another tau-directed therapy (tideglusib) in PSP, no clinical benefits were seen. Although the tideglusibtrialidentified a treatment effect on brain atrophy rate, the significance of this finding is unclear since there were no associated clinical benefits. Importantly, neither the tideglusib trial nor ourstudy included pharmacodynamic measurements that demonstrated that the investigational agent engaged its molecular target in the trial participantsand had the predicted mechanistic effect. Therefore the biological hypotheses underlying both of these trials were not adequately tested. Future studies of tau-directed therapeutics should be pursued in PSP, butmust incorporate phamacodynamic measures of target engagement. Tau PET imaging33 is a promising new biomarker that may help to demonstrate target engagement and allow identification of PSP patients at earlier stages of disease when tau-directed therapeutics are most likely to be effective.
Research in Context
Systematic review
We searched Pubmed using the following terms: “progressive supranuclear palsy,” “placebo” and “trial.” We identified 13 previous randomized, placebo-controlled trials of treatments for PSP between 1983 and 2014. Previous studies investigated the effects of multiple cholinergic agents including donepezil,25 riluzole,12 co-enzyme Q1024 and the GSK-3 inhibitor, tideglusib,23 on the symptoms of PSP. A small, beneficial treatment effect of co-enzyme Q10 was identified after 6 weeks of treatment in study that included 21 participants enrolled at a single center.24 Only two other studies followed participants for one year or longer,12,23 and no other beneficial clinical treatment effects were identified, although tideglusib appeared to decrease the rate of brain atrophy in a small MRI substudy.32
Interpretation
Similar to previous clinical trials of other potential therapies, we found no benefit of davunetide administered intranasally in PSP.To date, no therapies have been demonstrated to be effective in ameliorating the symptoms or slowing the rate of clinical progression of PSP over the course of one year or longer in a randomized, placebo-controlled clinical trial.
Supplementary Material
Acknowledgements
We thank Jennifer Whitwell and Matthew Senjem for their contributions to developing the volumetric MRI measurement methods.
Footnotes
The AL-108-231 Study Group
Australia: David Williams
Canada: Anne Louise Lafontaine, Connie Marras, Mandar Jog, Michael Panisset
France: Jean-Christophe Corvol, Jean-Philippe Azulay, Philippe Couratier
Germany: Brit Mollenhauer, Stefan Lorenzl, Albert Ludolph, Reiner Benecke, Günter Höglinger, Axel Lipp, Heinz Reichmann, Dirk Woitalla
United Kingdom: Dennis Chan, Adam Zermansky, David Burn
United States: Adam Boxer, Erik Roberson, Lawrence Honig, Edward Zamrini, Rajesh Pahwa, Yvette Bordelon, Erika Driver-Dunkley, Stephanie Lessig, Mark Lew, Kyle Womack, Brad Boeve, Joseph Ferrara, Argyle Hillis, Daniel Kaufer, Rajeev Kumar, Tao Xie, Steven Gunzler, Theresa Zesiewicz, Praveen Dayalu, Lawrence Golbe, Murray Grossman, Joseph Jancovic, Scott McGinnis, Anthony Santiago, Paul Tuite, Stuart Isaacson, Julie Leegwater-Kim, Irene Litvan
Author Contributions The AL-108-231 protocol was adapted from a grant proposal written by ALB, DSK, M. Grossman and BLM, and further modified by SW, AJS, BHM and M. Gold. The planned statistical analyses were performed by JH. The analyses were replicated, and new exploratory analyses were performed by ALB and IVL in the process of manuscript preparation. ALB wrote the manuscript with assistance from the other authors after Allon Therapeutics ceased to exist as a company. ALB participated in study leadership, data collection and interpretation. AEL, MG, DSK, BLM, LSS, RSD and AL participated in study design and protocol development, data collection and edited the manuscript. LIG, GUH, DRW, IL, MK, CRJ, VVD and CR participated in protocol development, data collection and manuscript revision. AL, DB, SL, EDR, GUH and MK participated in data collection and manuscript revision. IVL performed statistical analyses and participated in manuscript revision. HWH participated in design and analysis of the ocular motor substudy, and manuscript revision. IG invented davunetide and participated in manuscript revision. SW, JH, AJS, MG, LP and BHM participated in study design and protocol development, study leadership, data interpretation and manuscript revision. All authors reviewed and edited the manuscript and gave approval for its submission.
Conflicts of Interest
SW, BHM, AJS, M.Gold and LP were employees of Allon Therapeutics and JH was a paid contractor. AEL, M. Grossman, DSK, RSD, LSS, AJL and BLM were paid consultants for Allon Therapeutics. ALB received research support from Allon Therapeutics for enrolling participants and assistance with trial management in the form of a contract between Allon Therapeutics and the University of California, San Francisco. By contractual agreement with Allon Therapeutics, ALB, on behalf of the AL-108-231 steering committee (AEL, ALB, MG, LSS, RSD and AJL) took possession of all data and biological samples from the clinical trial.
In addition, ALB served as a consultant for Acetylon, Archer, Ipierian, Isis and Neurophage and received research support from BMS, C2N,Eli Lilly, Genentech, Janssen, Pfizer and TauRx for conducting clinical trials. He is funded by the NIH, the Alzheimer’s Association, the Bluefield Project to Cure FTD, CBD Solutions and the Tau Research Consortium.
AEL has served as an advisor for Abbott, Abbvie, Allon Therapeutics, Avanir Pharmaceuticals, Biogen Idec, Boerhinger-Ingelheim, Ceregene, Lilly, Medtronic, Merck, Novartis, NeuroPhage Pharmaceuticals, Teva and UCB; received honoraria from Teva, UCB, AbbVie; received grants from Brain Canada, Canadian Institutes of Health Research, Edmond J Safra Philanthropic Foundation, Michael J. Fox Foundation, the Ontario Brain Institute, National Parkinson Foundation, Parkinson Society Canada, Tourette Syndrome Association, W. Garfield Weston Foundation; received publishing royalties from Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press; and has served as an expert witness in cases related to the welding industry.
DSK serves as Deputy Editor for Neurology®; served on a Data Safety Monitoring Board for Lilly Pharmaceuticals; will serve on a Data Safety Monitoring Board for Lundbeck
Pharmaceuticals and for the DIAN study; served as a consultant to TauRx Pharmaceuticals, was an investigator in clinical trials sponsored by Baxter and Elan Pharmaceuticals in the past 2 years; and receives research support from the NIH.
BLM serves on a scientific advisory board for the Alzheimer’s Disease Clinical Study; serves as an Editor for Neurocase and as an Associate Editor of ADAD; receives royalties from the publication of Behavioral Neurology of Dementia (Cambridge, 2009), Handbook of Neurology (Elsevier, 2009), and The Human Frontal Lobes (Guilford, 2008); serves as a consultant for Lundbeck Inc., Elan Corporation, and Allon Therapeutics, Inc.; serves on speakers’ bureaus for Novartis and Pfizer Inc.; and receives research support from Novartis and the NIH and the State of California.
RSD has received grant funds from NIH, and serves as PI on clinical trials for which her institution receives support from Accera, Avanir, Genentech, Pfizer and Takeda. She has served as a consultant for Abbvie, Accera, AC Immune, Avanir, AZ Therapies, Baxter, Biote, Chiesi, Hoffman LaRoche, Merck, Novartis, Targacept, and Toyama. She receives research support from the State of Texas. She has stock options in AZTherapies, QR Pharma, Sonexa and Transition and serves on the editorial boards of AD Research and Therapy and Dementia and Geriatric Cognitive Disorders.
LSS has received grants from the NIH, the State of California, the Alzheimer’s Association for a registry for dementia and cognitive impairment trials, and grants or research support from Baxter, Genentech, Johnson & Johnson, Eli Lilly, Lundbeck, Novartis, Pfizer, and Tau Rx. He has served as a consultant for and received consulting fees from Abbott Laboratories, AC Immune, Allon, AstraZeneca, Baxter, Biogen Idec, Biotie, Bristol-Myers Squibb, Chiesi, Elan, Eli Lilly, EnVivo, GlaxoSmithKline, Johnson & Johnson, Lundbeck, MedAvante, Merck, Novartis, Piramal, Pfizer, Roche, Sanofi, Servier, Takeda, Tau Rx, Toyama and Zinfandel.
JCC received personal fees from Allon Therapeutics during the conduct of the study; personal fees from Impax Laboratory, grants from Sanofi-Aventis, personal fees from Novartis, personal fees from BIAL - Portela & Ca, S., personal fees from Addex, personal fees from Lunbeck, outside the submitted work.
IG served on the Board of Directors of Allon Therapeutics until its purchase by Paladin Labs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stamelou M, de Silva R, Arias-Carrion O, et al. Rational therapeutic approaches to progressive supranuclear palsy. Brain. 2010;133(Pt 6):1578–90. doi: 10.1093/brain/awq115. [DOI] [PubMed] [Google Scholar]
- 2.Steele JC, Richardson JC, Olszewski J, Progressive Supranuclear Palsy A Heterogeneous Degeneration Involving the Brain Stem, Basal Ganglia and Cerebellum with Vertical Gaze and Pseudobulbar Palsy, Nuchal Dystonia and Dementia. Arch Neurol. 1964;10:333–59. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- 3.Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009;8(3):270–9. doi: 10.1016/S1474-4422(09)70042-0. [DOI] [PubMed] [Google Scholar]
- 4.Nath U, Ben-Shlomo Y, Thomson RG, et al. The prevalence of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) in the UK. Brain. 2001;124(Pt 7):1438–49. doi: 10.1093/brain/124.7.1438. [DOI] [PubMed] [Google Scholar]
- 5.Hoglinger GU, Melhem NM, Dickson DW, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nature genetics. 2011;43(7):699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reed LA, Wszolek ZK, Hutton M. Phenotypic correlations in FTDP-17. Neurobiol Aging. 2001;22(1):89–107. doi: 10.1016/s0197-4580(00)00202-5. [DOI] [PubMed] [Google Scholar]
- 7.Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol. 2010;23(4):394–400. doi: 10.1097/WCO.0b013e32833be924. [DOI] [PubMed] [Google Scholar]
- 8.Boxer AL, Gold M, Huey E, et al. The advantages of frontotemporal degeneration drug development (part 2 of frontotemporal degeneration: The next therapeutic frontier) Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2012 doi: 10.1016/j.jalz.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiryaev N, Jouroukhin Y, Giladi E, et al. NAP protects memory, increases soluble tau and reduces tau hyperphosphorylation in a tauopathy model. Neurobiol Dis. 2009;34(2):381–8. doi: 10.1016/j.nbd.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto BH, Schmechel D, Hirman J, Blackwell A, Keith J, Gold M. A Double-Blind, Placebo-Controlled, Ascending-Dose, Randomized Study to Evaluate the Safety, Tolerability and Effects on Cognition of AL-108 after 12 Weeks of Intranasal Administration in Subjects with Mild Cognitive Impairment. Dementia and geriatric cognitive disorders. 2013;35(5-6):322–36. doi: 10.1159/000348347. [DOI] [PubMed] [Google Scholar]
- 11.Brown RG, Lacomblez L, Landwehrmeyer BG, et al. Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain : a journal of neurology. 2010;133(Pt 8):2382–93. doi: 10.1093/brain/awq158. [DOI] [PubMed] [Google Scholar]
- 12.Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, Leigh PN. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain. 2009;132(Pt 1):156–71. doi: 10.1093/brain/awn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain. 2007;130(Pt 6):1552–65. doi: 10.1093/brain/awm032. [DOI] [PubMed] [Google Scholar]
- 14.Schwab R, England A. Projecton technique for evaluating surgery in Parkinson’s disease. In: Gillingham F, Donaldson M, editors. Third Symposium on Parkinson’s Disease Research. ES Livingston; Edinburgh, Scotland: 1969. [Google Scholar]
- 15.Payan CA, Viallet F, Landwehrmeyer BG, et al. Disease severity and progression in progressive supranuclear palsy and multiple system atrophy: validation of the NNIPPS--Parkinson Plus Scale. PLoS ONE. 2011;6(8):e22293. doi: 10.1371/journal.pone.0022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl 2):S22–32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 17.Whitwell JL, Jack CR, Jr., Parisi JE, et al. Rates of cerebral atrophy differ in different degenerative pathologies. Brain. 2007;130(Pt 4):1148–58. doi: 10.1093/brain/awm021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of clinical and experimental neuropsychology. 1998;20(3):310–9. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 19.Yesavage JA, Brink TL, Rolse TL, et al. Development and validity of a Geriatric Depression Scale: A preliminary report. J Psychiatric Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 20.Scherling CS, Hall T, Berisha F, et al. CSF neurofilament concentration reflects disease severity in frontotemporal degeneration. Ann Neurol. 2013 doi: 10.1002/ana.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lachin JM. Worst-rank score analysis with informatively missing observations in clinical trials. Control Clin Trials. 1999;20(5):408–22. doi: 10.1016/s0197-2456(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 22.LaVange LM, Durham TA, Koch GG. Randomization-based nonparametric methods for the analysis of multicentre trials. Stat Methods Med Res. 2005;14(3):281–301. doi: 10.1191/0962280205sm397oa. [DOI] [PubMed] [Google Scholar]
- 23.Tolosa E, Litvan I, Hoglinger GU, et al. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov Disord. 2014 doi: 10.1002/mds.25824. [DOI] [PubMed] [Google Scholar]
- 24.Stamelou M, Reuss A, Pilatus U, et al. Short-term effects of coenzyme Q10 in progressive supranuclear palsy: a randomized, placebo-controlled trial. Mov Disord. 2008;23(7):942–9. doi: 10.1002/mds.22023. [DOI] [PubMed] [Google Scholar]
- 25.Litvan I, Phipps M, Pharr VL, Hallett M, Grafman J, Salazar A. Randomized placebo-controlled trial of donepezil in patients with progressive supranuclear palsy. Neurology. 2001;57(3):467–73. doi: 10.1212/wnl.57.3.467. [DOI] [PubMed] [Google Scholar]
- 26.Hall S, Ohrfelt A, Constantinescu R, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Archives of neurology. 2012;69(11):1445–52. doi: 10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- 27.Gunnarsson M, Malmestrom C, Axelsson M, et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Annals of Neurology. 2011;69(1):83–9. doi: 10.1002/ana.22247. [DOI] [PubMed] [Google Scholar]
- 28.Blennow K, Zetterberg H, Rinne JO, et al. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol. 2012;69(8):1002–10. doi: 10.1001/archneurol.2012.90. [DOI] [PubMed] [Google Scholar]
- 29.Kester MI, Scheffer PG, Koel-Simmelink MJ, et al. Serial CSF sampling in Alzheimer’s disease: specific versus non-specific markers. Neurobiol Aging. 2012;33(8):1591–8. doi: 10.1016/j.neurobiolaging.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Paviour DC, Price SL, Jahanshahi M, Lees AJ, Fox NC. Longitudinal MRI in progressive supranuclear palsy and multiple system atrophy: rates and regions of atrophy. Brain. 2006;129(Pt 4):1040–9. doi: 10.1093/brain/awl021. [DOI] [PubMed] [Google Scholar]
- 31.Josephs KA, Xia R, Mandrekar J, et al. Modeling trajectories of regional volume loss in progressive supranuclear palsy. Movement disorders : official journal of the Movement Disorder Society. 2013;28(8):1117–24. doi: 10.1002/mds.25437. [DOI] [PubMed] [Google Scholar]
- 32.Hoglinger GU, Huppertz HJ, Wagenpfeil S, et al. Tideglusib reduces progression of brain atrophy in progressive supranuclear palsy in a randomized trial. Mov Disord. 2014 doi: 10.1002/mds.25815. [DOI] [PubMed] [Google Scholar]
- 33.Maruyama M, Shimada H, Suhara T, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79(6):1094–108. doi: 10.1016/j.neuron.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.