Abstract
Background
Surgical management of incidental Meckel’s diverticulum (MD) is a highly debated controversial issue that has never been discussed from the oncological standpoint.
Objective
To describe the epidemiology and risk of Meckel’s diverticulum cancer (MDC) and compare it with other ileal malignancies.
Methods
Data were obtained from 163 cases of MDC and 6214 cases of non-Meckelian ileal cancer, between 1973 and 2006, from the Surveillance, Epidemiology, and End Results database.
Results
Mean annual incidence was 1.44 (± 1.12) per 10 million population, with a 5-fold increase in the last few decades. Incidence increases with age, with a mean age at diagnosis of 60.6 (±15.1) years. Adjusted risk of cancer in the MD was at least 70 times higher than any other ileal site. Disease was localized in 67% at presentation and malignant carcinoids constituted the major histologic type (77%). One-third of patients have had lifetime occurrence of other malignancies and in 13% of these patients, MDC was the first malignancy. Median tumor size was 7 mm. Median overall survival was 173 months (95% confidence interval [CI], 124–221 months), with 1- and 5-year relative survival rates of 85.8% (95% CI, 76.9%-91.4%) and 75.8% (95% CI, 64.9%-83.8%), respectively. Cox proportional hazards model revealed that age, histologic type, and metastatic disease were independent factors affecting survival.
Conclusions
MD is a “hot-spot” or high-risk area for cancer in the ileum. With risk that increases with age and high possibility of curative resection with negligible operative mortality, incidental MD is best treated with resection.
In 1598, Fabricius Hildanus described the presence of a diverticulum of the small bowel occurring in the distal part of the ileum, which later came to be called by the last name of the German anatomist, Johann Friedrich Meckel who described its embryology in 1809.1 The Meckel’s diverticulum (MD) is a true congenital diverticulum that is a remnant of the omphalomesenteric duct, the incomplete obliteration of which can result in a range of anomalies, the most common (90%) being MD.2 It is the most common congenital anomaly of the GI tract, with an estimated prevalence of 2%,3 although several autopsy studies describe a general population prevalence in the range of 0.3% to 3%.4,5 About 90% of MDs occur within 100 cm of the ileocecal valve1 and measure approximately 3 cm in length.1,6,7 Though there is a significant prevalence of ectopic tissue nests in the MD (10%–60%), they are commonly asymptom-atic.1,5,6,8,9 Malignancies are reported to account for only 0.5% to 3.2% of the complications.4,10
Several tumors of the MD have been described, with carcinoids being the most common.11–14 Other pathologic types include adenocarcinoma,15–18 pancreatic carcinoma,19 intraductal papillary mucinous neoplasm,20 gastrointestinal stromal tumors (GIST) and leiomyosarcomas,4,21,22 lymphoma,23,24 lipoma,25 adenomyoma,26 and villous adenoma.27 In 1992, Neis et al summarized 104 published cases of Meckel diverticulum cancer (MDC).13 In their review of 13,715 carcinoids from the Surveillance, Epidemiology, and End Results (SEER) database, Modlin et al observed that 0.48% to 0.74% of all carcinoids in the body occurred in the MD.28 To the best of our knowledge, there have been no single population-based analysis of the epidemiological and prognostic features of primary MD malignancies. In this study, we have performed such an analysis of patients with MDC identified in the SEER database and from that analysis have identified implications for the management of incidentally found MDs.
METHODS
SEER Database
The SEER program is a National Cancer Institute-based authoritative source of cancer data in the United States, with a coverage of 26% of the US population.29 SEER receives mortality data from the National Center for Health Statistics and population data periodically from the Census Bureau. The population-based cancer registries of SEER program include Alaska Native Tumor Registry, Arizona Indians, Los Angeles, San Francisco-Oakland, San Jose-Monterey, Greater California, Connecticut, Detroit, Atlanta, Rural Georgia, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, Seattle-Puget Sound, and Utah. However, there are several grouping of these registries for analysis such as SEER 9, SEER 11, SEER 13, and SEER 17, the number following “SEER” referring to the number of registries included in the grouping. For the most extensive grouping SEER 17, statistics that do not require population data such as survival are often reported for years prior to 2000. For population data-dependent statistics such as incidence, SEER 9 is used as it contributes cases diagnosed from 1973 to 2006, except Seattle-Puget Sound that contributes cases from 1974 and Atlanta that contributes cases from 1975. More information is available at: http://seer.cancer.gov/registries/terms.html.
Statistical Analysis
Apart from some basic statistical analysis performed using Microsoft Excel, the authors used SEER* Stat software provided by the SEER program, which is an intuitive tool that derives simple population-based epidemiological calculations. The data were analyzed using SPSS software (SPSS 17, IBM, Chicago, IL). Survival analysis was performed using Kaplan-Meier curves for overall survival (OS) and statistical significance (P) was tested using Log rank (Mantel-Cox) test. Breslow (Generalized Wilcoxon) and Tarone-Ware tests were also used to confirm statistical significance in OS comparisons. Relative survival values were derived using the SEER*Stat software. An alpha value (P) of 0.05 to identify statistical significance was uniformly adopted for all statistical analyses. Mean values were expressed along with the standard deviation (SD), and statistical significance was tested using Student t test with 2-tailed P values. Standard error (SE) or 95% confidence intervals (CI) were provided for all median survival estimates. A Cox proportional hazards model was executed to identify hazard ratios (HR) for all factors that independently affected survival. Most analyses used a sample space of 158 patients, as 5 patients with MDC diagnosed at autopsy were excluded. Patients who had some other primary malignancy in addition to MDC were excluded for survival analyses. To analyze whether cancer of the MD deserves any special mention or consideration from ileal cancers in general, we made some comparisons between Meckel malignancies and non-Meckelian ileal cancers (NMIC), using the same exclusion criteria.
RESULTS
A detailed description of patient characteristics is provided in Table 1.
TABLE 1.
Patient Characteristics of All Cases of Meckel Tumors
| Patient Characteristic | No. Patients (n = 158) |
Percentage* |
|---|---|---|
| Sex | ||
| Male | 101 | 64% |
| Female | 57 | 36% |
| Race | ||
| White | 148 | 94% |
| Black | 6 | 4% |
| Other | 4 | 2% |
| Major pathological subtypes | ||
| Carcinoid | 121 | 76.5% |
| Adenocarcinoma | 18 | 11.4% |
| GIST/leiomyosarcoma and sarcoma | 17 | 10.8% |
| Lymphoma | 2 | 1.3% |
| Tumor behavior | ||
| Benign | 0 | 0% |
| Malignant | 158 | 100% |
| Tumor size* | ||
| <1 cm | 14 | 48.3% |
| 1–5 cm | 6 | 20.7% |
| >5 cm | 6 | 20.7% |
| Unknown | 3 | 10.3% |
| Stage | ||
| Localized | 106 | 67.1% |
| Regional (node-positive) | 24 | 15.2% |
| Metastatic | 16 | 10.1% |
| Unstaged | 12 | 7.6% |
| Surgical treatment | ||
| Performed | 151 | 95.6% |
| Recommended but not performed | 6 | 3.8% |
| Not recommended | 1 | 0.6% |
| Radiation | ||
| No radiation | 156 | 98.8% |
| Beam radiation | 1 | 0.6% |
| Unknown | 1 | 0.6% |
Using data for patients diagnosed since 2004 (n = 29). Percentages might slightly differ as they were rounded off to the nearest whole number.
GIST indicates gastrointestinal stromal tumors.
Age at Diagnosis, Sex, and Race Distribution
The mean age at diagnosis of MDC was 60.6 years (± SD, 15.1 years). Adenocarcinomas appeared to present earlier (mean age, 55.9 years; ± 10.2 years) than malignant carcinoids (mean age, 61.0 years; ± 15.3 years) and GIST/leiomyosarcoma (mean age, 61.5 years; ± 16.5 years), although these groups were not statistically significant. Similarly, there was no statistically significant difference in the mean age at diagnosis of MDC between the genders or between Whites and African-Americans. The mean age at diagnosis of MDC is not statistically different from the mean age at diagnosis of NMIC (62.9 ± 15.8 years) in the same database during the same time period (1973–2006). However, the mean age at diagnosis of ileal adenocarcinoma of non-Meckelian origin was 65.9 years (± SD, 14.6 years), which is almost a decade later than the mean age at diagnosis of adenocarcinoma of MD. This difference was statistically significant (2 tailed P < 0.01). There was also a small yet statistically significant difference between mean ages at diagnosis of malignant carcinoid tumors of MD and non-Meckelian ileum (61.3 ± 15 years vs. 63.6 ± 13.4 years, respectively; t test, 2-tailed P < 0.05).
MDC was more common in men with a male:female ratio of 1.7:1. For adenocarcinoma and carcinoid types, the ratios were 2:1 and 2.02:1, respectively. Of total, 93% of MDC were white, with a white:black ratio of 25.3:1. In contrast, NMIC has an almost equal sex distribution of 1.1:1, which appears similar for both carcinoid and adenocarcinoma. The racial difference in cancer incidence with a white preponderance was steeper with MDC than with NMIC (white: black ratio, 10.4:1).
Incidence and Temporal Trends of MDC
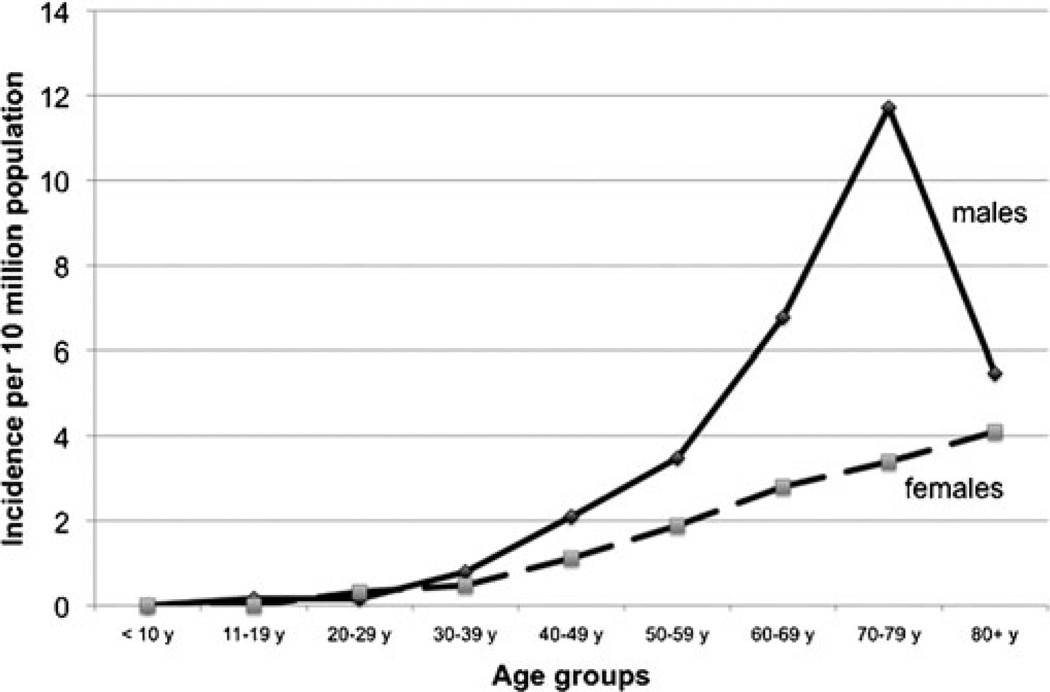
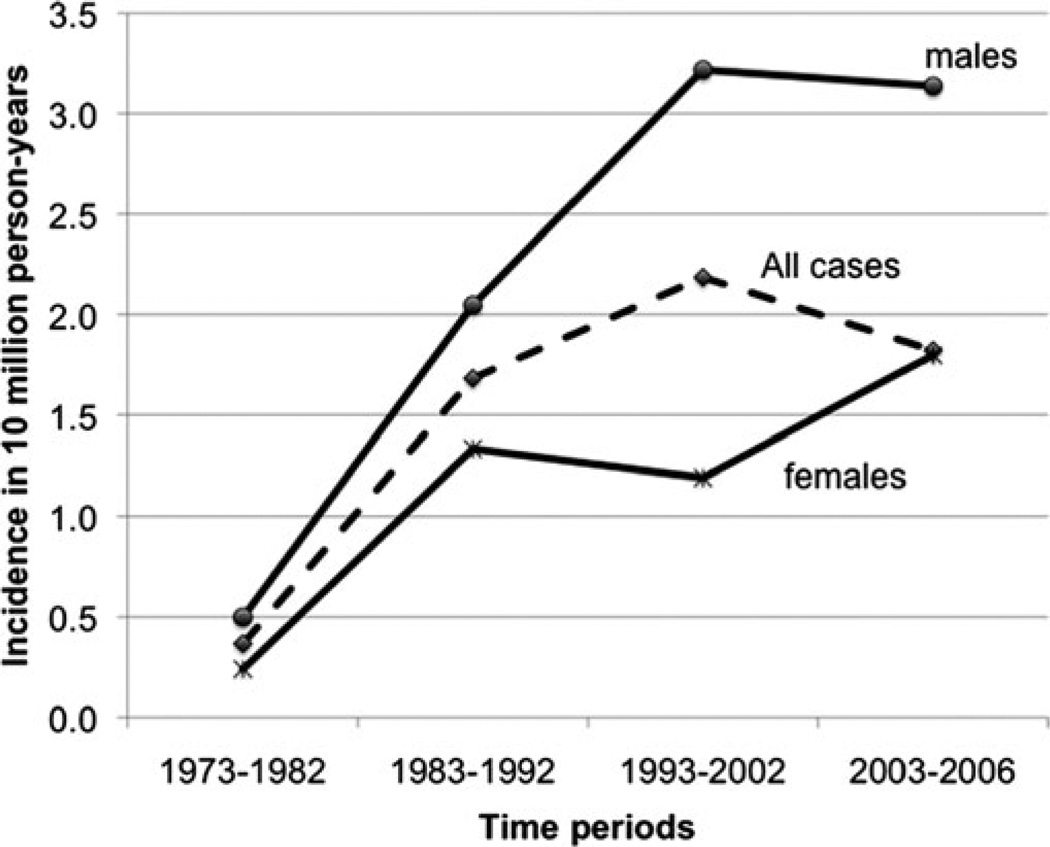
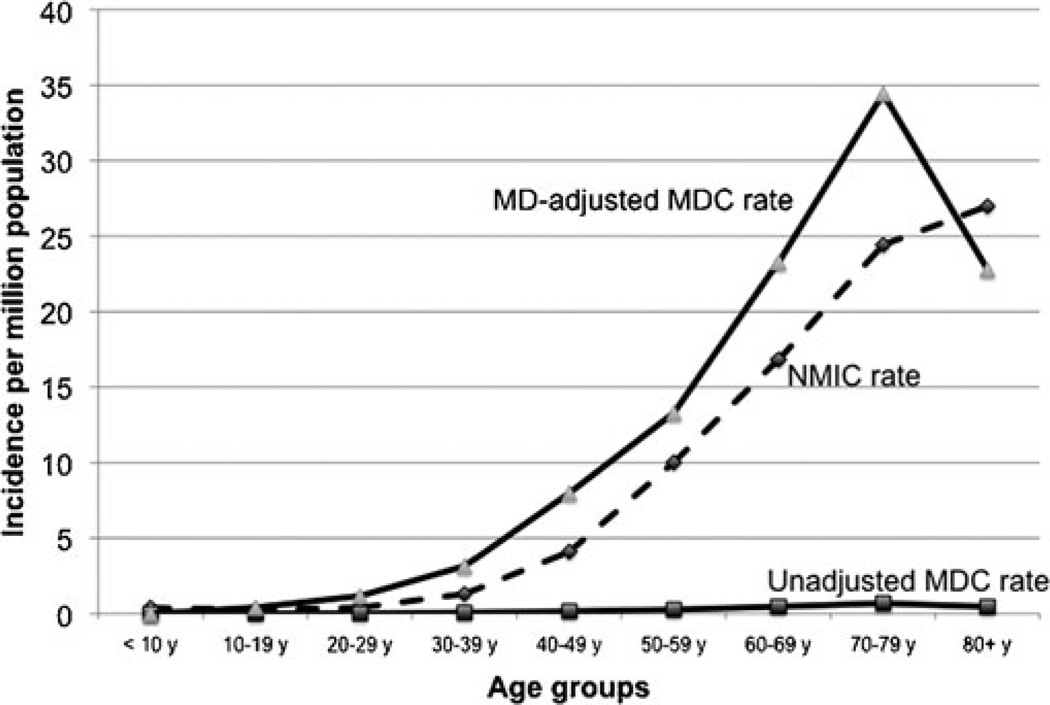
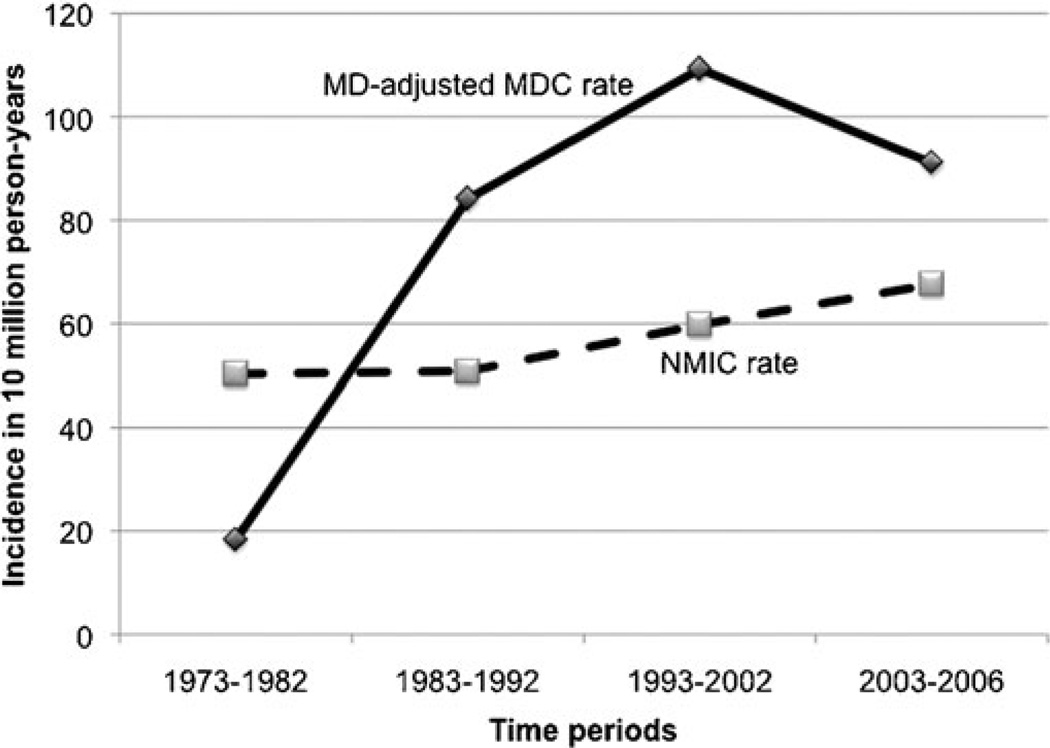
The mean annual incidence rate of MDC calculated for years 1973 to 2006 was 1.44 per 10 million population (±SD, 1.12). Incidence increased progressively with every decade of age, peaking in the eighth decade (Fig. 1). No cases were recorded below 10 years of age. The mean annual incidence rate in males was 2.07 per 10 million population (±SD; 1.8), which was 2.3 times higher than the incidence rate in females, 0.9 per 10 million population (±SD, 0.8), and this difference was statistically significant t test; 2-tailed p value < 0.001). The relatively higher incidence of MDC in males was 1.6 fold in the fourth decade of age, increasing thereafter during every decade until peaking at 3.4 fold in the eighth decade, after which it declined. Only in the third decade, was the incidence in females higher than the males. Incidence trends were analyzed by dividing the study period (1973–2006) into three 10-year periods (1973–1982, 1983–1992, and 1993–2002) and a 4-year period (2003–2006), and the rates were expressed per 10 million person-years (Fig. 2). MDC incidence has increased almost 5 fold, from 0.36 per 10 million person-years during 1973–1982 to 1.8 per 10 million person-years during 2003–2006, peaking at 2.1 per 10 million person-years (∼7 fold increase) during 1993–2002. Interestingly, this increase in incidence across time was more pronounced in females who experienced a 7.5-fold increase from 0.23 to 1.79 per 10 million person-years than inmen who showed a 6.2-fold increase from 0.49 to 3.13 per 10 million person-years. The mean incidence rate of MDC mentioned above was almost 38 times lower than the mean annual incidence rate of NMIC calculated for the same time period (55.47 ±8.16 per 10 million population) with high statistical significance t test; 2 tailed p < 0.001). However, this calculation of MDC incidence did not take into account that MD is prevalent in only 0.3% to 3% of the population. So, we calculated the MD prevalence-adjusted MDC incidence rate, by choosing the of t-quoted 2% prevalence rate1,3,30 in the general population. When done so, the mean-adjusted incidence rate of MDC was 72 per 10 million population (±SD, 56.15), which was higher than the mean incidence rate of NMIC (Fig. 3). When applied to temporal trends in incidence rates, the adjusted MDC-rate was lower than the NMIC rate (1.83 vs. 5.02 per 10 million person-years) during 1973–1982. However, the MD-adjusted MDC incidence was 1.6, 1.8, and 1.3 times higher than NMIC incidence for the periods 1983–1992, 1993–2002, and 2003–2006, respectively (Fig. 4). There was high statistical significance (paired t test; 2 tailed P= 0.001) for the higher adjusted-MDC incidence rate from 1982 to 2006. The incidence of ileal cancer increased only marginally (1.3 fold) during the entire period (1973–2006) from 50.23 to 67.55 per 10 million person-years whereas MDC increased by 5-fold.
FIGURE 1.
Mean annual incidence of MDC by age, calculated from SEER 9 registry (1973–2006).
FIGURE 2.
Temporal trends in incidence rates of MDC, calculated from SEER 9 registry (1973–2006).
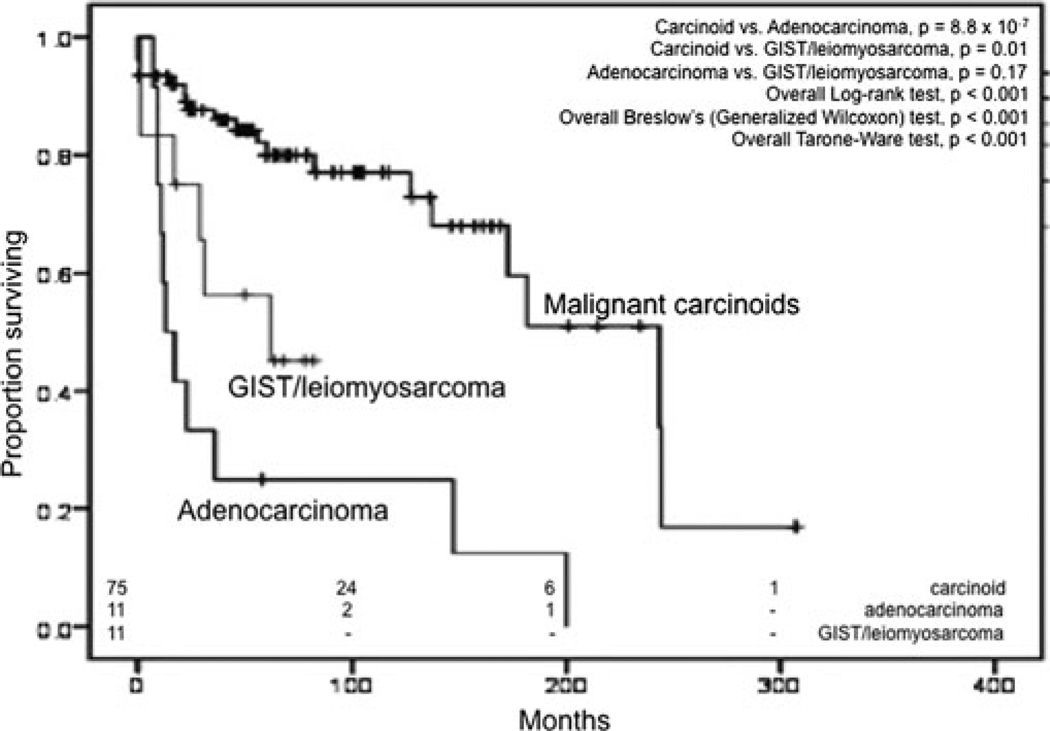
FIGURE 3.
Kaplan-Meier curves for overall survival for MDC based on histologic type. Overall significance values denote significance in at least 1 pair of groups.
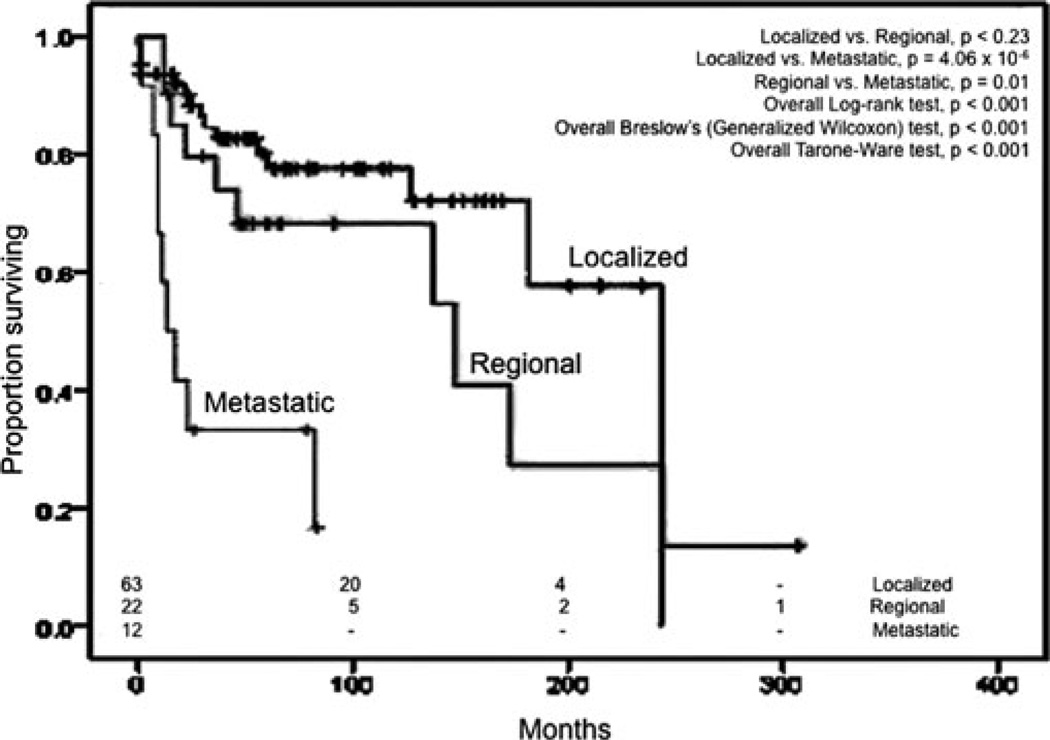
FIGURE 4.
Kaplan-Meier curves for overall survival for MDC based on our staging system. Overall significance values denote significance in at least 1 pair of groups.
The mean annual incidence rate of MDC in whites was 2.64 per 10 million, which was higher than the mean incidence rate in African- Americans of 1.04 per 10 million population. This difference approached but did not achieve statistical significance (t test; 2 tailed P = 0.068).
Pathologic Types
There were no reported benign tumors of the MD in the SEER database. Most of cancers of MDC (77.3%) were malignant carci noid tumors and 11.04% were adenocarcinoma. Stromal and smooth muscle tumors (GIST/leiomyosarcoma) and sarcomas constituted 10.43% and lymphomas 1.23%. The adenocarcinomas reported in this database included mucinous and signet-ring varieties. There was also a case of an adenocarcinoma arising in a tubulovillous adenoma. The lymphomas were of non-Hodgkin variety, one of them belonging to the diffuse large B-cell type. Carcinoids constituted a smaller majority in NMIC (56%) compared with MDC (77% carcinoids). Of 6214 ileal cancers, staging information was available only for 3225 patients, of which 27%, 47%, and 26% were localized, regional, and metastatic at the time of diagnosis. However, the high rate of unavailable staging data precluded any meaningful stage-forstage comparison between MDC and NMIC.
Stage at Diagnosis and Treatment
We staged Meckel malignancies based on the extent of disease involvement as localized (confined to MD and node-negative), regional (direct extension to adjacent sites without peritoneal spread or node-positive), and metastatic (Table 2). By this staging system, 67.1%, 15.2%, and 10.1% of MDC were localized, regional, and metastatic, respectively, at the time of diagnosis; staging information was not available for the remaining 7.6% in the database. Since 2004, the SEER program began recording accurate T, N, and M staging information. For these patients diagnosed since 2004 (n = 29), the authors derived the American Joint Committee on Cancer (AJCC) stage for small bowel adenocarcinoma.31,32 Accordingly, 66%, 10%, 14%, and 10% were at stages I, II, III, and IV, respectively, at the time of diagnosis. Most of them (95.6%) had surgical resection of the tumor with very few who did not have surgery recommended or performed for unknown reasons.
TABLE 2.
Stage Distribution and Median Overall Survival
| Extent of Disease |
Description | Corresponding AJCC Staging System* |
Percentage of Staged Patients† |
Median Survival (mo) |
95% CI‡ |
|
|---|---|---|---|---|---|---|
| Upper | Lower | |||||
| Localized | Confined to primary site | Stages 0,I | 72.6% | >243.0 | – | – |
| Regional | Extension by direct invasion or nodal involvement | Stages II, III | 16.4% | 147.0 | 28.8 | 265.1 |
| Distant | Metastatic disease, includes peritoneal spread | Stage IV | 11% | 13.0 | 2.8 | 23.2 |
AJCC stages corresponding to our system may not be equated absolutely as staging discrepancies may apple.
Analyses done using 146 after exclusing of 12 cases with inadequate staging data.
95%CI and SE for median OS for localized cancer were incalculable because more than 50% patients were alive at the time of last follow-up. However, calculated mean OS for this group is 179.36 mo (95% CI, 149–210 mo.
CI indicates confidence interval; SE, standard error; OS, overall survival.
Tumor Size
Tumor size analysis was performed only in the subset of patients diagnosed since 2004, for which SEER provided accurate tumor information. For this subset of patients (n = 29), 48.3%, 20.7%, and 20.7% of the patients had tumor sizes in the range of less than 1 cm, 1 to 5 cm, and greater than 5 cm, respectively (Table 1). Three patients (10.3%) did not have any tumor size recorded. The median tumor size was 0.7 cm.
Risk of Other Malignancies
A significant proportion of patients with Meckel malignancies were found to have other primary malignancies at some point during their lifetime. Of 158 patients, 51 (33%) with MDC had another primary malignancy at a non-Meckelian site, during their lifetime. In 13% of these patients (n = 7), MDC was the first malignancy to occur.
Forty-five patients had 1 additional malignancy and 4 patients had 2 other malignancies. One patient had 4 additional malignancies and 1 patient had 5 additional malignancies. These 51 patients had a total of 62 additional tumors, of which 37.1% were of GI and genitourinary origin each. A detailed analysis is shown in Table 3.
TABLE 3.
Distribution of Additional Primary Malignancies (n = 62) in 51 Patients With MDC
| Location of Other Primary Malignancies |
Distribution | Percentage |
|---|---|---|
| Gastrointestinal | ||
| Esophagus | 1 | 37.1% |
| Stomach | 1 | |
| Liver | 1 | |
| Pancreas | 1 | |
| Small bowel | 3 | |
| Colon | 16 | |
| Genitourinary | ||
| Kidney | 3 | 37.1% |
| Prostate | 6 | |
| Bladder | 6 | |
| Uterus | 2 | |
| Cervix | 1 | |
| Ovary | 5 | |
| Skin/breast | ||
| Skin | 7 | 17.74% |
| Breast | 4 | |
| Bone marrow | 2 | 3.23% |
| Bronchus | 1 | 1.61% |
| Retroperitoneum | 1 | 1.61% |
| Unknown | 1 | 1.61% |
| Total | 62 | 100% |
MDC indicates Meckel diverticulum cancer
Survival Analysis
After excluding patients who were diagnosed at autopsy (n = 5), had other primary malignancies (n = 51), or did not undergo surgery (n = 7), only 100 of the 163 patients were used for survival analyses. The median OS for all Meckel malignancies was 173 months (95% CI, 124–221 months) and appeared dependent on the pathologic type of malignancy (Fig. 5) and the stage (Fig. 6). Carcinoids had the best survival with a median OS of 243 months (95% CI, 157–329 months). This is significantly different (Mantel-Cox test, P < 0.001) compared to the median OS of adenocarcinoma, which was 13 months (95% CI, 5–21 months) and from GISTs/leiomyosarcomas, which was 62 months (95% CI, 0–146 months). There was statistical significance (P < 0.001), in all statistical tests employed (Mantel-Cox, Breslow, and Tarone-Ware). Relative survival rates for MDC derived using SEER*Stat software are shown in Table 4. Stage-forstage comparisons between the different histologic types were precluded by the small sample size in each subtype that was insufficient to adequately power the analyses.
FIGURE 5.
Comparison of actual and MD–prevalence adjusted MDC rates, along with non–Meckelianileal cancer(NMIC)rates.
FIGURE 6.
Comparison of temporal trends in incidence rates of actual and MD–prevalence, along with non–Meckelian ileal cancer(NMIC)rates.
TABLE 4.
Yearly Observed and Relative Survival Rates for MDC
| Yearly [-2pt] Survival Rate |
Observed Survival |
Relative Survival |
Standard Error for Relative Survival |
95% CI |
|
|---|---|---|---|---|---|
| Upper | Lower | ||||
| 1–yr | 84.10% | 85.80% | 3.60% | 76.9% | 91.40% |
| 3–yr* | 73.20% | 77.4% | 4.6% | 66.7% | 85.0% |
| 5–yr* | 68.50% | 75.8% | 4.8% | 64.9% | 83.8% |
The survival rates were derived by the SEER* Stat software by an actuarial method (n = 109) from the SEER 17 registry, with no adjustment for heterogeneity.
The values for relative survival rates, their standard errors and confidence intervals have been adjusted for increase in cumulative rates from the prior interval.
MDC indicates Meckel diverticulum cancer; CI, confidence interval.
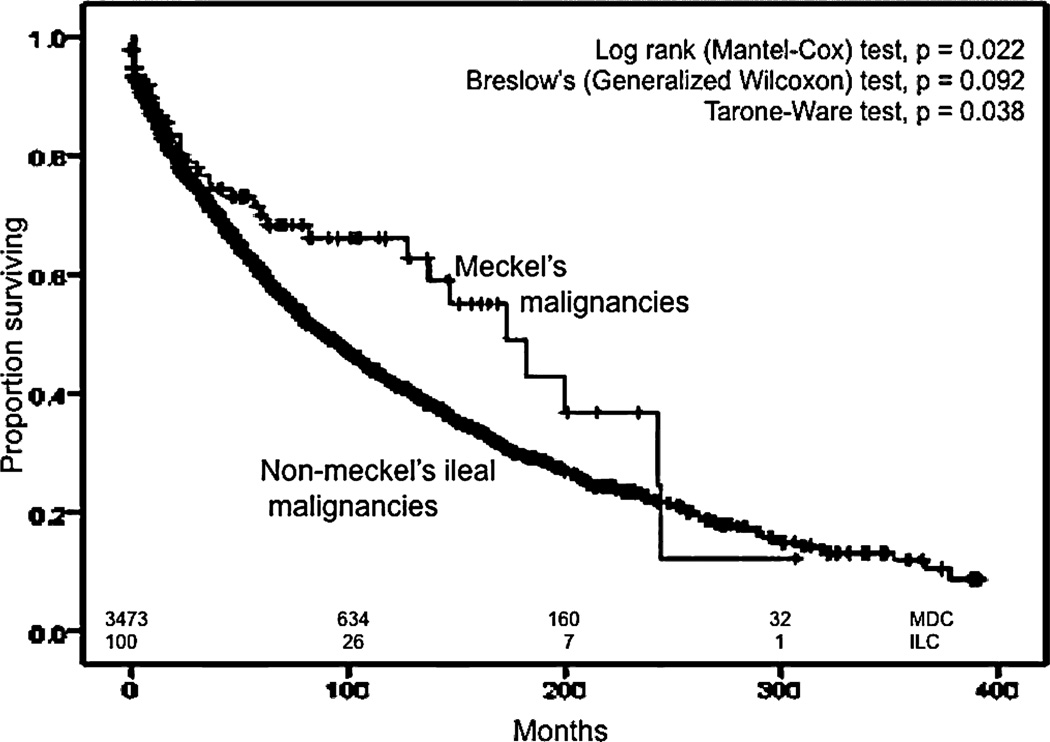
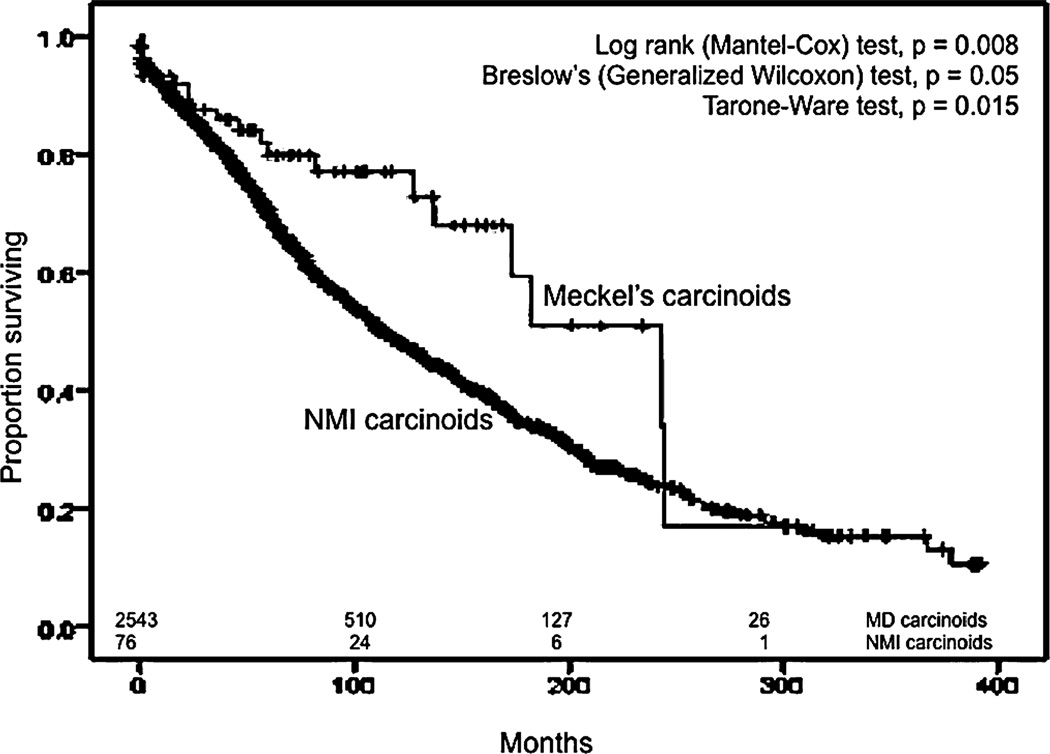
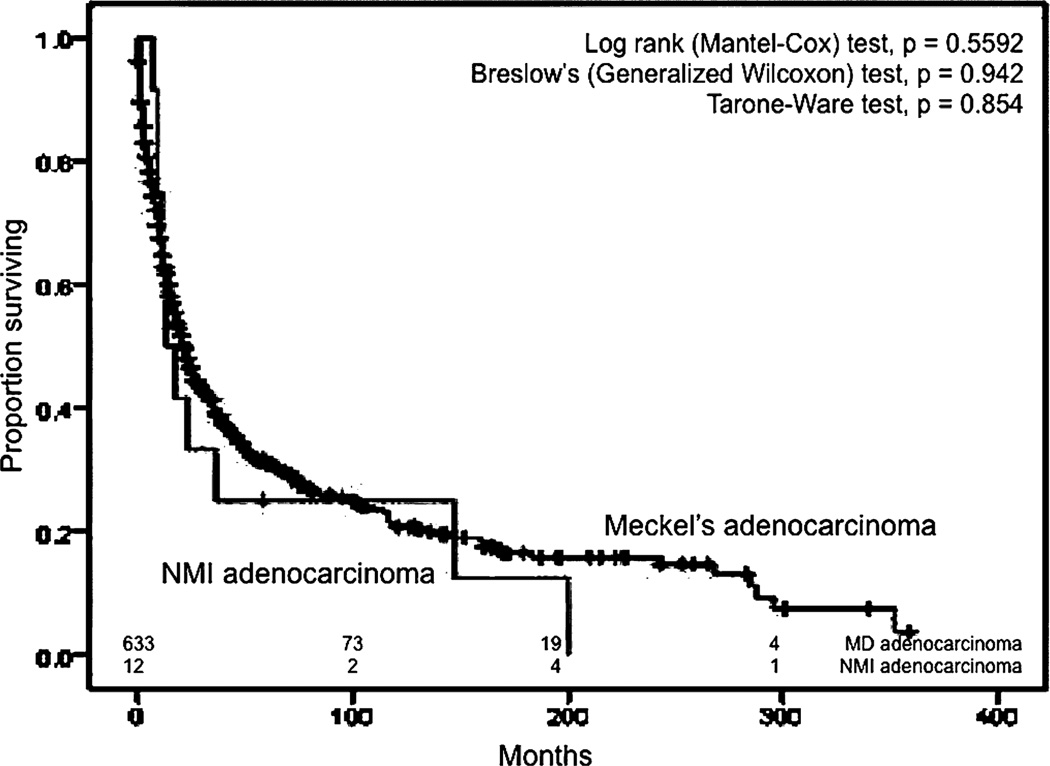
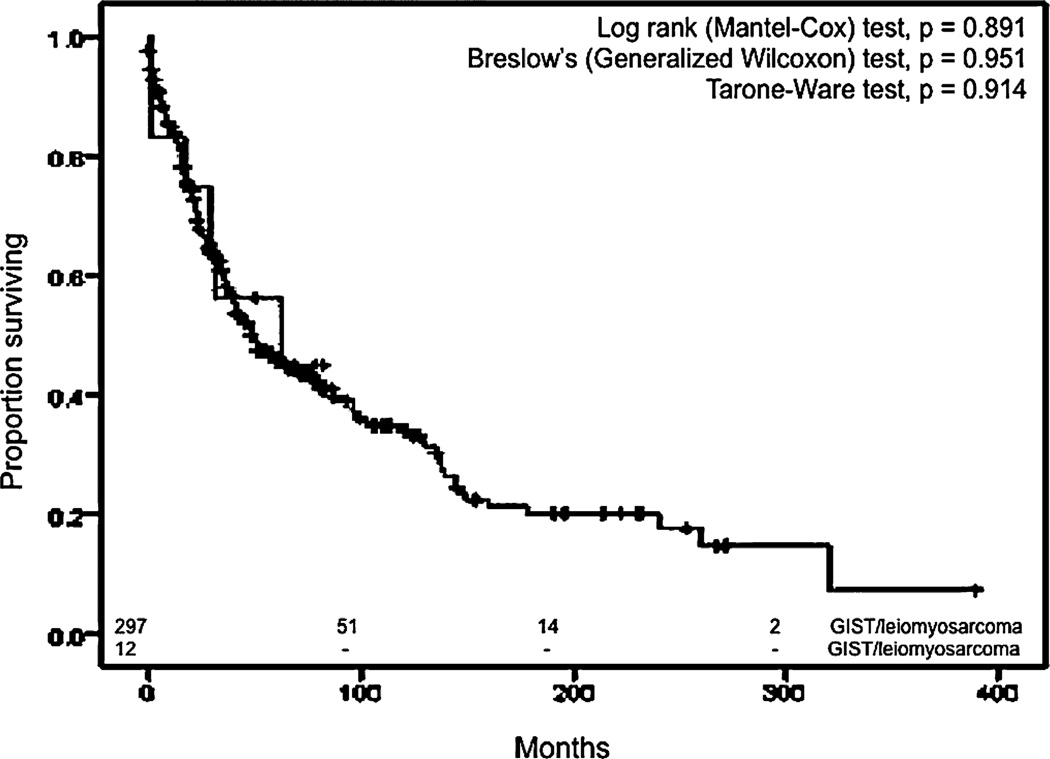
We also compared ileal malignancies to Meckel malignancies in terms of survival. We used the same exclusion criteria in addition to the inclusion of only the pathologic types of NMIC that corresponded to the spectrum of MDC, resulting in 3472 patients with NMIC. The median OS for all MDC was higher than the median OS of NMIC (89 months; 95% CI, 82–96 months) in a statistically significant manner (P = 0.022) (Fig. 7). Similar results were obtained when all pathologic types of NMIC were included and only those with other primary malignancies during their lifetime were excluded. The median OS for all NMI carcinoids was 115 months (95% CI, 105–125 months), which was less than the median OS for all Meckel carcinoids in a statistically significant manner (Mantel-Cox, P < 0.01) (Fig. 8). The median OS of Meckel adenocarcinoma was worse than the median OS of NMI adenocarcinoma, which was 22 months (95% CI, 18.7–25.3 months), although this difference did not achieve statistical significance (Fig. 9). Similarly, there was no statistical difference between the median OS of Meckel GIST/ leiomyosarcoma and those of NMI origin (48 months; 34.1–62 months) (Fig. 10). The small sample size available precluded stagefor-stage comparisons with respect to pathologic types.
FIGURE 7.
Kaplan–Meier curves for overall survival for Meckel diverticulum cancers(MDC) and non–Meckelian ileal cancers(NMIC).
FIGURE 8.
Kaplan–Meier curves for overall survival for malignant carcinoids of Mecfel diverticulum and non–Meckelian ileal (NMI) carcinoids.
FIGURE 9.
Kaplan–Meier curves for overall survival for Meckel diverticulum (MD) adenocarcinoma and non-Meckelian ileal (NMI) adenocarcinoma.
FIGURE 10.
Kaplan–Meier curves for overall survival for Meckel diverticulum (MD) GISTs/leiomyosarcomas and non–Meckelian ileal (NMI) GISTs/leiomyosarcomas
We also performed a Cox proportional hazards model to identify independent factors that affected survival in MDC. The model obtained by using the various covariates (Table 5) had an overall fit, which was statistically significant to a very high degree (P = 1.19 × 106). Age turned out to be an independent factor with an HR of 1.09 (95% CI, 1.05–1.13) in a statistically significant manner (P < 0.001). Certain histologic types also adversely impacted survival. Adenocarcinoma and GISTs/leiomyosarcomas had an HR of 7.68 and 5.72, respectively, when compared to malignant carcinoids as a reference. When our staging system was used, regional and metastatic disease had an HR of 2.01 and 3.84, respectively, when compared to Stage I disease. Metastatic disease was an independent factor affecting survival in a statistically significant manner (P < 0.05).
TABLE 5.
Cox Proportional Hazards Model for the Determination of Independent Factors Affecting Survival for All MDC
| Factor | P | Hazard Ratio |
95% CI |
|
|---|---|---|---|---|
| Upper | Lower | |||
| Age | <0.001 1.09 | 1.05 | 1.13 | |
| Sex | >0.05 | – | – | – |
| Race | >0.05 | – | – | – |
| Histological subtypes | ||||
| Carcinoid | Reference* | 1 | – | – |
| Adenocarcinoma | 0.003 | 7.68 | 2.0 | 29.43 |
| GIST/leiomyosarcoma | 0.007 5.72 | 1.61 | 20.35 | |
| Year of diagnosis | >0.05 | – | – | – |
| Proposed staging system | ||||
| Stage I (localized) | Reference* | 1 | – | – |
| Stage II (regional) | >0.05 | 2.01 | 0.71 | 5.72 |
| Stage III (metastatic) | 0.04 | 3.84 | 1.04 | 14.22 |
Assumed as reference for calculation of hazard ratios.
GIST indicates gastrointestinal stromal tumors; CI, confidence interval; MDC, Meckel diverticulum cancer.
Length of Bowel at Risk
Another interesting issue is that the “length of bowel at risk” for malignancy is drastically different—about 3 cm for MD1,6,7 and 165 to 516 cm for the ileum.33–35 We calculated the risk of cancer per unit length of bowel at risk per unit time adjusting for 2% prevalence of 3 cm long MD and 100% prevalence of the most conservative estimate of ileal length, 165 cm. The risk of MDC thus calculated was 2.28 cancers per km of bowel per decade (±SD, 1.78), which was 70 times higher than the risk of NMIC (0.03 cancers per km of bowel per decade, ±0.006). This difference was highly statistically significant (t test, 2 tailed P < 0.0001).
DISCUSSION
MD, being the most common anomaly of the GI tract has received a lot of attention, with nearly 1600 publications in the decade preceding 2005.5 But most of these studies either pertained to short case reports or small series of patients or were single institutional and usually nonpopulation based. A recent comprehensive study by Zani et al performed a systematic review of 244 publications concerning MD.36 Their analysis of more than 19 autopsy studies estimated a general population prevalence of MD to be 1.23%. However, we chose to use the 2% prevalence rate, as traditional teaching has advocated a “rule of 2” for characterizing the features of MD—2% prevalence, 2 feet from ileocecal valve, and 2 inches long.3 If a prevalence of 1.23% was used to calculate the MD-adjusted MDC incidence rate, it would have been still higher than NMIC incidence rate. Again, if a 1.23%) prevalence adjustment was applied to the estimation of risk of cancer per unit length of bowel per unit time, the risk of MDC would be 1.6 fold higher than the estimate mentioned above. These calculations undoubtedly established that MD was a “hot spot” or “high-risk” region for cancer in the ileum.
The incidence of MDC has also shown a dramatic rise in the last 3.5 decades that span the entire duration of the database. During this time, there has been only a marginal rise in NMIC incidence. Although there have been conflicting reports on gender differences in the occurrence of MD, complications are well known to have a high male predominance.3,5,37 Our study not only showed a male predominance in MDC (which was not so pronounced in NMIC), but also that there been a higher fold increase in MDC in females in the last few decades than the males. In summary, MDC tended to occur earlier, was more commonly carcinoid and was more often localized at presentation than its ileal counterparts, accounting for the better trend in survival. We also chose to classify MDC as localized, regional, or metastatic to uniformly and reliably assimilate the way in which stage information was coded in SEER database. We have made the best possible effort to extrapolate the AJCC stages of small bowel carcinoma from our staging system; however, these may not be absolute comparisons because of changes in stage coding over the years that do not adjust for lack of detail in earlier staging systems.
Although there have been a plethora of publications about MD, there is little consensus with regard to the appropriate surgical management of the incidentally discovered MD. Only 4% of those with MD would require a MD-related hospital admission and 2.9% would require surgery.36 The lifetime risk of developing complications is 6.4%, the most common being bleeding and obstruction.38 Only approximately 3% of the complications have been attributed to tumors.10,11 In 1976, Soltero and Bill analyzed 202 cases of diseased MD from several hospitals in a certain county.30 Using a 2% prevalence rate, they calculated the risk of developing MD complications to be 4.2%, which tapers with increasing age. They also estimated that 800 asymptomatic Meckel’s diverticula need to However, there have been multiple reports that suggest otherwise. In 1994, Cullen et al, through an epidemiological population-based study noted that the risk of complications from MD do not decrease with age,38 as was suggested by Soltero and Bill. They also went ahead to show that cumulative rate of long-term complications was much less with incidental diverticulectomies than with diverticulectomies done for complications. They also showed operative mortality and morbidity to be lower with incidental diverticulectomy. Following this, there have been multiple reports advocating the safety of incidental diverticulectomy with near zero or zero mortality.1,6,8,39,40 A recent analysis of 1476 patients with MD at the Mayo Clinic (Rochester, MN) by Park et al showed no MD-related complications or deaths with diverticulectomy.5 They did not explicitly favor or reject incidental diverticulectomy, but suggested a selected approach for resection of incidental MD in the presence of any of 4 factors that turned out to correlate in a statistically significant manner with MDs producing complications, namely age less than 50 years, male sex, diverticulum length greater than 2 cm, and presence of histologically abnormal tissue.
Our study that looks at this problem from the oncological standpoint appeared to favor resection of incidental MD, especially in light of the study by Park et al.5 Our study identified increasing age as a risk factor for MDC, especially above 50 years of age (Fig. 1 and Table 5) and identified a larger increase in MDC incidence in females over the last few decades. Histologically, abnormal tissue may be found in up to 60% of MDs3,5,6,8,36 and only 38% of time will such tissue be palpable in asymptomatic MDs.5 With a high chance of a malignancy in the MD being localized, implying a high potential for curative resection and long-term survival as suggested by our analyses, it seems most prudent to resect asymptomatic MDs. This is compounded by the fact that preoperative diagnosis of any MD-related pathology is rare,8,41 implying that resection during incidental intraoperative discovery not only removes MDC from the list of late diagnoses, but also MD complications from the list of diagnostic problems for obscure abdominal pain or GI bleeding for the general surgeon. With accumulating reports of minimally invasive techniques that can be successfully employed for Meckel’s diverticulectomy11,42,43 and negligible complication rate with incidental diverticulectomy,5,8,40 we consider the benefits of resection of this high-risk area for ileal cancer to outweigh the risks. However, the authors refrain from advocating planned surgical resection of the MD when found incidentally during radiologic investigation for unrelated reasons.
ACKNOWLEDGMENTS
Dr. Kothai Divya Guruswamy Sangameswaran, MBBS for her support in data collection and analyses; Meredith Mahan, MS for review of statistical methods and analyses.
REFERENCES
- 1.Yahchouchy EK, Marano AF, Etienne JC, et al. Meckel’s diverticulum. J Am Coll Surg. 2001;192:658–662. doi: 10.1016/s1072-7515(01)00817-1. [DOI] [PubMed] [Google Scholar]
- 2.Turgeon DK, Barnett JL. Meckel’s diverticulum. Am J Gastroenterol. 1990;85:777–781. [PubMed] [Google Scholar]
- 3.Sagar J, Kumar V, Shah DK. Meckel’s diverticulum: a systematic review. JR Soc Med. 2006;99:501–505. doi: 10.1258/jrsm.99.10.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorusso R, Forte A, Urbano V, et al. Small bowel stromal tumors in a “meckelian” location. About a clinical observation [in Italian] Ann Ital Chir. 2003;74:707–711. [PubMed] [Google Scholar]
- 5.Park JJ, Wolff BG, Tollefson MK, et al. Meckel diverticulum: the Mayo Clinic experience with 1476 patients (1950–2002) Ann Surg. 2005;241:529–533. doi: 10.1097/01.sla.0000154270.14308.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bani-Hani KE, Shatnawi NJ. Meckel’s diverticulum: comparison of incidental and symptomatic cases. World J Surg. 2004;28:917–920. doi: 10.1007/s00268-004-7512-3. [DOI] [PubMed] [Google Scholar]
- 7.Mackey WC, Dineen P. A fifty year experience with Meckel’s diverticulum. Surg Gynecol Obstet. 1983;156:56–64. [PubMed] [Google Scholar]
- 8.Ludtke FE, Mende V, Kohler H, et al. Incidence and frequency or complications and management of Meckel’s diverticulum. Surg Gynecol Obstet. 1989;169:537–542. [PubMed] [Google Scholar]
- 9.Varcoe RL, Wong SW, Taylor CF, et al. Diverticulectomy is inadequate treatment for short Meckel’s diverticulum with heterotopic mucosa. ANZ J Surg. 2004;74:869–872. doi: 10.1111/j.1445-1433.2004.03191.x. [DOI] [PubMed] [Google Scholar]
- 10.Morcillo Rodenas MA, Planells Roig M, Garcia Espinosa R, et al. Neoplasms of the Meckel diverticulum. Apropos of 2 new cases [in Spanish] Rev Esp Enferm Dig. 1990;77:143–146. [PubMed] [Google Scholar]
- 11.Anderson DJ. Carcinoid tumor in Meckel’s diverticulum: laparoscopic treatment and review of the literature. J Am Osteopath Assoc. 2000;100:432–134. [PubMed] [Google Scholar]
- 12.Moyana TN. Carcinoid tumors arising from Meckel’s diverticulum. A clinical, morphologic, and immunohistochemical study. Am J Clin Pathol. 1989;91:52–56. doi: 10.1093/ajcp/91.1.52. [DOI] [PubMed] [Google Scholar]
- 13.Nies C, Zielke A, Hasse C, et al. Carcinoid tumors of Meckel’s diverticula. Report of two cases and review of the literature. Dis Colon Rectum. 1992;35:589–596. doi: 10.1007/BF02050541. [DOI] [PubMed] [Google Scholar]
- 14.Shebani KO, Souba WW, Finkelstein DM, et al. Prognosis and survival in patients with gastrointestinal tract carcinoid tumors. Ann Surg. 1999;229:815–821. doi: 10.1097/00000658-199906000-00008. discussion 822–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusumoto H, Yoshitake H, Mochida K, et al. Adenocarcinoma in Meckel’s diverticulum: report of a case and review of 30 cases in the English and Japanese literature. Am J Gastroenterol. 1992;87:910–913. [PubMed] [Google Scholar]
- 16.Lippe P, Berardi R, Latini L, et al. Severe prognosis of signet-ring cell adenocarcinoma occurring in Meckel’s diverticulum. Ann Oncol. 2001;12:277. doi: 10.1023/a:1008350819956. [DOI] [PubMed] [Google Scholar]
- 17.Parente F, Anderloni A, Zerbi P, et al. Intermittent small-bowel obstruction caused by gastric adenocarcinoma in a Meckel’s diverticulum. Gastrointest Endosc. 2005;61:180–183. doi: 10.1016/s0016-5107(04)02450-2. [DOI] [PubMed] [Google Scholar]
- 18.Rieber JM, Weinshel EH, Nguyen T, et al. Synchronous gastric adenocarcinomas in a patient with Meckel’s diverticulum. J Clin Gastroenterol. 2001;33:78–80. doi: 10.1097/00004836-200107000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Koh HC, Page B, Black C, et al. Ectopic pancreatic-type malignancy presenting in a Meckel’s diverticulum: a case report and review of the literature. World J Surg Oncol. 2009;7:54. doi: 10.1186/1477-7819-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cates JM, Williams TL, Suriawinata AA. Intraductal papillary mucinous adenoma that arises from pancreatic heterotopia within a Meckel diverticulum. Arch Pathol Lab Med. 2005;129:e67–e69. doi: 10.5858/2005-129-e67-IPMATA. [DOI] [PubMed] [Google Scholar]
- 21.Khoury MG, II, Aulicino MR. Gastrointestinal stromal tumor (GIST) presenting in a Meckel’s diverticulum. Abdom Imaging. 2007;32:78–80. doi: 10.1007/s00261-006-9019-x. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu N, Kuramoto S, Mimura T, et al. Leiomyosarcoma originating in Meckel’s diverticulum: report of a case and a review of 59 cases in the English literature. Surg Today. 1997;27:546–549. doi: 10.1007/BF02385809. [DOI] [PubMed] [Google Scholar]
- 23.Beyrouti MI, Ben Amar M, Beyrouti R, et al. Complications of Meckel’s diverticulum. Report of 42 cases [in French] Tunis Med. 2009;87:253–256. [PubMed] [Google Scholar]
- 24.Kim J, Kim YS, Chun HJ, et al. Laparoscopy-assisted exploration of obscure gastrointestinal bleeding after capsule endoscopy: the Korean experience. JLa-paroendosc Adv Surg Tech A. 2005;15:365–373. doi: 10.1089/lap.2005.15.365. [DOI] [PubMed] [Google Scholar]
- 25.Krespis EN, Sakorafas GH. Partial intestinal obstruction caused by a lipoma within a Meckel’s diverticulum. Dig Liver Dis. 2006;38:358–359. doi: 10.1016/j.dld.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Yao JL, Zhou H, Roche K, et al. Adenomyoma arising in a Meckel diverticulum: case report and review of the literature. Pediatr Dev Pathol. 2000;3:497–500. doi: 10.1007/s100240010097. [DOI] [PubMed] [Google Scholar]
- 27.Minimo C, Talerman A. Villous adenoma arising in Meckel’s diverticulum. J Clin Pathol. 1998;51:485–186. doi: 10.1136/jcp.51.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 29.Yu JB, Gross CP, Wilson LD, et al. NCI SEER public-use data: applications and limitations in oncology research. Oncology (Williston Park) 2009;23:288–295. [PubMed] [Google Scholar]
- 30.Soltero MJ, Bill AH. The natural history of Meckel’s Diverticulum and its relation to incidental removal. A study of 202 cases of diseased Meckel’s Diverticulum found in King County, Washington, over a fifteen year period. Am J Surg. 1976;132:168–173. doi: 10.1016/0002-9610(76)90043-x. [DOI] [PubMed] [Google Scholar]
- 31.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 32.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 33.Grant JP. Anatomy and physiology of the luminal gut: enteral access implications. J Parenter Enteral Nutr. 2006;30(suppl 1):S41–S46. doi: 10.1177/01486071060300S1S41. [DOI] [PubMed] [Google Scholar]
- 34.Nightingale JM, Lennard-Jones JE. Adult patients with a short bowel due to Crohn’s disease often start with a short normal bowel. Eur J Gastroenterol Hepatol. 1995;7:989–991. doi: 10.1097/00042737-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Nordgren S, McPheeters G, Svaninger G, et al. Small bowel length in inflammatory bowel disease. Int J Colorectal Dis. 1997;12:230–234. doi: 10.1007/s003840050095. [DOI] [PubMed] [Google Scholar]
- 36.Zani A, Eaton S, Rees CM, et al. Incidentally detected Meckel diverticulum: to resect or not to resect? Ann Surg. 2008;247:276–281. doi: 10.1097/SLA.0b013e31815aaaf8. [DOI] [PubMed] [Google Scholar]
- 37.Pinter A, Szemledy F, Pilaszanovich I. Remnants of vitelline duct: analysis of 66 cases. Acta Paediatr Acad Sci Hung. 1978;19:113–123. [PubMed] [Google Scholar]
- 38.Cullen JJ, Kelly KA, Moir CR, et al. Surgical management of Meckel’s diverticulum. An epidemiologic, population-based study. Ann Surg. 1994;220:564–568. doi: 10.1097/00000658-199410000-00014. discussion 568–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peoples JB, Lichtenberger EJ, Dunn MM. Incidental Meckel’s diverticulec-tomy in adults. Surgery. 1995;118:649–652. doi: 10.1016/s0039-6060(05)80031-5. [DOI] [PubMed] [Google Scholar]
- 40.Zulfikaroglu B, Ozalp N, Zulfikaroglu E, et al. Is incidental Meckel’s diverticulum resected safely? N Z Med J. 2008;121:39–44. [PubMed] [Google Scholar]
- 41.Ymaguchi M, Takeuchi S, Awazu S. Meckel’s diverticulum. Investigation of 600 patients in Japanese literature. Am J Surg. 1978;136:247–249. doi: 10.1016/0002-9610(78)90238-6. [DOI] [PubMed] [Google Scholar]
- 42.Bona D, Schipani LS, Nencioni M, et al. Laparoscopic resection for incidentally detected Meckel diverticulum. World J Gastroenterol. 2008;14:4961–4963. doi: 10.3748/wjg.14.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivas H, Cacchione RN, Allen JW. Laparoscopic management of Meckel’s diverticulum in adults. Surg Endosc. 2003;17:620–622. doi: 10.1007/s00464-002-8613-4. [DOI] [PubMed] [Google Scholar]