Abstract
OBJECTIVES
: To evaluate the neuroprotective effect of epidural hypothermia in rats subjected to experimental spinal cord lesion.
METHODS:
Wistar rats (n = 30) weighing 320-360 g were randomized to two groups (hypothermia and control) of 15 rats per group. A spinal cord lesion was induced by the standardized drop of a 10-g weight from a height of 2.5 cm, using the New York University Impactor, after laminectomy at the T9-10 level. Rats in the hypothermia group underwent epidural hypothermia for 20 minutes immediately after spinal cord injury. Motor function was assessed for six weeks using the Basso, Beattie and Bresnahan motor scores and the inclined plane test. At the end of the final week, the rats' neurological status was monitored by the motor evoked potential test and the results for the two groups were compared.
RESULTS:
Analysis of the Basso, Beattie and Bresnahan scores obtained during the six-week period indicated that there were no significant differences between the two groups. There was no significant difference between the groups in the inclined plane test scores during the six-week period. Furthermore, at the end of the study, the latency and amplitude values of the motor evoked potential test were not significantly different between the two groups.
CONCLUSION:
Hypothermia did not produce a neuroprotective effect when applied at the injury level and in the epidural space immediately after induction of a spinal cord contusion in Wistar rats.
Keywords: Spinal Cord Injuries, Hypothermia, Neuroprotective Agents, Rats, Wistar
INTRODUCTION
Each year in the United States, approximately 11,000 to 12,000 individuals suffer spinal cord lesions resulting from motor vehicle accidents, sports-related injuries or direct trauma. Many victims are young adult males and most individuals continue to experience severe paralysis and functional deficits for the remainder of their lives (1). In Brazil, the public health system (Sistema Único de Saúde, SUS) treated at least 6,517 victims of spinal cord injury or fracture in 2012 (2).
Many cases of paralysis after spinal cord injury result from the ischemia that is secondary to the increased epidural venous pressure and arterial spasm, both of which are reversible (3). Extensive research in previous decades has investigated techniques to maximize neurological recovery after spinal cord injury (4,5) and various therapeutic methods have been advocated, including surgical decompression and early stabilization, pharmacological agents (6), modification of certain modulators of cellular inflammation and hypothermia (5,7). Whether these methods significantly contribute to neurological recovery remains unclear.
The procedure of cooling the spinal cord after an injury was successfully applied for the first time in an experiment by Alvin et al. (8). Since then, the neuroprotective effect of hypothermia has been demonstrated in different experimental models of spinal cord injury. Promising preliminary results from clinical trials and experimental studies were shown in the 1970s (4,5,8). Induced hypothermia for the treatment of brain trauma and spinal cord injuries is currently under investigation in studies using both animal models and humans. Hypothermia can be induced systemically or locally (4,5). Traditionally, until the 1980s, it was induced systemically; however, the target temperature range was limited (32 to 33 degrees Celsius) to avoid the complications that can arise as the body temperature decreases, such as the desaturation of hemoglobin, which leads to ischemia in polytrauma patients (5,7).
Considering these risks and given the limited clinical applicability of the systemic method, other researchers have induced hypothermia directly at the lesion site through the skin, subcutaneous tissue, paraspinal muscles and the posterior neural arch, with favorable results (5). However, local cooling of the spinal cord at the level of the injury requires the use of special devices and the performance of emergency surgery with complicated postoperative management (7). Because of the difficulties of the clinical application of local and systemic hypothermia, uncertainties remain regarding which approach provides a better neuroprotective effect. Therefore, the objective of this study was to evaluate the neuroprotective effect of hypothermia applied at the level of spinal cord injuries in rats.
MATERIALS AND METHODS
In this study, the ethical protocols for animal experimentation established by the Brazilian College of Animal Experimentation and the Institute of Laboratory Animal Resources, as well as those of the U.S. National Academy of Sciences, were followed. The study was approved by the Ethics Committee of Faculdade de Medicina da Universidade de São Paulo (USP).
Thirty male and female Wistar rats, aged 12 weeks and weighing 320 to 360 g, were used. A standardized experimental procedure was used to induce spinal cord lesions in all rats. The animals were randomly divided into two groups; the first group underwent epidural hypothermia as described below and the second group comprised the controls that did not undergo hypothermia. The exclusion criteria were: death immediately after the surgical procedure, abnormalities in the spinal cord (malformations) observed during surgery and animals that retained normal movement after the spinal cord lesion (i.e., absence of paraplegia).
Prior to the experimental spinal cord lesion procedures and the evoked potential tests, the rats were anesthetized by intraperitoneal injection of 55 to 75 mg/kg pentobarbital and intramuscular injection of 55 to 75 mg/kg of ketamine. Euthanasia was performed on day 42 by inducing anesthesia with intraperitoneally administered thiopental and, after confirmation of anesthesia, the intravenous administration of potassium chloride.
The standardized spinal cord lesion procedure after laminectomy has been previously described by our team (9). The procedure consists of producing an injury using a computerized device (the NYU Impactor, New York University Spinal Cord Contusion System) and a 10-g weight dropped from a 25-mm height, which compresses the spinal cord for 15 seconds. In this study, the rats received prophylactic cephalothin subcutaneously (25 mg/kg body weight) immediately after the procedure and once per day for the next seven days thereafter to prevent wound and urinary tract infection.
The 15 rats in the experimental group underwent hypothermia at T9-T10 after the spinal cord lesion was induced. Cool water stored in a polystyrene tank dripped through a catheter directly in the region of the lesion for 20 minutes. The distance from the tip of the catheter to the spinal cord tissue was standardized at 5 mm. The water in the tank was maintained at 9-10°C. It was not necessary to use an infusion pump because the water tank was placed above the animal; thus, gravity was sufficient. The drip speed was controlled; the system provided 10 drops per minute, which was equivalent to 0.5 ml per minute. The spinal temperature of the animals was measured using a thermometer with a laser tip (InfraRed Thermometer TI-860) immediately after inducing the spinal cord lesion and once every minute thereafter to ensure that the 25°C temperature was maintained throughout the 20 minutes. The drip rate was manually changed if the thermometer showed any change in the tissue surface temperature.
For motor function analysis, we used the BBB (Basso, Beattie and Bresnahan) scale, the inclined plane test and the evoked potential test that is described below (4,8). The study period was six weeks. The BBB scale was used daily throughout the entire study period, after induction of the spinal cord lesion. The inclined plane test was conducted weekly and the evoked potential test was performed at the end of the final week of the study.
Each animal's weight, length (from the head to the beginning of the tail) and body temperature (in the posterior limb) were measured prior to performing the evoked potential test to enable calculation of the dose of anesthetic and to allow comparison of the latency between rats.
For the BBB evaluation, the rats were allowed to move freely and a single researcher observed the rat for at least one minute per day during the six weeks of the study; data were recorded on the movements of the joints, hips, knees and ankles in the posterior limbs. The BBB score was then calculated (10).
The inclined plane test measured the ability of the animal to remain on top of a ramp the slope of which increased every 15 degrees. The test was also used as an index of hind limb strength. The maximum angle at which the animal remained on the ramp without slipping for at least five seconds defined the score on the inclined plane test as follows: zero points for a ramp without inclination, 1 point for ramp with a slope up to 15 degrees, 2 points for up to 30 degrees and 3 points for up to 45 degrees.
The motor evoked potential was obtained with a four-channel electromyography machine, which included two monopolar needle electrodes, corkscrew type, model EO401 from Neuromedical Supplies for transcranial stimulation; one monopolar needle electrode used as a ground and four pairs of monopolar needle electrodes used to capture motor responses in the anterior and posterior limbs. The instrument calibration was performed in two ways. The transcranial electrical stimulation was performed with a single stimulus for 0.2 ms and the muscular responses were captured within a 20-ms window with a sensitivity of 2 mV/div, a low frequency filter of 10 Hz, filter and a high frequency filter of 10 kHz. The stimulus intensity was considered supramaximal.
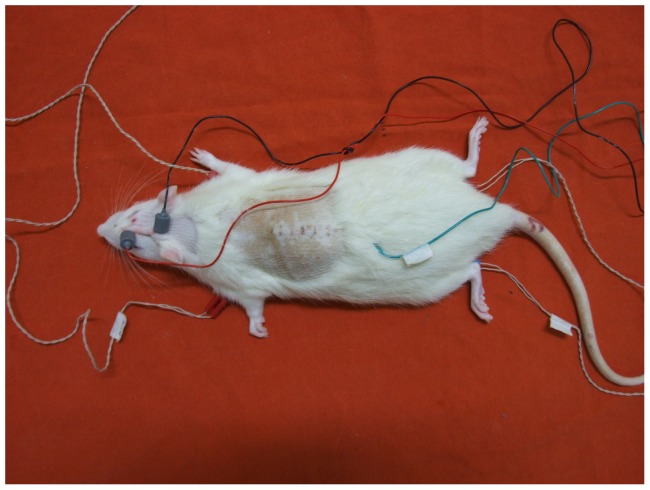
The muscle responses were captured by inserting the monoplanar electrode pairs (acquisition and reference) with a fixed interelectrode distance in the proximal and ventral muscles of the anterior and posterior limbs of the rats. The ground electrode was placed in the lumbar region using a monopolar needle electrode. The transcranial electric stimulation was performed by placing two needle electrodes, corkscrew-type, on the animal's scalp in the frontal (anode) and occipital (cathode) regions on the interhemispheric line for bilateral simultaneous stimulation (Figure 1). After placement of the electrodes on the rat, the machine was turned on and the impedance of the electrodes was checked to demonstrate good adaptability to obtain sharp, safe and reliable responses.
Figure 1.
Placement of electrodes for the evoked motor potential exam.
Continuous data, such as weight, BBB score and evoked potentials, were evaluated using the Kolmogorov-Smirnov test, which indicated that the data were normally distributed. The scale produced by the inclined plane test was analyzed throughout the week using the Friedman test and with a post hoc analysis using the Wilcoxon signed-rank test. The Mann-Whitney U test was used to detect differences between groups. Analysis of variance (ANOVA) was used to compare the scores of the BBB scale across weeks; when a difference between weeks was identified in both groups, a post hoc Bonferroni test was performed to determine the specific weeks on which the scores differed. Student's t test was used to analyze the BBB scores between groups and the amplitude and latency of the evoked potential. In all analyses, any value less than or equal to 5% was considered an alpha error, which is an acceptable error for significant differences.
RESULTS
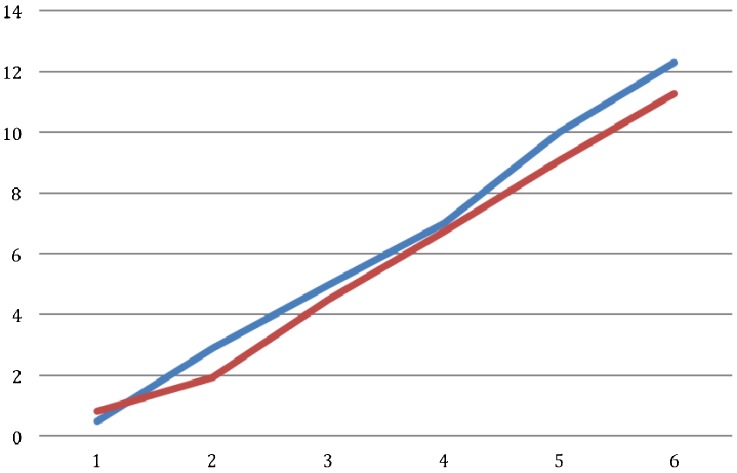
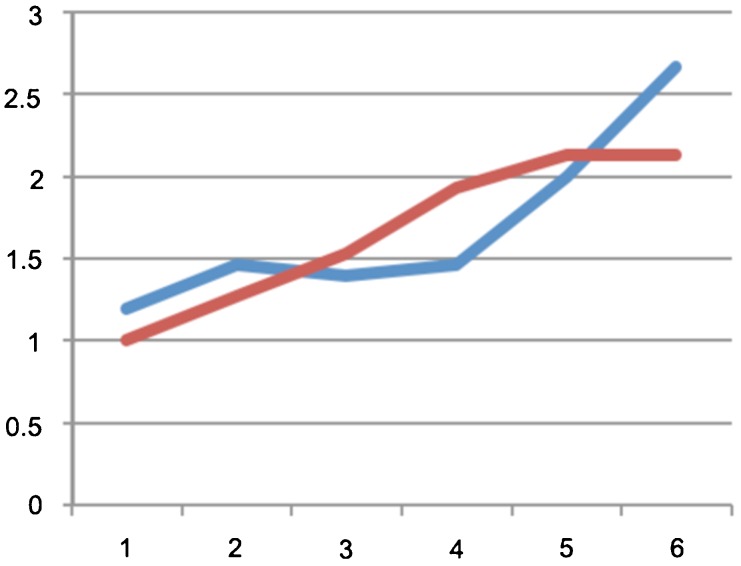
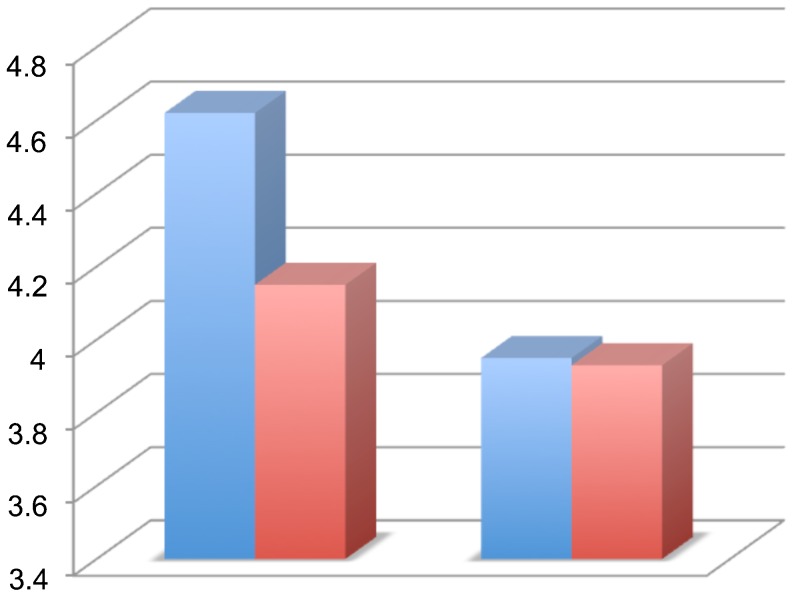
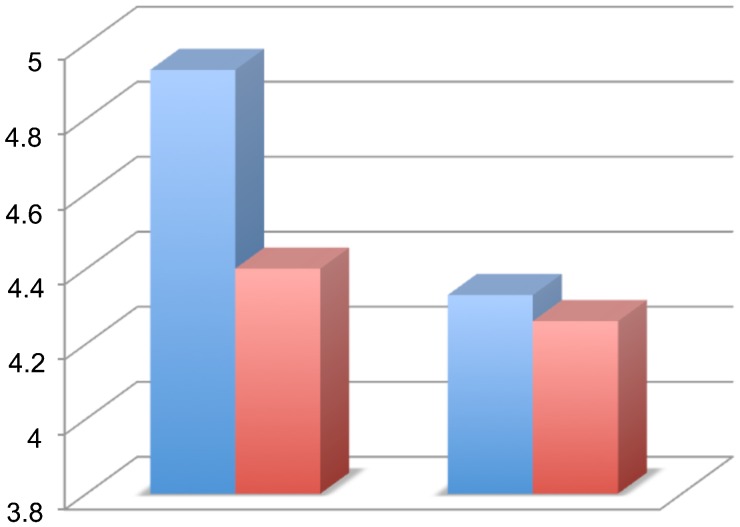
There was no significant difference between the groups regarding the weight of the rats. There was no between-group difference in the BBB score at the end of each week (p>0.05; Figure 2). The average score on the inclined plane test was compared on a weekly basis and no significant difference was identified (p>0.06; Figure 3). At the end of the final week, the evoked potential was measured in the left and right posterior limbs of the rats and no difference was identified between the experimental and control groups (p = 0.11 for the left limb and p = 0.63 for the right limb; (Figure 4). The average latency values were also similar between groups (p = 0.182 for the left limb and p = 0.737 for the right limb; Figure 5). Because no differences were identified between the amplitudes for the right and left posterior limbs within each group, we pooled the samples and compared the amplitudes and latencies of the hind limbs between groups. We did not identify significant differences in the amplitudes of the motor evoked potential or in the latency in the hind limbs (Table 1).
Figure 2.
Comparison of BBB scores between groups along six weeks: hypothermia group in blue and control group in red.
Figure 3.
Comparison of the scores of the inclined plane test between groups along six weeks: hypothermia group in blue and control group in red.
Figure 4.
Comparison of evoked potential results of amplitude in the groups comparing the left and right posterior limbs (in the left and right side of the graph, respectively): hypothermia group in blue and control group in red.
Figure 5.
Comparison of evoked potential results of latency in the groups comparing the left and right posterior limbs (in the left and right side of the graph, respectively): hypothermia group in blue and control group in red.
Table 1.
The means and standard deviations of the amplitude and latency in the evoked motor potential test in the posterior limbs of rats.
| Evoked potential result | Group 1 (n = 30) | Group 2 (n = 30) | p-value |
| Amplitude | 4.39±1.36 | 3.94±0.89 | 0.143 |
| Latency | 4.66±1.42 | 4.3±0.75 | 0.217 |
DISCUSSION
Numerous studies are currently being conducted to identify methods to minimize or prevent secondary damage after a spinal cord lesion. However, the feasibility of the therapies studied remains limited. The concept of inducing systemic or local hypothermia arose more than 40 years ago, and clinical and experimental trials were performed to examine ischemic lesions secondary to vascular procedures. The results of these studies showed that lowering the spinal cord or brain temperature slowed the metabolism, which offered protection against the lesions (11-15). However, the reactions caused by ischemic spinal cord injury are different from those caused by traumatic spinal cord injury. We have experienced great difficulty in comparing the scientific studies because the model of induction of hypothermia, the target temperature, the duration and the method used to cause the experimental spinal cord injury vary widely among the studies.
Casas et al. (16) reported the absence of a neuroprotective effect of epidural hypothermia with saline infusion in rats after six weeks of evaluation. In their study, which was published in 2005, the authors used the BBB scale and the same method of inducing the spinal cord lesion (contusion) used in the current study, i.e., the NYU Impactor. They applied hypothermia 30 minutes after the lesion for three hours and compared three levels of hypothermia (mild, moderate and intense) with one control. Exactly as in our study, Casas et al. found no significant differences between the groups, indicating that hypothermia did not protect nerves or spinal cord tissues or improve locomotor function. On the contrary, the authors stated that hypothermia could potentially exacerbate secondary damage by reducing the blood flow to the injured site. In a similar study, Maybhate et al. (17) induced a moderate lesion (by a fall from a height of 12.5 mm with the NYU Impactor) and conducted a two-hour session of systemic hypothermia. The session began two hours after the lesion was induced and the results were evaluated by the evoked potential test and the BBB scale. The authors concluded that early systemic hypothermia provided significant neuroprotection at four weeks after spinal cord injury through improved sensory electrophysiological signals in Lewis rats; furthermore, higher BBB scores were identified in acute and post-acute periods after injury.
In the present study, we used a spinal cord contusion model induced by the NYU Impactor, with a drop of twice the height (25 mm) of that used in the study conducted by Casas et al. (16) to ensure the production of an effective spinal cord injury. In contrast to their study, we applied hypothermia for 20 minutes immediately after the spinal cord injury. We initiated hypothermia procedures immediately after the spinal cord injury because our goal was to interfere with the effects of secondary damage, which begin immediately after the trauma. We also chose to induce intense hypothermia in the epidural space, with temperature ranging from 9°C to 14°C and we were aware of the necessity to break through the protective barrier of the membranes' dura, arachnoid and pia mater and the cerebrospinal fluid to achieve low medullary temperatures of approximately 25°C. It was possible to maintain the cooling procedure for 20 minutes without compromising the estimated epidural temperature. The drip system we described in the Methods is of low cost, easy to apply and a single device can be used for all of the rats in an experiment.
Dimar et al. (5) conducted an experimental study on hypothermia using two different methods of spinal cord injury: either placement of a 50% spacer in the epidural space or induction of a spinal cord contusion with an NYU Impactor at a height of 25 mm (the same approach as that used in the present study). The authors tested different temperatures: 37°C (normothermia) and 19°C (hypothermia); they also evaluated motor function with the BBB scale and used the motor evoked potential test. The authors reported significant improvement in the motor score of the rats subjected to epidural spacer injury and hypothermia compared with the normothermic controls, but these improvements were not observed in the group subjected to severe spinal cord injury or in the rats that were injured with the spacer and the NYU device (mixed contusion model). The parameters used in the group subjected to severe spinal cord injury were approximately the same as the parameters used in our study, with minimal differences in the follow-up (five weeks compared with six weeks in our study) and in the temperature used (19°C compared with 9°C-14°C used in our duty). Dimar et al. (5) obtained very similar results to those obtained in our study. These findings suggest that mild, moderate, severe and profound epidural hypothermia do not produce the effects that are observed in the model of spinal cord ischemia when using the traumatic model.
Ha and Kim (4) induced moderate epidural hypothermia (30°C) in rats for 48 hours after inducing spinal cord injury with the NYU Impactor at a height of 25 mm. To assess motor function, they used the Gale et al. (18) scale, which ranges from 0 to 6 and the inclined plane test. The follow-up period was seven days. The authors identified significant improvements in motor function in both tests in the group subjected to hypothermia compared with the controls and they attributed the improvement to the fact that the secondary damage after spinal trauma continued for at least a week; the maximum time for the apoptosis of oligodendrocytes is 48 hours. The authors concluded that the positive results occurred because hypothermia was applied for a sufficiently long time to produce neuroprotective effects. Those authors justified the use of moderate hypothermia because it was safer and produced fewer adverse effects.
The study by Ha and Kim (4), published in 2008, inspired the idea that hypothermia should be maintained for a longer period to obtain neuroprotective effects. However, the scale they used to evaluate motor function (the Gale scale) was different from ours and from that used in other studies on the same subject. Their study also had a short follow-up period considering the extent of the traumatic lesion; therefore, one cannot say that the neuroprotective effect from hypothermia was maintained because the rats were sacrificed on the seventh day after the lesion. It must be considered that the pathophysiology of spinal cord trauma has two distinct phases, which include primary and secondary injury; thus, the trauma-induced lesion is distinct from the ischemic injury induced by compression of the spinal cord; therefore, the response to hypothermia might also be different. Inflammatory activity is one of the mechanisms involved in secondary injury in addition to apoptosis of neuronal cells and glia. Hypothermia has been shown to reduce the migration of inflammatory cells to the site of injury and it has an effect on the expression of markers involved in cell apoptosis (19). However, the mechanisms that produce an irreversible spinal cord injury remain unclear.
Our results differed from those recently published by Ok et al. (15). Their study compared systemic and epidural moderate hypothermia and demonstrated neuroprotective effects after induction of spinal cord lesions. According to the authors, the systemic hypothermia showed more neuroprotective effects via antiapoptotic and anti-inflammatory effects. One potential explanation for this difference in the results could be the length of hypothermia administration (48 hours) and the temperature used (28°C).
Four limitations of the present study should be considered. First, it was not possible to control the exact temperature of the infusion solution, and the temperature varied slightly during the experiment as the water dripped from the tank. Nevertheless, the temperature was monitored and only varied from 9°C to 14°C. Moreover, the tissue temperature was maintained at 25°C, was measured minute by minute and was controlled by increasing or reducing the drip rate.
Second, we did not perform a histopathological study of the tissues in the injured area, which could enable verification of the efficacy of the spinal cord contusion model. However, the contusion model has been widely used and our team has substantial experience with it. The primary outcome analyzed in our study was the functional evaluation of the neurological recovery in the rats using the BBB scale and the inclined plane test; both tests have been validated for this type of study and have been used to identify functional changes in weekly evaluations for periods of six weeks. We understand that immunohistochemical analysis represents a potential secondary outcome to be investigated at the end of the study. However, even if this analysis did indicate changes, we would not be able to identify the moment at which the tissue changes occurred.
Third, and more importantly, the cooling system used in our study did not allow epidural hypothermia to be maintained for long periods. Because the maximum time for the apoptosis of oligodendrocytes is 48 hours, it is possible that longer periods of epidural hypothermia could interfere in cell death and thereby alter the motor function results in the rats.
Fourth, it is possible that the vascular spasm that was naturally caused by cooling would interfere in the recovery of the neural tissues in the area of the injured spinal cord. However, no study has identified a way to detect or measure a vascular spasm. Therefore, this issue remains a limitation of any study that addresses hypothermia in spinal cord lesions.
We expect that studies with more uniform methodologies and more sophisticated cooling systems can be undertaken to reveal which factors determine the irreversibility of spinal cord lesions and the specific role of hypothermia in spinal cord trauma treatment.
The present study indicated that there were no neuroprotective effects of epidural hypothermia when applied at the injury site immediately after inducing traumatic spinal cord injury in rats.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.National Spinal Cord Injury Statistical Center. Alabama: National Spinal Cord Injury Statistical Center; 2010. The 2010 Annual Statistical Report for the Spinal Cord Injury Model Systems. Disponível em: https://www.nscisc.uab.edu/PublicDocuments/reports/pdf/2010%20NSCISC%20Annual%20Statistical%20Report%20-%20Complete%20Public%20 Version.pdf. Acessado em 2013 (17 out) [Google Scholar]
- 2.Brasil. Ministério da Saúde. Datasus. Informações de Saúde. Procedimentos hospitalares do SUS – por local de internação – Brasil. Disponível em: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sih/cnv/qiuf.def. Acessado em 2013 (12 dez)
- 3.Crock HV, Yoshizawa H, Yamagishi M, Crock MC. Commentary on the prevention of paralysis after traumatic spinal cord injury in humans: the negleted factor—urgent restoration of spinal cord circulation. Eur Spine J. 2005;14(9):910–4. doi: 10.1007/s00586-005-0924-4. [DOI] [PubMed] [Google Scholar]
- 4.Ha KY, Kim YH. Neuroprotective effect of moderate epidural hypothermia after spinal cord injury in rats. Spine (Phila Pa 1976) 2008;33(19):2059–65. doi: 10.1097/BRS.0b013e31818018f6. [DOI] [PubMed] [Google Scholar]
- 5.Dimar JR, 2nd, Shields CB, Zhang YP, Burke DA, Raque GH, Glassman SD. The role of directly applied hypothermia in spinal cord injury. Spine (Phila Pa 1976) 2000;25(18):2294–302. doi: 10.1097/00007632-200009150-00006. [DOI] [PubMed] [Google Scholar]
- 6.Schiaveto-de-Souza A, da-Silva CA, Defino HL, Del Bel EA. Effect of melatonin on the functional recovery from experimental traumatic compression of the spinal cord. Braz J Med Biol Res. 2013;46(4):348–58. doi: 10.1590/1414-431X20132322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu CG, Jimenez O, Marcillo AE, Weider B, Bangerter K, Dietrich WD, et al. Beneficial effects of modest systemic hypothermia on locomotor function and histopathological damage following contusion-induced spinal cord injury in rats. J Neurosurg. 2000;93(1 Suppl):85–93. doi: 10.3171/spi.2000.93.1.0085. [DOI] [PubMed] [Google Scholar]
- 8.Albin MS, White RJ, Locke GS, Massopust LC, Jr, Kretchmer HE. Localized spinal cord hypothermia—anesthetic effects and application to spinal cord injury. Anesth Analg. 1967;46(1):8–16. [PubMed] [Google Scholar]
- 9.Souza FI, Cristante AF, Marcon RM, Ferreira R, Santos GB, Barros Filho TEP. Monossialogangliosídeo transdérmico com laser no tratamento de lesão medular espinal de ratos [Transdermal monosialoganglioside with laser in the treatment of spinal cord lesion in rats] Acta Ortop Bras. 2013;21(2):87–91. doi: 10.1590/S1413-78522013000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 11.Arrica M, Bissonnette B. Therapeutic hypothermia. Semin Cardiothorac Vasc Anesth. 2007;11(1):6–15. doi: 10.1177/1089253206297409. [DOI] [PubMed] [Google Scholar]
- 12.Busto R, Dietrich WD, Globus MY, Valdés I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7(6):729–38. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- 13.Inamasu J, Ichikizaki K. Mild hypothermia in neurologic emergency: an update. Ann Emerg Med. 2002;40(2):220–30. doi: 10.1067/mem.2002.123697. [DOI] [PubMed] [Google Scholar]
- 14.Bernard SA, Buist M. Induced hypothermia in critical care medicine: a review. Crit Care Med. 2003;31(7):2041–51. doi: 10.1097/01.CCM.0000069731.18472.61. [DOI] [PubMed] [Google Scholar]
- 15.Ok JH, Kim YH, Ha KY. Neuroprotective effects of hypothermia after spinal cord injury in rats: comparative study between epidural hypothermia and systemic hypothermia. Spine (Phila Pa 1976) 2012;37(25):E1551–9. doi: 10.1097/BRS.0b013e31826ff7f1. [DOI] [PubMed] [Google Scholar]
- 16.Casas CE, Herrera LP, Prusmack C, Ruenes G, Marcillo A, Guest JD. Effects of epidural hypothermic saline infusion on locomotor outcome and tissue preservation after moderate thoracic spinal cord contusion in rats. J Neurosurg Spine. 2005;2(3):308–18. doi: 10.3171/spi.2005.2.3.0308. [DOI] [PubMed] [Google Scholar]
- 17.Maybhate A, Hu C, Bazley FA, Yu Q, Thakor NV, Kerr CL, All AH. Potential long-term benefits of acute hypothermia after spinal cord injury: assessments with somatosensory-evoked potentials. Crit Care Med. 2012;40(2):573–9. doi: 10.1097/CCM.0b013e318232d97e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gale K, Kerasidis H, Wrathall JR. Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol. 1985;88(1):123–34. doi: 10.1016/0014-4886(85)90118-9. [DOI] [PubMed] [Google Scholar]
- 19.Chatzipanteli K, Yanagawa Y, Marcillo AE, Kraydieh S, Yezierski RP, Dietrich WD. Posttraumatic hypothermia reduces polymorphonuclear leukocyte accumulation following spinal cord injury in rats. J Neurotrauma. 2000;17(4):321–32. doi: 10.1089/neu.2000.17.321. [DOI] [PubMed] [Google Scholar]