Abstract
OBJECTIVES:
Breast hamartoma is an uncommon breast tumor that accounts for approximately 4.8% of all benign breast masses. The pathogenesis is still poorly understood and breast hamartoma is not a well-known disorder, so its diagnosis is underestimated by clinicians and pathologists. This study was designed to present our experience with breast hamartoma, along with a literature review.
METHOD:
We reviewed the demographic data, pathologic analyses and imaging and results of patients diagnosed with breast hamartoma between December 2003 and September 2013.
RESULTS:
In total, 27 cases of breast hamartoma operated in the Ankara University Medicine Faculty's Department of General Surgery were included in the study. All patients were female and the mean age was 41.8±10.8 years. The mean tumor size was 3.9±2.7 cm. Breast ultrasound was performed on all patients before surgery. The most common additional lesion was epithelial hyperplasia (22.2%). Furthermore, lobular carcinoma in situ was identified in one case and invasive ductal carcinoma was observed in another case. Immunohistochemical staining revealed myoid hamartoma in one case (3.7%).
CONCLUSION:
Breast hamartomas are rare benign lesions that may be underdiagnosed because of the categorization of hamartomas as fibroadenomas by pathologists. Pathologic examinations can show variability from one case to another. Thus, the true incidence may be higher than the literature indicates.
Keywords: Breast, Hamartoma, Carcinoma
INTRODUCTION
Breast hamartoma is an uncommon breast tumor that accounts for approximately 4.8% of all benign breast masses (1) and that contains lobular breast tissue involving various fibrous, fibrocystic and adipose tissues (2). However, with increasing social awareness and widespread breast cancer screening, hamartomas are being routinely diagnosed with greater frequency. The pathogenesis of hamartomas remains unclear and its diagnosis is underestimated by clinicians and pathologists. In the literature, breast hamartoma is presented only in case reports and reviews of wide series are very rare. Thus, this study was designed to present the clinicopathologic findings of twenty-seven patients with breast hamartoma.
METHODS
Between December 2003 and September 2013, 27 cases of breast hamartoma operated in the Ankara University Medicine Faculty's Department of General Surgery were included in this study. The histopathology records, clinical follow-up data and previous breast imaging results of the patients were obtained. These data were again analyzed and annotated by clinicians and pathologists.
RESULTS
Demographic features, tumor sizes, preoperative imaging procedures, pathologic results and treatment modalities are shown in Table 1). Clinically, most (24) patients had painless, soft-to-firm, palpable breast lumps and three patients had breast asymmetry. Breast ultrasonography (US) was performed on all of the patients as the first preoperative imaging procedure. In addition to US, mammography was performed in 6 cases and magnetic resonance imaging (MRI) was conducted in one case. In total, 23 cases showed features characteristic of fibroadenoma or hamartoma. In 4 patients (14.8%), US revealed a partial irregular border, and fine-needle aspiration cytology (FNAC) was performed and revealed benign lesions. Pathologic examination usually revealed a well-circumscribed mass with normal breast components, such as a terminal ductal lobular unit, fat and a hyalinized stroma (Figures 1A-B). The lesions usually showed fibrocystic changes, columnar cell changes and adenosis. No proliferative lesions were observed in 14 patients (51.9%). The most common additional proliferative lesion was ductal epithelial hyperplasia, which was diagnosed in 6 patients (22.2%) (Figure 2). We identified lobular carcinoma in situ (LCIS) in one case and invasive ductal carcinoma (IDC) in another case, near the breast hamartoma (3.7%). Pseudoangiomatous hyperplasia was observed in 7 cases (25.9%) (Figure 3). One case (3.7%) was pathologically reported as breast myoid hamartoma and this hamartoma stained positive for desmin, smooth muscle actin (SMA) and CD34 (Figures 4A-C). Mastectomy and sentinel lymph node biopsy were performed on the patient with IDC and lumpectomies were performed on the other patients.
Table 1.
Demographics and clinicopathologic features of 27 patients with breast hamartoma.
| Number of patients | 27 |
| Age, mean ± SD (min-max) | 41.8±10.8 (19-56 years) |
| Gender | Female (all) |
| Tumor size, mean ± SD (min-max) | 3.9±2.7 (0.3-15 cm) |
| Additional lesion | |
| Ductal epithelial hyperplasia | 6 (22.2%) |
| Pseudoangiomatous hyperplasia | 7 (25.9) |
| LCIS | 1 (3.4%) |
| IDC | 1 (3.4%) |
| Myoid hamartoma | 1 (3.4%) |
| SMA | + |
| CD34 | + |
| Desmin | + |
| Preoperative evaluation | |
| Ultrasonography | 27 (100%) |
| Mammography | 6 (22.2%) |
| FNAC | 4 (14.8%) |
| MRI | 1 (3.4%) |
| Surgery | |
| Lumpectomy | 27 (100%) |
| Mastectomy + SLNB | 1 (3.4%), second surgery for IDC |
SD: standard deviation, LCIS: lobular carcinoma in situ, IDC: invasive ductal carcinoma, SMA: smooth muscle actin, FNAC: fine-needle aspiration cytology, MRI: magnetic resonance imaging, SLNB: sentinel lymph node biopsy.
Figure 1.
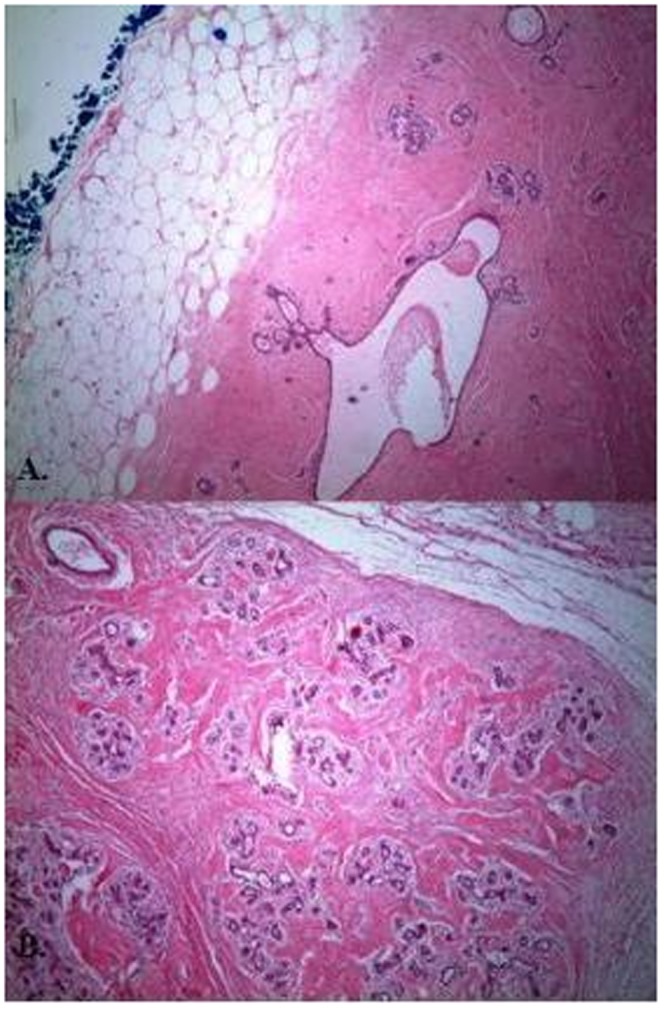
(A) and (B) Well-circumscribed mass with normal terminal ductal lobuler unit, fat and hyalinized stroma, Hematoksilen Eosin (H&E), ×40.
Figure 2.
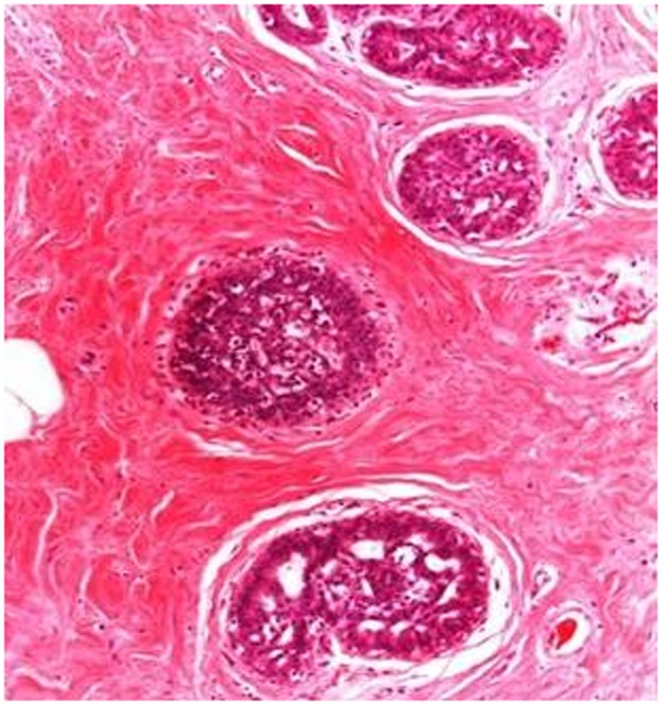
Ductal epithelial hyperplasia, (H&E), ×100.
Figure 3.
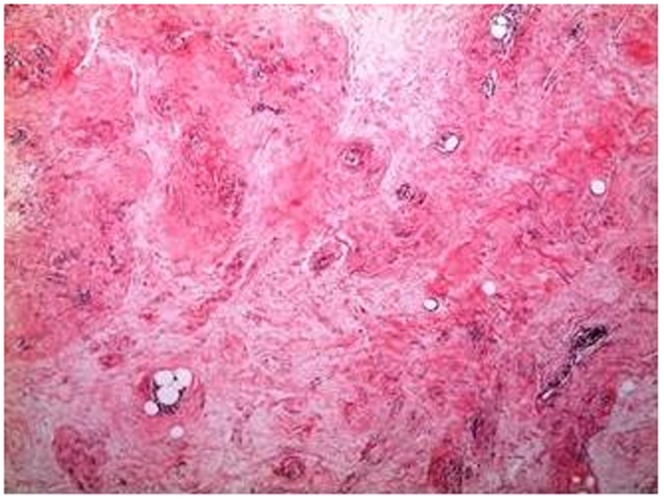
Pseudo-angiomatous hyperplasia, (H&E), ×40.
Figure 4.
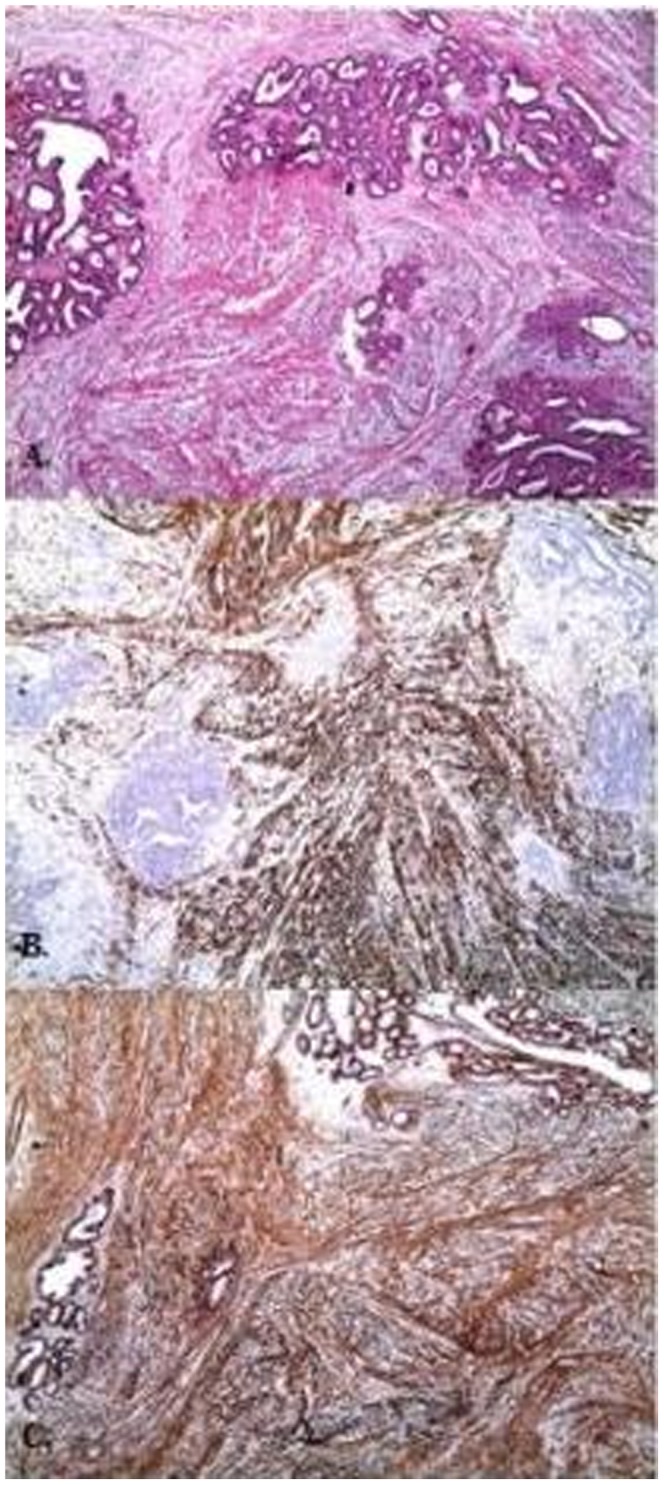
Myoid hamartoma, (A) (H&E), ×40 (B) desmin, ×40, (C) SMA, ×40.
DISCUSSION
Breast hamartomas are poorly defined, rare, benign breast neoplasms. Hamartomas were initially defined as mastomas in 1928 by Prym (3). Afterward, several cases were reported and classified as adenolipomas, fibroadenolipomas or lipofibroadenomas (4). Arrigoni et al. (5) first used the term hamartoma in 1971. Hamartoma is referred to as myoid hamartoma, a rarer form, when it shows a significant smooth muscle component. This term was first used by Davies and Riddell (6) in 1973.
Articles related to breast hamartoma in the literature are mostly case reports and rarely case series; therefore, they are limited in number. Thus, there are few data about these tumors. We compiled the published case reports and case series in PubMed from between 1993 and 2013 in terms of demographic features, tumor size, applied imaging procedures, pathology reports and the results of additional staining, which are shown in Table 2) (7-43).
Table 2.
Literature review for breast hamartoma.
| Author and year | Age, gender | Clinical findings | Size (cm) | US (n) | MMG (n) | MRI (n) | FNAC (n) | Pathologic findings (n) | Additional staining | Recurrence |
| Nasit et al., 2012 (7) | 45, F | Lump | 9 | NR | Well-circumscribed, dense mass | NR | Few mammary lobules and ducts, with a fibrous stroma | Myoid hamartoma | Vimentin +, SMA +, Desmin +, ER, PR +Cytokeratin -, S-100 - CD34 - | No (one-year follow-up) |
| Mizuta et al., 2012 (8) | 38, F | Lump | 2.8 | Well-demarcated, hypoechoic lesion with slightly irregular margins | Well-demarcated, oval, isodense mass that was partly indistinct (BI-RADS-4) | Well-circumscribed mass with high signal intensity, showing strong enhancement and a microlobulated margin. Dynamic contrast-enhanced imaging demonstrated rapid enhancement of the mass | Fibroadenoma with focal mastopathic change | Myoid hamartoma | SMA +, Vimentin +, Desmin + Cytokeratin -, S-100 - | NR |
| Kai et al., 2012 (9) | 70, F | Lump | 3.2 | Irregular hypoechoic tumor both within and external to hamartoma | Typical hamartoma | Suspected malignancy | NR | HamartomaDCISIDC | NR | NR |
| Uchôa et al., 2010 (10) | 59, F | Lump | 2.5 | NR | NR | NR | NR | NR | Vimentin +, Desmin +, CD34 + Calponin +, bcl-2 +,SMA -Actin -,S-100 -,CD99 -,CD10 - | NR |
| Choi et al., 2010 (11) | 72, F | Lump | 9 | Spiculated, non-parallel hypoechoic nodule within hamartoma | Typical hamartoma with focal asymmetry | Suspected malignancy | NR | HamartomaIDC | NR | No (2-year follow-up) |
| Ko et al.,2010 (12) | 43, F | Lump | 2.5 | Irregular isoechoic mass with a microlobulated margin | Two oval isodense masses with a partially obscured margins in the left subareolar area | Suspected malignancy | Mammary lobules, ducts, and stromaNo malignant component | Myoid hamartoma | Vimentin +, SMA +,CD34 +,S-100 - | Recurrence in the first year |
| Gupta et al., 2010 (13) | 13, M | Lump | 10 | Solid heterogeneous mass with internal echogenic zones | NR | NR | Non-diagnostic | Hamartoma | NR | No (14-month follow-up) |
| Kajo et al., 2010 (14) | 46, F | Lump | 17 | NR | NR | NR | Fibroadenoma | Myoid hamartoma | Desmin +, SMA +,Caldesmon +,CD34 -,S-100 -,CD 10 - | NR |
| Khoo et al., 2009 (15) | 46, F | Lump | 9 | Solid breast mass | Dense, well-encapsulated mass with no calcifications | NR | Fibroadenoma | Myoid hamartoma,chondroid differentiation | Vimentin +,Myoglobin +, SMA + Desmin +,CD34 +,Cytokeratin -,S-100 - | NR |
| Pervatikar et al., 2009 (16) | 25, F | Lump | 15 | NR | NR | NR | Malignant | HamartomaIDC | No (one-year follow-up) | |
| Lee WF et al., 2008 (17) | 48, F | Lump | 4.5 | NR | Typical hamartoma | NR | NR | Hamartoma | NR | NR |
| Hernanz et al., 2008 (18) | 29, F | Breast asymmetry | 14 | NR | Well-circumscribed mass | NR | Core biopsy; hamartoma | Hamartoma | NR | NR |
| Stafyla et al., 2007 (19) | 60, F | Lump | 11.5 | Solid mass | Dense, well-defined mass | NR | NR | Myoid hamartoma | NR | No (4-year follow-up) |
| Murat et al., 2007 (20) | 42, F | No complaints, MMG, US | 5 | Smooth-edged,solid mass lesion with heterogeneous echogenicity | Smooth-edged, heterogeneous,oval opacity and surrounding radiolucency | Mass lesion with heterogeneous intensity | Core biopsy; hamartoma | Hamartoma | NR | NR |
| Murugesan et al., 2006 (21) | 45, F | Breast pain, MMG, US | 1.6 | Irregular hypoechoic, lobulated, ill-defined borders | Irregular, partly ill-defined lesion with no microcalcification | NR | Core biopsy; myoid hamartoma | Myoid hamartoma | SMA +,Desmin +, S-100 - | NR |
| Borges da Silva et al., 2006 (22) | 33, F | Axillary mass | 10 | Compatible with the diagnosis of a voluminous nodule | NP | NP | NP | Hamartoma | NP | No (2-year follow-up) |
| Ruiz-Tovar et al., 2006 (23) | 43.2 (mean), F, 8 cases | Lump (all) | 7.25 (mean) | Heterogeneous mass with hypoechogenic areas inside (2) | Well-circumscribed masses combining radiolucent and dense areas (all) | NR | Benign (5)Core biopsy;hamartoma (1)Incisional biopsy; hamartoma (1) | Hamartoma (all) | NR | Recurrence in 1 patient (after 6 months) |
| Kuroda et al., 2006 (24) | 57, F | Bilateral axillary lump | 3.5, 3.3 | NP | NP | NP | NP | Bilateral hamartoma | NP | NP |
| Breucq et al., 2005 (25) | 42, F | Lump | 2 | Well-circumscribed, oval heterogeneous nodule | Denser aspect | NP | NR | Myoid hamartoma,LCIS | NR | NR |
| Barbaros et al., 2005 (26) | 36, F | Lump | 15 | Fibroadenolipoma | Fibroadenolipoma | NR | NR | Hamartoma | NR | NR |
| Gatti et al., 2005 (27) | 43, F | Lump | 4.5 | Oval shape, well-circumscribed margins and internal echogenicity | No sign of disease | NR | NR | Hamartoma | NR | NR |
| Giannotti Filho et al., 2004 (28) | 51 (mean), F, 3 cases | Lump | 1.3 (mean) | NR | Well-circumscribed lump | NR | NR | Myoid hamartoma | SMA +, Vimentin +, Desmin +, S-100 - | NR |
| Lee et al., 2003 (29) | 66.5 (mean), F, 2 cases | Axillary mass (1)MMG (1) | 5.5 (mean) | Well-defined hypoechoic nodule within hamartoma (1)NP (1) | Typical hamartoma | NR | Malignant (1)NP (1) | Hamartoma, DCIS, IDC (1)Hamartoma, IDC (1) | NR | No (6-month follow-up) (1)No (5-year follow-up) (1) |
| Baron et al., 2003 (30) | 54 | Lump | 5 | NR | Well-circumscribed, oval, mixed fatty and fibroglandular lesion | NR | Benign epithelial cells | HamartomaILC | High ERLow PR | NR |
| Tse et al., 2002 (31) | 50 (mean), F2 cases | Lump | 2 (mean) | Well-defined lobulated mass (1)NP (1) | NP | NR | NP (1)Cellular atypia (1) | Hamartoma, DCIS (1)Hamartoma, DCIS, mucinous carcinoma (1) | NR | No (5-year follow-up) (1)No (4-year follow-up) (1) |
| Kuroda et al., 2002 (32) | 53, F | Lump | 6 | İsoechoic solid mass with capsule suspected to be a lipomatous tumor | Circumscribed mass with a thin pseudocapsule, no microcalcification, and no spiculation | NR | NR | Hamartoma, ILC | Β-catenin - | NR |
| Herbert et al., 2002 (33) | 48 (mean)F, 24 cases | Lump (all) | 2-5 (range) | NR | NR | NR | NR | HamartomaFocal apocrine metaplasia (in a few cases)Pseudoangiomatous hyperplasia (7) | NR | NR |
| Tse et al., 2002 (34) | 38 (mean)F, 24 cases | Lump (23)Breast asymmetry (1) | 3.8 (mean) | Typical hamartoma (18) | Mixed fibrous adipose tissueOvoid-rounded, well-circumscribed masses of mixed heterogeneous density with a mottled center and thin smooth capsules with peripheral radiolucent zones | Typical hamartoma (4)Well capsulated, with a dark smooth rim; ovoid in shape, with internal heterogeneity and heterogeneous gadolinium enhancement | Hamartoma (11)Non-diagnostic (3)Benign (7)More cellular (4) | HamartomaPseudoangiomatous changes (8)Mild epithelial hyperplasia (10)Cystic changes (8)Apocrine metaplasia (4)Adenosis (1)DCIS (3)Stromal hyalinization (3) | NR | Recurrence in 1 patient (after 10 months) |
| Ravakhah et al., 2001 (35) | 36, M | Lump | 3 | NR | NR | NR | Non-diagnostic | Myoid Hamartoma | NR | NR |
| Weinzweig et al., 2001 (36) | 15, F | Breast asymmetry | 27 | Mixed echogenic pattern | NR | NR | NR | Hamartoma (lobular proliferation) | NR | NR |
| Wahner-Roedler et al., 2001 (37) | 50 (mean), F, 35 cases | Lump (18)MMG (18) | 3.2 (mean) | Solid hypoechoic lesion (12)Solid hypoechoic with definite cystic areas (5) | Irregular margin (1)Indistinct margin (4)Water density (21)Mixed density (2) | NR | Core biopsy; benign (3) | Hamartoma (all)Calcifications (4)Ductal hyperplasia (9) | NR | NR |
| Takeuchi et al., 2001 (38) | 74, F | Lump | 1.8 | Oval-shaped hypoechoic mass with slightly irregular margin | Well marginated and oval-shaped isodense nodule with no microcalcification | NR | NR | Myoid hamartoma | SMA +,S-100 -, Myoglobin -, Keratin -, Vimentin - | NR |
| Mester et al., 2000 (39) | 59, F | Lump | 7 | NR | Circumscribed, predominantly fatty mass. Multiple macro- and microcalcifications were contained in the mass | NR | Nondiagnostic | HamartomaDCIS | NR | NR |
| Chiacchio et al., 1999 (40) | 40.4 (mean)F, 10 cases | Lump | 5.4 (mean) | NR | Nodular densities | NR | NR | HamartomaPseudoangiomatous hyperplasia | CK +,S-100 +,Vimentin + | NR |
| Blomqvist et al., 1997 (41) | 33.5 (mean), F, 2 cases | Lump (1)Breast asymmetry (1) | NR | NP | NP | NP | Benign hamartoma (1)Benign (1) | Hamartoma | NP | NR |
| Anani et al., 1996 (42) | 65.5 (mean), F, 2 cases | Lump, skin ulcer (1)Lump (1) | 6 (mean) | Hypoechoic absorbing lesion suggestive of carcinoma (1) | Typical hamartoma with irregular opacity containing microcalcifications (1)Typical hamartoma with spiculated mass (1) | NR | NR | HamartomaIDC (all) | NR | NR |
| Garfein et al., 1996 (43) | 50-59 (range), F, 6 cases | Lump (3)MMG (3) | 1.1-7 (range) | Solid lesion (4) | Well-circumscribed lesion (1)Heterogeneous radiodensity (4)Homogeneously radiodense (1)Slightly irregular border (1) | NR | Nondiagnostic (1) | Myoid Hamartoma | Actin +,Desmin +,Vimentin +,Cytokeratin -, S-100 + | No (follow-up of 2-19 months) |
| This study | 41.8 (mean), F, 27 cases | Lump (24)Breast asymmetry (3) | 3.9 (mean) | Well-circumscribed, oval heterogeneous nodule (23)Slightly irregular margin(4) | Typical hamartoma (5)Malignant lesion (1) | Malignancy (1) | Benign (4) | Hamartoma (26)Myoid hamartoma (1)IDC (1)LCIS (1) | SMA + (1),Desmin + (1), CD34 + (1) | No recurrence to date |
F: female, M: male, NR: not reported, NP: not performed, SMA: smooth muscle actin, ER: estrogen receptor, PR: progesterone receptor, DCIS: ductal carcinoma in situ, LCIS: lobular carcinoma in situ, IDC: invasive ductal carcinoma, ILC: invasive lobular carcinoma.
The incidence of breast hamartoma among benign breast tumors was reported to be 4.8% by Charpin et al. (1) in a large case series in 1994. In total, 27 hamartomas (3.1%) were diagnosed among 864 benign breast tumors at our center over the last ten years. In the literature (23,28,29,31,33,34,37), the average age of the patients with breast hamartoma ranged between 33.5 and 66.5 years. In our study, the age range was from 19-56 years and the mean age was 41.8 years. There was only one patient under 20 years of age and 7 patients were over 50 years of age.
The etiopathogenesis of breast hamartomas is not clear but they are thought to result from dysgenesis (44) rather than a true tumorous process. However, female sex steroid hormones (34) have been implicated in the development of breast hamartomas. In one study, Herbert et al. (33) reported estrogen receptor (ER) and progesterone receptor (PR) positivity in epithelial cells and stromal cells in all 24 cases with breast hamartomas. Additionally, there are no clear data on the source of smooth muscle for myoid hamartomas, but this muscle could derive from vessels, the nipples, undifferentiated breast stromal tissue or myoepithelial cells (4,14). Another hypothesized smooth muscle source is the metaplasia of breast stromal cells (14) into smooth muscle cells. The existence of CD34 on smooth muscles (21,34) is an important sign of the metaplasia of stromal cells into smooth muscle cells, but there are no clear data on this subject. Myoid hamartomas stain strongly positive for SMA, desmin and vimentin (8) by immunohistochemical staining. In our study, only 1 patient of 27 was diagnosed with myoid hamartoma and immunohistochemical staining for desmin, SMA and CD34 was positive, consistent with the literature.
With increasing social awareness and the use of numerous diagnostic procedures on breast lumps, including mammography, ultrasound, and MRI, it is expected that more hamartomas will be reported. Breast hamartomas have a typical mammographic appearance. The radiolucent lesions include fat; various amounts of fibrous and adenomatous tissues, with smooth rims; and occasionally, a thin capsule (4). US evaluates breast hamartomas as having sonolucent fat and a heterogeneous internal echo pattern with echogenic fibrous components (45). Alongside US, MRI characteristically shows a smooth and well-defined hypointense rim, internal heterogeneous enhancement and the presence of fat density (34). In the present study, US was used for all patients as the first imaging procedure and in addition to US, mammography was utilized for 6 patients. MRI was used only for 1 patient with IDC.
The pathologic features of hamartomas are not well known. The original definition consisted of a fibrous fatty stroma including various amounts of epithelial elements, as well as nodular lesions. The fibrous and fatty tissues were used for the early classification of hamartomas: McGuire et al. (46) classified breast hamartomas as fibrous, fatty or fibrous-fatty. Jones et al. (47) suggested 4 classification groups for breast hamartomas: encapsulated fibrocystic changes, fibroadenoma with a fibrous stroma, fibroadenoma-like and surrounded adenolipoma. These classifications were not widely accepted. No criteria with detailed explanations of breast hamartomas are used by pathologists. Fechner et al. (48) described the lobular distribution and the presence of fat in breast hamartomas as distinguishing features compared with fibroadenomas. A fibrotic stroma surrounding the lobules and extending into the interlobular areas, causing obliteration, is the most frequently observed feature; most authors (2,47,48) refer to this feature as interlobular fibrosis. However, interlobular fibrosis is not specific to breast hamartomas. In sclerosing hyperplasia, an increasing number of interlobular glands are observed as round masses with interlobular fibrosis, which can mimic breast hamartomas. The absence of fatty tissue in the stromal structure is often associated with fibroadenomas, so this condition can be distinguished from sclerosing lobular hyperplasia and breast hamartomas (33,34,45). Fischer et al. (2) defined the presence of a pseudoangiomatous stroma particularly in breast hamartomas. This condition was revealed to be a consistent observation in subsequent reports and the incidence rate (34,49) varied between 16 and 71% (34). We also found the rate to be 25.9% in our series, in line with the literature.
Malignancies associated with breast hamartomas (9,11,16,29,31,42,50) are rarely observed, and only a few studies have reported invasive breast cancer associated with breast hamartoma. We identified IDC in only 1 case and LCIS in 1 other case. Although epithelial hyperplasia is not a characteristic feature of breast hamartomas, ductal epithelial hyperplasia was identified in 6 cases (22.2%). Wahner-Rodler et al. (37) reported this incidence rate as 26% in their large case series. These high coincidence rates are very important. Patients with a certain or suspected diagnosis of hamartoma must be operated and these patients should never be followed, even if their tumors are small.
In conclusion, we present our institutional experience with breast hamartomas in 27 patients. Although breast hamartomas are rare, benign lesions, these lesions can reach large sizes. A diagnosis can be made by core needle biopsy, along with an appropriate correlation of clinical and radiologic features. Breast hamartomas may be underdiagnosed because pathologists may categorize these lesions as fibroadenomas instead of hamartomas. Thus, the true incidence may be higher than the literature indicates.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Charpin C, Mathoulin MP, Andrac L, Barberis J, Boulat J, Sarradour B, et al. Reappraisal of breast hamartomas. A morphological study of 41 cases. Pathol Res Pract. 1994;190(4):362–71. doi: 10.1016/S0344-0338(11)80408-5. [DOI] [PubMed] [Google Scholar]
- 2.Fisher CJ, Hanby AM, Robinson L, Millis RR. Mammary hamartoma—a review of 35 cases. Histopathology. 1992;20(2):99–106. doi: 10.1111/j.1365-2559.1992.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 3.Prym P. Pseudoadenome, Adenome und Mastome der weiblithen Brustdrfise; Studien fiber die Entstehung umschriebener adenom∼ hnlicher Herde in der Mamma und fiber die Nachahmung der Brustdrfisengewebes durch echte Adenome und Fibroadenome. Beitr Pathol Anat. 1928;81:1–44. [Google Scholar]
- 4.Altermatt HJ, Gebbers JO, Laissue JA. Multiple hamartomas of the breast. Appl Pathol. 1989;7(2):145–8. [PubMed] [Google Scholar]
- 5.Arrigoni MG, Dockerty MB, Judd ES. The identification and treatment of mammary hamartoma. Surg Gynecol Obstet. 1971;133(4):577–82. [PubMed] [Google Scholar]
- 6.Davies JD, Riddell RH. Muscular hamartomas of the breast. J Pathol. 1973;111(3):209–11. doi: 10.1002/path.1711110309. [DOI] [PubMed] [Google Scholar]
- 7.Nasit JG, Parikh B, Trivedi P, Shah M. Myoid (muscular) hamartoma of the breast with chondroid metaplasia. Indian J Pathol Microbiol. 2012;55(1):121–2. doi: 10.4103/0377-4929.94883. [DOI] [PubMed] [Google Scholar]
- 8.Mizuta N, Sakaguchi K, Mizuta M, Imai A, Nakatsukasa K, Morita M, et al. Myoid hamartoma of the breast that proved difficult to diagnose: a case report. World J Surg Oncol. 2012;10:12. doi: 10.1186/1477-7819-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kai M, Tada K, Tamura M, Gomi N, Horii R, Akiyama F, et al. Breast cancer associated with mammary hamartoma. Breast Cancer. 2012;19(2):183–6. doi: 10.1007/s12282-009-0147-3. [DOI] [PubMed] [Google Scholar]
- 10.Uchoa DM, Cruz DB, Schaefer PG, Pegas KL, Cambruzzi E. Myofibroblastoma arising in mammary hamartoma: a case report. Patholog Res Int. 2010;2010 doi: 10.4061/2010/726829. 4 pages. Article ID726829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi N, Ko ES. Invasive ductal carcinoma in a mammary hamartoma: case report and review of the literature. Korean J Radiol. 2010;11(6):687–91. doi: 10.3348/kjr.2010.11.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko MS, Jung WS, Cha ES, Choi HJ. A rare case of recurrent myoid hamartoma mimicking malignancy: imaging appearances. Korean J Radiol. 2010;11(6):683–6. doi: 10.3348/kjr.2010.11.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta SS, Singh O, Hastir A, Arora G, Sabharwal G, Mishra H. Breast hamartoma with intrathoracic extension in a 13-year-old boy. J Cancer Res Ther. 2010;6(1):86–8. doi: 10.4103/0973-1482.63559. [DOI] [PubMed] [Google Scholar]
- 14.Kajo K, Zubor P, Danko J. Myoid (Muscular) Hamartoma of the Breast: Case Report and Review of the Literature. Breast Care (Basel) 2010;5(5):331–4. doi: 10.1159/000321341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoo JJ, Alwi RI, Abd-Rahman I. Myoid hamartoma of breast with chondroid metaplasia: a case report. Malays J Pathol. 2009;31(1):77–80. [PubMed] [Google Scholar]
- 16.Pervatikar SK, Rao R, Dinesh US, Parameswaraiah S. Large mammary hamartoma with focal invasive ductal carcinoma. Indian J Pathol Microbiol. 2009;52(2):249–51. doi: 10.4103/0377-4929.48935. [DOI] [PubMed] [Google Scholar]
- 17.Lee WF, Sheen-Chen SM, Chi SY, Huang HY, Ko SF. Hamartoma of the breast: an underrecognized disease. Tumori. 2008;94(1):114–5. doi: 10.1177/030089160809400120. [DOI] [PubMed] [Google Scholar]
- 18.Hernanz F, Vega A, Palacios A, Fleitas MG. Giant hamartoma of the breast treated by the mammaplasty approach. ANZ J Surg. 2008;78(3):216–7. doi: 10.1111/j.1445-2197.2007.04414.x. [DOI] [PubMed] [Google Scholar]
- 19.Stafyla V, Kotsifopoulos N, Grigoriadis K, Bakoyiannis CN, Peros G, Sakorafas GH. Myoid hamartoma of the breast: a case report and review of the literature. Breast J. 2007;13(1):85–7. doi: 10.1111/j.1524-4741.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 20.Murat A, Ozdemir H, Yildirim H, Poyraz AK, Ozercan R. Hamartoma of the breast. Australas Radiol. 2007;51 Spec No.:B37–9. doi: 10.1111/j.1440-1673.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- 21.Murugesan JR, Joglekar S, Valerio D, Bradley S, Clark D, Jibril JA. Myoid hamartoma of the breast: case report and review of the literature. Clin Breast Cancer. 2006;7(4):345–6. doi: 10.3816/CBC.2006.n.050. [DOI] [PubMed] [Google Scholar]
- 22.da Silva BB, Rodrigues JS, Borges US, Pires CG, Pereira da Silva RF. Large mammary hamartoma of axillary supernumerary breast tissue. Breast. 2006;15(1):135–6. doi: 10.1016/j.breast.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Tovar J, Reguero-Callejas ME, Arano-Bermejo JI, Gonzalez-Palacios F, Cabanas-Navarro L. [Mammary hamartoma] Cir Esp. 2006;79(3):186–8. doi: 10.1016/s0009-739x(06)70848-x. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda N, Goishi K, Ohara M, Hirouchi T, Mizumo K, Nakagawa K. Bilateral hamartoma arising in axillary accessory mammary glands. Case report. APMIS. 2006;114(1):77–8. doi: 10.1111/j.1600-0463.2006.apm_349.x. [DOI] [PubMed] [Google Scholar]
- 25.Breucq C, Verfaillie G, Perdaens C, Vermeiren B, Stadnik T. Lobular carcinoma located in a breast hamartoma. Breast J. 2005;11(6):508–9. doi: 10.1111/j.1075-122X.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- 26.Barbaros U, Deveci U, Erbil Y, Budak D. Breast hamartoma: a case report. Acta Chir Belg. 2005;105(6):658–9. doi: 10.1080/00015458.2005.11679798. [DOI] [PubMed] [Google Scholar]
- 27.Gatti G, Mazzarol G, Simsek S, Viale G. Breast hamartoma: a case report. Breast Cancer Res Treat. 2005;89(2):145–7. doi: 10.1007/s10549-004-1656-6. [DOI] [PubMed] [Google Scholar]
- 28.Filho OG, Gordan AN, Mello Rde A, Neto CS, Heinke T. Myoid hamartomas of the breast: report of 3 cases and review of the literature. Int J Surg Pathol. 2004;12(2):151–3. doi: 10.1177/106689690401200211. [DOI] [PubMed] [Google Scholar]
- 29.Lee EH, Wylie EJ, Bourke AG, Bastiaan De Boer W. Invasive ductal carcinoma arising in a breast hamartoma: two case reports and a review of the literature. Clin Radiol. 2003;58(1):80–3. doi: 10.1053/crad.2003.1133. [DOI] [PubMed] [Google Scholar]
- 30.Baron M, Ladonne JM, Gravier A, Picquenot JM, Berry M. Invasive lobular carcinoma in a breast hamartoma. Breast J. 2003;9(3):246–8. doi: 10.1046/j.1524-4741.2003.09313.x. [DOI] [PubMed] [Google Scholar]
- 31.Tse GM, Law BK, Pang LM, Cheung HS. Ductal carcinoma in situ arising in mammary hamartoma. J Clin Pathol. 2002;55(7):541–2. doi: 10.1136/jcp.55.7.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuroda N, Sugimoto T, Numoto S, Enzan H. Microinvasive lobular carcinoma associated with intraductal spread arising in a mammary hamartoma. J Clin Pathol. 2002;55(1):76–7. doi: 10.1136/jcp.55.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbert M, Sandbank J, Liokumovich P, Yanai O, Pappo I, Karni T, et al. Breast hamartomas: clinicopathological and immunohistochemical studies of 24 cases. Histopathology. 2002;41(1):30–4. doi: 10.1046/j.1365-2559.2002.01429.x. [DOI] [PubMed] [Google Scholar]
- 34.Tse GM, Law BK, Ma TK, Chan AB, Pang LM, Chu WC, et al. Hamartoma of the breast: a clinicopathological review. J Clin Pathol. 2002;55(12):951–4. doi: 10.1136/jcp.55.12.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravakhah K, Javadi N, Simms R. Hamartoma of the breast in a man: first case report. Breast J. 2001;7(4):266–8. doi: 10.1046/j.1524-4741.2001.20079.x. [DOI] [PubMed] [Google Scholar]
- 36.Weinzweig N, Botts J, Marcus E. Giant hamartoma of the breast. Plast Reconstr Surg. 2001;107(5):1216–20. doi: 10.1097/00006534-200104150-00019. [DOI] [PubMed] [Google Scholar]
- 37.Wahner-Roedler DL, Sebo TJ, Gisvold JJ. Hamartomas of the breast: clinical, radiologic, and pathologic manifestations. Breast J. 2001;7(2):101–5. doi: 10.1046/j.1524-4741.2001.007002101.x. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi M, Kashiki Y, Shibuya C, Yamamoto S, Kitamura F, Nagao Y, et al. A case of muscular hamartoma of the breast. Breast Cancer. 2001;8(3):243–5. doi: 10.1007/BF02967516. [DOI] [PubMed] [Google Scholar]
- 39.Mester J, Simmons RM, Vazquez MF, Rosenblatt R. In situ and infiltrating ductal carcinoma arising in a breast hamartoma. AJR Am J Roentgenol. 2000;175(1):64–6. doi: 10.2214/ajr.175.1.1750064. [DOI] [PubMed] [Google Scholar]
- 40.Chiacchio R, Panico L, D'Antonio A, Delrio P, Bifano D, Avallone M, et al. Mammary hamartomas: an immunohistochemical study of ten cases. Pathol Res Pract. 1999;195(4):231–6. doi: 10.1016/S0344-0338(99)80039-9. [DOI] [PubMed] [Google Scholar]
- 41.Blomqvist L, Malm M, Fernstad R. Hamartoma of the breast: surgical treatment and reconstruction. Case report. Scand J Plast Reconstr Surg Hand Surg. 1997;31(4):365–9. doi: 10.3109/02844319709008985. [DOI] [PubMed] [Google Scholar]
- 42.Anani PA, Hessler C. Breast hamartoma with invasive ductal carcinoma. Report of two cases and review of the literature. Pathol Res Pract. 1996;192(12):1187–94. doi: 10.1016/S0344-0338(96)80149-X. [DOI] [PubMed] [Google Scholar]
- 43.Garfein CF, Aulicino MR, Leytin A, Drossman S, Hermann G, Bleiweiss IJ. Epithelioid cells in myoid hamartoma of the breast: a potential diagnostic pitfall for core biopsies. Arch Pathol Lab Med. 1996;120(7):676–80. [PubMed] [Google Scholar]
- 44.Guray M, Sahin AA. Benign breast diseases: classification, diagnosis, and management. Oncologist. 2006;11(5):435–49. doi: 10.1634/theoncologist.11-5-435. [DOI] [PubMed] [Google Scholar]
- 45.Kopans DB. Pathologic, mammographic and sonographic correlation. In: Kopans D B, editor. Breast Imaging. 2nd ed. Boston, Massachusetts: Lippincott-Raven; 1998. pp. 558–560. [Google Scholar]
- 46.McGuire LI, Cohn D. Hamartoma of the breast. Aust N Z J Surg. 1991;61(9):713–6. doi: 10.1111/j.1445-2197.1991.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 47.Jones MW, Norris HJ, Wargotz ES. Hamartomas of the breast. Surg Gynecol Obstet. 1991;173(1):54–6. [PubMed] [Google Scholar]
- 48.Fechner RE. Fibroadenoma and related lesions. In: Page D L, Anderson T J, editors. Diagnostic Histopathology of the Breast. Edinburgh: Churchill Livingstone; 1987. pp. 72–85. [Google Scholar]
- 49.Daya D, Trus T, D'Souza TJ, Minuk T, Yemen B. Hamartoma of the breast, an underrecognized breast lesion. A clinicopathologic and radiographic study of 25 cases. Am J Clin Pathol. 1995;103(6):685–9. doi: 10.1093/ajcp/103.6.685. [DOI] [PubMed] [Google Scholar]
- 50.Kronsbein H, Bassler R. [Metaplasias and malignant transformations in hamartomas of the breast] Verh Dtsch Ges Pathol. 1985;69:310–5. [PubMed] [Google Scholar]


