Abstract
Aim: The aim of this study is to investigate the molecular identification of Giardia lamblia in patients with diarrhea.
Background: Giardiasis caused by Giardia lamblia is a common intestinal disease. Although this parasitic infection found in mammals including human, pets and livestock, but few species within the genus Giardia can infects humans. G. lamblia have seven complex genotypes termed (A-H). Genotype A and B the main causes of human infections.
Patients and methods: Sixty seven microscopically positive G. Lamblia samples were collected from clinical laboratories in Isfahan province between June 2013 and February 2014. Extraction of genomic DNA was performed for 65 concentrated cysts and 2 cultured trophozoites. Partial sequences of tpi including 148-bp and 81-bp were amplified for detection the genotypes A and B using RFLP- PCR protocol respectively.
Results: PCR results showed that out of 67 patients with giardiasis infection, genotype A (148 bp) was detected in 40 isolates (59.70%) compared to genotype B (81 bp) isolated was detected in 25 isolates (37.31%). Also two isolates (2.98%) had mix infection infected with genotype A and B. By comparing the frequency of genotype A (81.8%) and genotype B (13.6%), we found that genotype A is six times higher prevalence than genotype B in patients with diarrhea.
Conclusion: We suggest that using sensitive techniques and larger sample for detection of G. lamblia genotypes and their subtypes would be necessary for investigation the immune system respond and correlation with diarrhea in the future studies in Iran.
Custom Keyword Group: Giardia lamblia, Tpi, PCR-RFLP, Isfahan, Iran
Introduction
Giardia lamblia, synonymous with G. intestinalis and G. duodenalis, is a intestinal flagellant protozoa. Giardiasis caused by Giardia lamblia is a common intestinal disease. Usually G. lamblia transmitted by contaminated water with cyst (1). Although this parasitic infection found in mammals including human, pets and livestock, but few species within the genus Giardia can infect humans (2). Giardiasis is one of the most common intestinal protozoa infections in human that have been reported worldwide (3). In Asia, Africa, and Latin America, about 200 million people have symptomatic giardiasis, with at least 500,000 new cases reported each year (4). G. lamblia have seven complex genotypes termed (A-H). Genotypes A and B are the main and only causes of human infections (4, 5). Genotype A have two subtype, subtype AI a main zoonotic subtype and subtype AII belonging to anthroponotic infections, however, in a few studies have been reported in animals. Assemblage B divided to two subtypes named BIII and BIV (1, 2, 6). Genotypic characterization of G. lamblia has been shown a useful tool in epidemiological studies or outbreak investigations (7, 8). PCR techniques for genotyping of G. lamblia are based on polymorphic genes encoding 18S rRNA, glutamate dehydrogenase (gdh), elongation factor 1-alpha (ef1-α), triose phosphate isomerase (tpi), and β-giardin (5, 9).
The current study was conducted to determine G. lamblia genotypes in Isfahan, using tpi markers. PCR-RFLP method was performed based on tpi, as this is suitable technique for direct typing of G. lamblia in crude specimens. The main purpose of this study was to evaluation the correlation between genotypes of G. lamblia and patients with diarrhea.
Patients and Methods
Sample collection
Sixty seventy stool samples from microscopically positive G. Lamblia cysts were collected from clinical laboratories in Isfahan province between June 2013 and February 2014. Samples were collected from both males and females aged 5 months to 70 years. After direct analysis by microscope, the samples without any preservation were kept at 4°C. Cysts of 67 Giardia-positive specimens were concentrated from the specimens by flotation on 4 layers (0.5, 0.75, 1, and 1.5 M) and single-layer (0.85 M) sucrose. Among samples, the fresh specimens with a high number of G. lamblia (cysts > 205) were isolates and cultured on TYI-S-33 medium (10). Finally, purified cysts and cultured trophozoites were stored at -20°C until further analysis.
DNA extraction
Genomic DNA was extracted from 65 concentrated cysts and 2 cultured trophozoites. Those isolates didn’t have suitable DNA were excluded from the study. The trophozoite's DNA was extracted using QIAamp DNA Stool Mini Kit (QIAgen Company, Germany) according to the manufacturer's instruction with some modification and used as template for PCR assay. Extraction of genomic DNA from Giardia’s cysts was carried out according to our previous published paper (11).
PCR amplification
Partial sequences of tpi including 148-bp and 81-bp were amplified for detection the genotypes A and B using RFLP- PCR protocol respectively. For detection of genotype A the following primers were used; forward primer (5´-GGAGACCGACGAGCAAAGC-3´) and reverse primer (5´-CTTGCCAAGCGCCTCAA-3´) and also to identifying genotype B the following primers were used; forward primer (5'-AATAGCAGCACARAACGTGTATCTG-3') and reverse primer (5'-CCCATGTCCAGCAGCATC-3') (12, 13).
The reaction mixture for PCR amplification contained 5 μL of 10× buffer (CinnaGen, Iran), 1.5 mM of MgCl2 (CinnaGen, Iran), 0.2 mM of each dNTPs, 1 U of Taq polymerase (CinnaGen, Iran), 20 P mole of each primers and 5–10 μL of extracted template DNA. Amplification of extracted DNA was performed according to the following conditions: One cycle of 94°C for 5 min (initial denaturation), followed by 35 cycles of 20 s at 94°C, 45 s at 60.5°C, 45 s at 72°C, and a final extension at 72°C for 5 min. The DNA sample extracted from cultured trophozoites and G. Lamblia standard strain (ATCC: 30888TM) were used as the positive control to monitor PCR success and distilled water was used as the negative control to check for false-positive results that may have arisen from carryover contamination in all experiments. PCR amplicons underwent electrophoresis in a 3% (W/V) agarose gel with Tris-acetate electrophoresis buffer and were stained with ethidium and visualized under a UV trans illuminator. After amplification, the tpi gene PCR products for genotype A (148-bp) and B (81-bp), fragment by the enzyme BspLI. 8 μL of PCR product was digested in a total of 20 μL reaction mixture containing 10 U of BspLI (Fermentase EU) and 2 μL of restriction buffer (Tango buffer fermentase). Then the reaction mixture was incubated at 37°C for 2 hours. The digested fragments were fractionated on 3% agarose gel stained by DNA green viewer.
Results
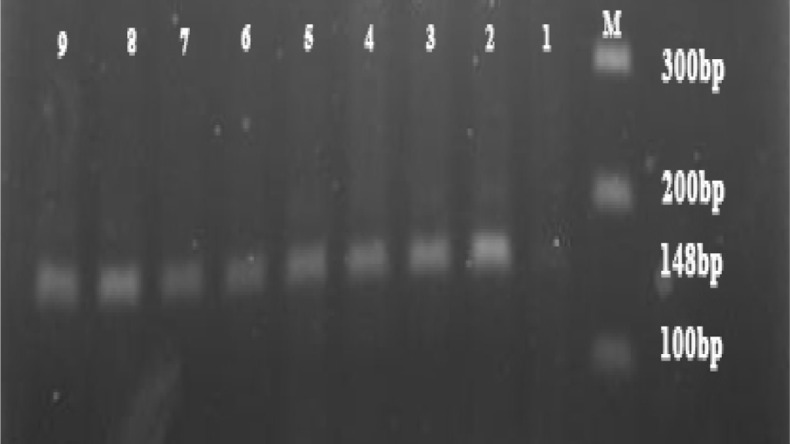
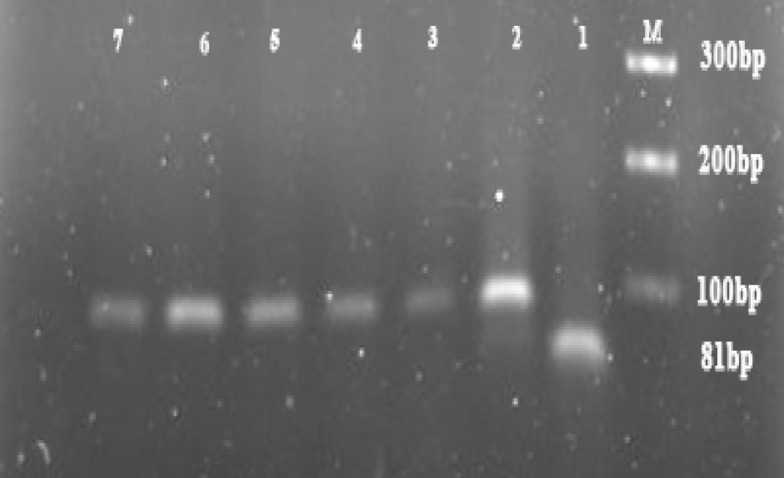
DNA was extracted successfully from all 67 samples and then selected for molecular analysis. PCR results showed that out of 67 patients with giardiasis infection, genotype A (148 bp) was detected in 40 isolates (59.70%) and genotype B (81 bp) was detected in 25 isolates (37.31%) (Figure 1, 2).
Figure 1.
PCR amplification of G. lamblia tpi on 3% agarose gel stained with DNA green viewer. (Lane M: 100 bp gene ruler (fermentase); Line 1: negative control and line 2-9 is belonged to genotype A (148bp).
Also two isolates (2.98%) had mixed infection with genotype A and B. By comparing the frequency of genotype A (81.8%) and genotype B (13.6%), we found that genotype A is six times higher prevalence than genotype B in patients with diarrhea. However, among patients without diarrhea, frequency of genotype B (82.6%) (17.39%) was significantly higher than genotype A. Statistical Fisher’s test showed, there was significant correlation (P value < 0.05) between patients with diarrhea and genotype A and also asymptomatic infections with genotype B
Figure 2.
PCR amplification of G. lamblia tpi on 3% agarose gel stained with DNA green viewer. (Lane M: 100 bp gene ruler (fermentase); showed genotype B in line 2 (81bp).
Table 1.
Frequency of G. Lamblia genotypes in patients with giardiasis base one clinical sign of diarrhea.
| Genotype | Non-diarrheal patients No. (%) | Diarrheal patients No. (%) | Total (%) |
|---|---|---|---|
| genotype A | 4(17.39) | 36(81.8) | 40(59.7) |
| genotype B | 19(82.6) | 6(13.6) | 25(37.31) |
| Mix genotype | --- | 2(4.5) | 2(2.98) |
| Total | 23(100) | 44(100) | 67(100) |
Discussion
Giardiasis in humans is caused by two entirely different genotypes A and B. So far various methods have been performed for molecular diagnosis and detection of G. lamblia in the feces and the environment as well. Giardiasis has highly variability symptoms. Some people without any obvious symptoms can be able to dispose infected cysts in feces. It’s not clear why some patients have clinical signs and others are asymptomatic. However, it seems host factors such as immunity conditions and variability in parasite strains, are involved in such differences. Although several studies shown that G. Lamblia strains are similar in morphologies but there are different in phenotypic and genetics analysis. In present study we used tpi marker and PCR-RFLP method for detection assemblages A and B of G. lamblia in stool collection samples from clinical laboratories in Isfahan province. Subtype of G. lamblia was not detected in this study. A main purpose of this study was to check the correlation between genotyping of G. lamblia and diarrhea in human. The result of this study showed a significant correlation between genotype A of G. lamblia and diarrhea. In recent years many studies have been done in the different parts of the World including Bangladesh, Australia, Turkey, Spain, India and Iran and showed the correlation between genotype A and diarrhea (14-20). The same result was reported by Manochehri et al. (2012) in Shahrekord province, Iran (21). In contrast, Rafiei et al. (2013) in Southwest of Iran did not find any correlation between clinical symptoms and assemblages of G. lamblia (23). In other study Etemadi et al. (2011) used glutamate dehydrogenase (gdh) marker for detect the genotypes of G. lamblia by PCR-RFLP method in human faces in Kerman, Southeastern of Iran. They reported both A and B genotype in this region (22).
On the hand in the study by Hamdan et al. in Saudi Arabia different result to our findings was reported (24). They Used High Resolution Melting (HRM) analysis technique for investigate the correlation between G. lamblia genotype and children with and without gastrointestinal symptomatic. They found the correlation between assemblage B and symptomatic patients (24). In support of Hamdan et al. study, Homan et al. in Netherlands and Gelanew et al. in Ethiopia found 100% correlation between the severity of diarrhea and genotype B of G. lamblia and obvious correlation between genotype B of G. lamblia with sustainable diarrhea respectively (25, 26).The relationship between diarrhea and G. lamblia genotypes not essentially means that certain genotypes have higher virulence with diarrhea compared to other genotype.
In this study we observe similar diversity in genotypes to other regions in Asia, some parts of Europe and America, though in contrast to some previous studies, we found similar levels of diarrheal symptoms in those individuals infected with assemblage A compared with those infected with assemblage B.
Future research on Giardia infection within sensitive technique like HRM analysis technique and using a large sample size could address whether those Giardia genotypes found in humans are being transmitted zoonotically and correlation with diarrhea and other clinical sign or not.
References
- 1.Sarkari B, Ashrafmansori A, Hatam G, Motazedian M, Asgari Q, Mohammadpour I. Genotyping of Giardia lamblia isolates from human in southern Iran. Trop Biomed. 2012;2003:366–371. [PubMed] [Google Scholar]
- 2.Cacci{\`o} S, Ryan U. Molecular epidemiology of giardiasis. Mol Biochem Parasitol. 2008;160:75–80. doi: 10.1016/j.molbiopara.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Thompson R. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet Parasitol. 2004;126:15–35. doi: 10.1016/j.vetpar.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 4.A M, J S, K W, A M.-A, M A.-M, Y L. Giardia intestinalis genotypes: Risk factors and correlation with clinical symptoms. Acta Trop. 2009;112:67–70. doi: 10.1016/j.actatropica.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Monis P, Andrews R, Mayrhofer G, Ey P. Genetic diversity within the morphological species Giardia intestinalis and its relationship to host origin. Infect. Genet. Evol. 2003;3:29–38. doi: 10.1016/s1567-1348(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 6.Yang R, Lee J, Ng J, Ryan U. High prevalence Giardia lamblia assemblage B and potentially zoonotic subtypes in sporadic human cases in Western Australia. Int J Parasitol. 2010;40:293–297. doi: 10.1016/j.ijpara.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Palmer C, Traub R, Robertson I, Devlin G, Rees R, Thompson R. Determining the zoonotic significance of Giardia and Cryptosporidium in Australian dogs and cats. Vet Parasitol. 2008;154:142–147. doi: 10.1016/j.vetpar.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 8.der Giessen J, Vries A, Roos M, Wielinga P, Kortbeek L, Mank T. Genotyping of Giardia in Dutch patients and animals: A phylogenetic analysis of human and animal isolates. Int J Parasitol. 2006;36:849–858. doi: 10.1016/j.ijpara.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Monis P, Andrews R, Mayrhofer G, Ey P. Molecular systematics of the parasitic protozoan Giardia intestinalis. Mol Bio Evol. 1999;16:1135–1144. doi: 10.1093/oxfordjournals.molbev.a026204. [DOI] [PubMed] [Google Scholar]
- 10.Yousefi H. Pure culture method: Giardia lamblia from different stool samples. J Res Med Sci. 2000;5:78–81. [Google Scholar]
- 11.Pestehchian N, H R, Z B, Ha Y, Aa E, M K, al e. Identification of genotypes of Giardia lamblia human isolates in Isfahan, Iran, using polymerase chain reaction–Restriction Fragment Length polymorphism. Adv Biomed Res. 2012;1:84–84. doi: 10.4103/2277-9175.105166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallah E, Nahavandi K, Jamali R, Poor B, Asgharzadeh M. Molecular Identification of Giardia lamblia Isolates from Human and Animal Reservoirs by PCR-RFLP. J Biol Sci. 2008;8:896–896. [Google Scholar]
- 13.Tungtrongchitr A, Sookrung N, Indrawattana N, Kwangsi S, Ongrotchanakun J, Chaicumpa W. Giardia intestinalis in Thailand: Identification of genotypes. J Health Popul Nutr. 2010;28:42–42. doi: 10.3329/jhpn.v28i1.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alam M, Ilias M, Siddique M, Kabir M, Nazib F, Khan M. Genotype-specific detection of Giardia lamblia in stool samples of diarrhoeal and non-diarrhoeal patients in Dhaka, Bangladesh. Dhaka Univ J Biol Sci. 2011;20:183–189. [Google Scholar]
- 15.Haque R, Roy S, Kabir M, Stroup S, Mondal D, Houpt E. Giardia assemblage A infection and diarrhea in Bangladesh. J Infect Dis. 2005;192:2171–2173. doi: 10.1086/498169. [DOI] [PubMed] [Google Scholar]
- 16.Petri W, R H, D M, A K, Ih M, A R, al e. Prospective case-control study of the association between common enteric protozoal parasites and diarrhea in Bangladesh. Clin Infect Dis. 2009;48:1191–1197. doi: 10.1086/597580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Read C, Walters J, Robertson I, Thompson R. Correlation between genotype of Giardia lamblia and diarrhoea. Int J Parasitol. 2002;32:229–231. doi: 10.1016/s0020-7519(01)00340-x. [DOI] [PubMed] [Google Scholar]
- 18.Aydin A, Besirbellioglu B, Avci I, Tanyuksel M, Araz E, Pahsa A. Classification of Giardia lamblia parasites in Turkey into groups A and B using restriction fragment length polymorphism. Diag Microbiol Infect Dis. 2004;50:147–151. doi: 10.1016/j.diagmicrobio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Sahagun J, A C, P G, C S, M L, F C, al e. Correlation between the presence of symptoms and the Giardia lamblia genotype. Eur J Clin Microbiol Infect Dis. 2008;27:81–83. doi: 10.1007/s10096-007-0404-3. [DOI] [PubMed] [Google Scholar]
- 20.Paintlia A, Mahajan R, Chakraborti A, Sehgal R, Ganguly N. Characterization of Giardia lamblia groups A and B from North India by isoenzyme and random amplified polymorphic DNA analysis. Parasitol Res. 1999;85:510–512. doi: 10.1007/s004360050588. [DOI] [PubMed] [Google Scholar]
- 21.K M, S A, A G, Z B, S T. Genotyping of Giardia Lamblia Isolates in Individuals with and without Chronic Diarrhea Using Polymerase Chain Reaction. J Mazandaran Univ Med Sci. 2012;22:38–38. [Google Scholar]
- 22.Etamadi S, N Z.-A, Z B, Mf H, A Z.-A, Z S, al e. The correlation between clinical signs and genotypes of Giardia lamblia isolated from patients with Giardiasis in Kerman city. J Kerman Univ Medi Sci. 2011;18:330–338. [Google Scholar]
- 23.Rafiei A, Es R, Ar S, Aa S, A S, Mp B. Investigation of Possible Correlation between Giardia lamblia Genotypes and Clinical Symptoms in Southwest of Iran. Iran j Parasitol. 2013;8:389–389. [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Mohammed H. Genotypes of Giardia intestinalis clinical isolates of gastrointestinal symptomatic and asymptomatic Saudi children. Parasitol Res. 2011;108:1375–1381. doi: 10.1007/s00436-010-2033-5. [DOI] [PubMed] [Google Scholar]
- 25.Homan W, Mank T. Human giardiasis: Genotype linked differences in clinical symptomatology. Int J Parasitol. 2001;31:822–826. doi: 10.1016/s0020-7519(01)00183-7. [DOI] [PubMed] [Google Scholar]
- 26.Gelanew T, Lalle M, Hailu A, Pozio E, Cacci{\`o} S. Molecular characterization of human isolates of Giardia lamblia from Ethiopia. Acta trop. 2007;102:92–99. doi: 10.1016/j.actatropica.2007.04.003. [DOI] [PubMed] [Google Scholar]