Abstract
Background:
Chronic obstructive pulmonary disease (COPD) is a syndrome of progressive airflow limitation caused by the abnormal inflammatory reaction of the airway and lung parenchyma. Osteoporosis is one of the major extrapulmonary manifestations of COPD. The, prevalence of osteoporosis in COPD patients in Indian population is unknown.
Objectives:
To study the prevalence of osteoporosis in COPD and to define various risk factors associated with reduced bone mineral density (BMD) in COPD.
Materials and Methods:
The study was done in the department of Pulmonary Medicine of a tertiary care hospital. All the diagnosed cases of COPD according to the Global Initiative for Obstructive Lung Disease (GOLD) guidelines were included in this study. The present study was a prospective study in for a period of 1 year. A brief history of the patients was taken, especially regarding duration of illness, number of exacerbations in the past 3 years, smoking in pack years, and history of steroid use (both systemic and inhaled steroids) after which cumulative dose of steroids was calculated. Spirometry was done in all these patients to stage the severity of COPD according to GOLD criteria. DEXA scan of the lumbar spine was done using bone densitometer to determine osteoporosis. A world Health Organization (WHO) criterion for definition of osteoporosis was applied and patients with T-score of > –2.5 standard deviation (SD) were diagnosed to have osteoporosis, –1 SD to –2.5 SD were diagnosed to have osteopenia and < –1 SD as normal. Statistical analysis for association of COPD with osteoporosis was done using chi-square test. Risk factors for osteoporosis were identified by univariate and multivariate logistic regression analysis.
Results:
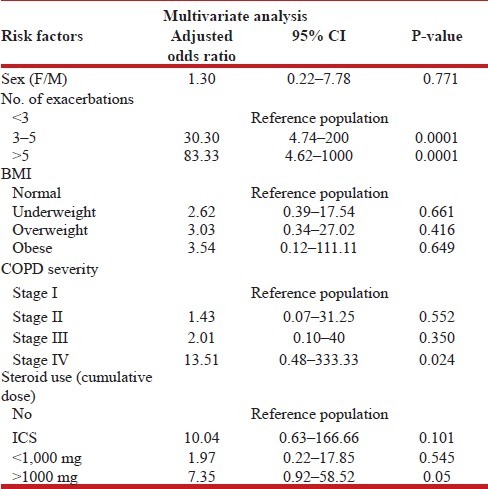
A total of 102 COPD patients were included in the study. Among these, 68 patients (66.6%) had osteoporosis and 20 patients (19.6%) had osteopenia. Majority (64.7%) of the patients who had osteoporosis had stage III and stage IV COPD disease. It was observed that as the severity grade of COPD increased, the risk of osteoporosis also increased. The bone mineral density (BMD) showed a significant difference among different stages of COPD. As the severity of the stage of COPD increased, BMD decreased. It was also observed that patients with lower body mass index (BMI) had higher prevalence of osteoporosis (37.3%) as compared to overweight patients. On univariate analysis, it was observed that risk factors for osteoporosis were female sex, higher number of exacerbations, BMI, and severity of COPD. After using multivariate logistic regression analysis, stage IV COPD (odds ratio (OR): 34.48, 95% confidence interval (CI): 1.59–1,000, P < 0.02), number of acute exacerbations >3 (OR: 30.3, 95% CI: 4.74–200, P < 0.01), and steroid cumulative dose >1,000 mg (OR: 7.35, 95% CI: 0.92–58.5, P < 0.04) were observed to be significant risk factors for osteoporosis in COPD patients.
Conclusions:
In the present study, the prevalence of osteoporosis was 66.6% and another 19.6% had osteopenia. As the severity of COPD increased, the risk of osteoporosis increased. GOLD stage III and stage IV patient had significantly lower BMD as compared to stage I and stage II of COPD disease. Stage IV COPD disease, use of oral or parenteral glucocorticoids, and repeated number of exacerbations were found to be independent risk factors for osteoporosis in COPD patients. Thus, high clinical suspicion and early diagnosis and treatment is required in the evaluation of osteoporosis in COPD patients so that the quality of life can be improved in these patients.
Keywords: COPD, correlates, DEXA scan, osteoporosis, repeated exacerbations, risk factors
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a syndrome of progressive airflow limitation caused by the abnormal inflammatory reaction of the airway and lung parenchyma. It is now considered a systemic disease with widespread extrapulmonary manifestations. The comorbid conditions, in association with pulmonary manifestations, pose additional problems in the management of the disease.[1] It remains a major public health problem and is projected to be rank fifth, in 2020 in burden of disease worldwide.[2] In India it is estimated that there are around 1.49 crore chronic cases of COPD in the age group of 30 years and above.[3] The prevalence rates of COPD in males varied from 2.12 to 9.4% in studies conducted in north India and from 1.4 to 4.08% in south India. These are projected to increase by nearly 50% by the year 2016.[3]
In the care of patients with COPD, the primary focus of the pulmonologist is improvement in their respiratory function. However, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines include “significant extrapulmonary effects” in the definition of COPD, indicating that COPD can be considered a multicomponent disease with marked extra-pulmonary effects.[2] The most common comorbidities responsible for the clinical manifestations and natural history of COPD are cachexia, skeletal muscle abnormalities, osteoporosis, metabolic syndrome, coronary artery disease, heart failure, pulmonary infections, cancer, and pulmonary vascular disease.[4] Osteoporosis, with resulting fractures is one such major comorbid condition in patients with advanced COPD. The prevalence of osteoporosis in COPD patients is 36-60% and that of osteopenia is 35-72%.[5] COPD patients have a higher risk of osteoporosis as compared to healthy subjects, and the loss of bone occurs over an extended period of years.[6] The etiology of osteoporosis in COPD is probably complex and various factors may contribute to its pathogenesis, with chronic inflammatory changes in the lung contributing to the majority of the consequences that lead to osteoporosis.[7] When fractures occur as a complication of osteoporosis, the quality of life of such patients, who are already restricted because of the lung disease, is further reduced. Therefore, awareness amongst healthcare providers and early diagnosis should trigger preventive and therapeutic measures that could avoid or reduce the consequences of osteoporosis.
The present study was undertaken to study the prevalence of osteoporosis in COPD among Indian patients, and to analyze the various risk factors contributing to the development of osteoporosis and osteopenia in these patients.
MATERIALS AND METHODS
The present study was conducted in the department of Pulmonary Medicine, KLES Dr. Prabhakar Kore Hospital and Medical Research Centre, Belgaum in COPD patients during the period of January 2010-December 2011. This was a cross-sectional study done in a tertiary care hospital. The study included both the admitted as well as outpatients of varying grades of COPD. The COPD grading was done according to the GOLD criteria.[2]
Inclusion criteria
All the patients diagnosed as a case of COPD, based on GOLD guidelines[2] were included in the study. The diagnosis of COPD was done based on clinical history and pulmonary function testing and staging was done as per GOLD criteria.
Exclusion criteria
Bronchogenic carcinoma, untreated thyroid dysfunction, rheumatic diseases, diseases affecting bone or calcium homeostasis, primary or secondary hyperparathyroidism, Cushing's syndrome, established osteoporosis, and patients taking treatment with bone active agents.
Procedure
The study was approved by the ethical and research committee of Jawaharlal Nehru Medical College, Belgaum. The selected patients were briefed about the study and written informed consent was obtained.
The study was designed as a prospective study and the enrolled patients were given a questionnaire concerning age, gender, previous bone fractures, present and previous medications, cigarette smoking in pack years, daily exercise, daily diet and duration of respiratory disease, and number of exacerbations in the past 3 years. The cumulative dose of corticosteroids was calculated by taking into account the total dose of parental steroids for the previous 3 years and equivalent dose of prednisolone was calculated. BMI of all the patients was calculated and they were classified as underweight, normal, overweight, and obese with a BMI <18.5, 18.5–24.9, 25–29.9, ≥30, respectively.
The patients were subjected to pulmonary function tests and dual energy X-ray absorptiometry (DEXA) scan to stage the severity of COPD and osteoporosis respectively. Pulmonary function test was done using RMS Helios 702 Spirometer (Recorders and Medicare Systems Pvt. Ltd, MEDSPIROR, India). The following values were obtained from the test: Forced expiratory volume (FEV)1, forced vital capacity (FVC), FEV1 /FVC ratio, slow vital capacity (SVC), and maximal voluntary ventilation (MVV). FEV1 and FVC and FEV1 /FVC ratio were the main parameters used to stage the COPD patients according to GOLD guidelines.[2] Post-bronchodilator spirometry (salbutamol 2.5 mg by nebulization) was performed in all the patients to exclude the diagnosis of bronchial asthma.
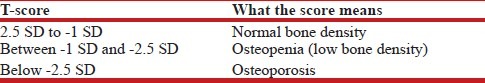
Bone mineral density (BMD) of the patient was determined using whole body densitometer, DEXA Scan (GE Healthcare Lunar prodigy advance, scanner serial no. PA + 302343, software version - ENCORE 2008 version 12.2, Germany). A patient's BMD was given a T-score, which is derived by comparing it to an average score for a healthy 30-year-old of the same sex and race. The difference between the “normal young” score and the patient's score is referred to as a standard deviation (SD)[8] [Table 1].
Table 1.
WHO Classification for Osteoporosis and Osteopenia
Statistical analysis
This is a cross-sectional study in which prevalence of osteoporosis in COPD was evaluated. Prevalence of osteoporosis in COPD was estimated by considering all the COPD patients attending the respiratory clinic. Severity of osteoporosis was correlated with stages of COPD by one-way analysis of variance (ANOVA) test. Various risk factors for osteoporosis in COPD patients were studied and relative risk for individual risk factors was analyzed by using univariate and multivariate logistic regression analysis.
RESULTS
Baseline characteristics
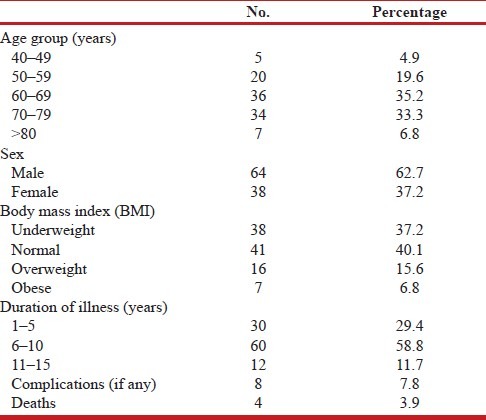
A total of 102 patients were included in the study. There were 64 male patients (62.7%) and 38 female patients (37.3%) [Table 2] A total of 68 patients had osteoporosis (66.7%), 20 patients (19.6%) had osteopenia, while the rest 14 patients (13.7%) had normal bone densitometry. Age of the patients ranged from 42 to 85 years. Mean age of the male patients was 66.6 ± 7.74 years and that of female patients was 64.4 ± 11.48 years. Eight patients had complications like pneumothorax, congestive cardiac failure, pulmonary thromboembolism, respiratory failure requiring intubation, and mechanical ventilation. Four of these patients expired.
Table 2.
Baseline demographic characteristics of the patients
Association of osteoporosis and COPD
Majority of the patients who had osteoporosis had stage III and stage IV COPD disease (64.7%). Stage I and stage II COPD disease had less prevalence of osteoporosis. It was observed that as the severity grade of COPD increased, the risk of osteoporosis had also increased. The total number of COPD exacerbations in the last 3 years the patient suffered, were compared with the severity of osteoporosis. Almost 87% of patients who had suffered ≥3 exacerbations in the past 3 years had osteoporosis, while all the patients with >5 exacerbations in the past 3 years had osteoporosis.
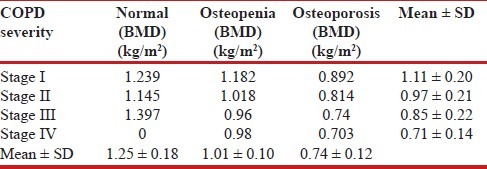
Mean BMD of all the patients was calculated by DEXA scan. The BMD showed a significant difference (P < 0.0001) among the different stages of COPD [Table 3]. The mean BMD reduced with higher stage of COPD. There was also significant difference in BMD (P < 0.0001) among normal, osteopenia, and osteoporosis group. As the severity of stage of COPD increased, the BMD decreased.
Table 3.
Bone mineral density and severity of COPD
Risk factors of osteoporosis
Various risk factors for osteoporosis in COPD such as body mass index (BMI), smoking, and use of corticosteroids in COPD patients were analyzed. It was observed that patients with low BMI had higher prevalence of osteoporosis (29.4%) as compared to overweight patients. Obese individuals had protective effect against development of osteoporosis (4.9%). But this association was not statistically significant (P < 0.073). In this study, all female patients were nonsmokers. Since female patients are already at high risk of osteoporosis, the number of patients with osteoporosis in nonsmokers was higher (P < 0.1). Smoking was found to have no association with COPD. But when compared only among smokers, osteoporosis was observed to be more prevalent in those with >10 pack years smoking history (19.6%) as compared to the patients having pack year history of ≤10 (10.8%). But this association was not statistically significant (P < 0.3).
Most of the patients with COPD are prescribed steroids during any exacerbations, and use of steroids may increase with number of exacerbations and hospitalizations. All the patients were categorized as those not on any steroids, those who used only inhaled steroids, those who used <1,000 mg of steroids (cumulative dose; equivalent of prednisolone) and those who used >1,000 mg (cumulative dose; equivalent of prednisolone). Osteoporosis was observed to be high in those using >1,000 mg (cumulative dose, prednisolone) and the association was statistically significant (P < 0.0001). It was also observed that risk of developing osteoporosis in COPD patients using inhaled corticosteroids was almost the same as those not using inhaled corticosteroids (3.9 versus 6.8%, respectively).
Univariate and Multivariate Analysis of Various Risk Factors for Osteoporosis in COPD
To run binary logistic regression, normal and osteopenia patients were clubbed and considered non-diseased and osteoporosis group was considered diseased. By performing simple univariate analysis, it was observed that the risk factors for osteoporosis in COPD were female sex, number of exacerbations, BMI, and severity of COPD [Table 4]. After running multivariate logistic regression analysis, the significant risk factors for the development of osteoporosis in COPD were: >3 excerbations, GOLD stage IV disease and cumulative use of > 1,000 of prednisone equivalent [Table 5].
Table 4.
Univariate analysis of various risk factors for osteoporosis in COPD
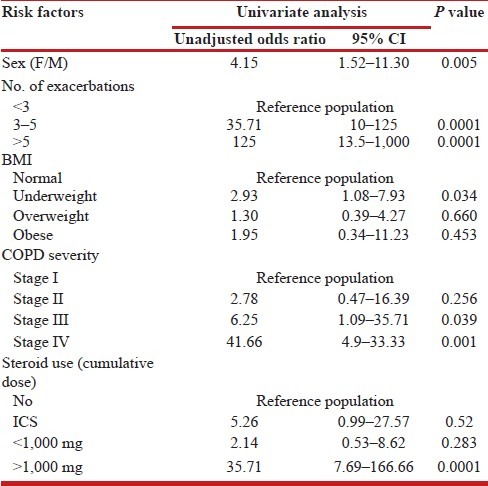
Table 5.
Multivariate analysis of risk factors for osteoporosis in COPD
DISCUSSION
Osteoporosis is more prevalent among COPD patients than among healthy subjects.[9] Thus, it is important to recognize the risk factors and strategies to manage osteoporosis in COPD patients in order to avoid osteoporotic fractures that deteriorate quality of life and prognosis. Thus, the present study was done to determine the prevalence of osteoporosis in COPD patients and also to evaluate various risk factors involved in reduced BMD in patients with COPD. There is no Indian study that has studied the occurrence of osteoporosis in COPD patients using standard DEXA scan.
In the present study, prevalence of osteoporosis observed was 66.7% and that of osteopenia 19.6%. Various studies done in different parts of the world showed prevalence of osteoporosis to be 9–69% in COPD patients versus 0-13% in healthy individuals.[6] Among various studies done, study by Graat-Verboom et al .,[10] and TORCH trial[11] were landmark studies. They found prevalence of osteoporosis to be 21 and 65% and that of osteopenia 41 and 65%, respectively. An Indian study[12] recently done among 37 patients showed a prevalence of osteoporosis of 21.6% and osteopenia 27%. But this study had used calcaneal ultrasonography (USG) for the diagnosis of osteoporosis, which is not a standard test. We have used DEXA scan for the diagnosis of osteoporosis, which is considered a gold standard test and the patients were classified according to the World Health Organization (WHO) criterias.[8] The varied difference in the prevalence of osteoporosis in different studies can be attributed to the methodological differences in the assessment of BMD and also the characteristics (age, sex, past use of bone medications, and stable COPD patients) of patient population chosen for the study.
It is a known fact that female sex is at increased risk for development of osteoporosis, due to the effect of estrogen, as compared to male sex. In the current study, it was observed that prevalence of osteoporosis was high in female population (P < 0.005). But by multivariate analysis, the difference in prevalence of osteoporosis in male and female patients was statistically insignificant. In a meta-analysis done by Graat-Verboom et al.,[6] involving 13 studies with a total of 775 COPD patients, it was observed that there were more male patients (67 versus 33%), and the prevalence of osteoporosis varied from 9 to 69%. In addition, the prevalence of osteopenia varied from 27 to 67%. Patients with osteoporosis consisted of a higher proportion of women. However, in our study there was no significant difference observed in the prevalence of osteoporosis among male and female population.
There is a definite relation between severity of COPD disease and risk of osteoporosis both in terms of T-score and BMD. In the present study, majority of patients who had osteoporosis had grade IV COPD (93.7%). Also mean BMD was comparable between stage II, stage III, and stage IV patients (which was statistically significant) (P < 0.004). In a study by Jψrgensen et al.,[13] it was observed that there was increased incidence of osteopenia and osteoporosis with advancing COPD stage. Only GOLD stage III and IV patients were included, and patients with already known osteoporosis were excluded. They observed that, 68% had either low bone mass (osteopenia or osteoporosis) or a previously undiagnosed vertebral fracture, with 25% of the included patients having a vertebral fracture. Consistent with the above studies, another study by de Vries, et al.,[14] observed that the risk of osteoporotic fracture increased in patients with COPD (crude odds ratio (OR) 1.61; 95% confidence interval (CI) 1.52-1.71). It was also observed that patients with more severe airway obstruction in COPD had increased risks of osteoporosis and bone fractures as compared with patients without the history of obstructive airway disease. Graat-Verboom et al.,[15] studied the osteoporosis with whole body and local DEXA scan in evaluation of osteoporosis in COPD patients. They observed that as severity of COPD increased, the prevalence of osteoporosis also increased. In another study by Vrieze et al.,[14] had similar findings of higher prevalence of osteoporosis in stage III and stage IV COPD disease as compared to stage I and stage II COPD.
In contrast to the above mentioned studies, Karadag et al.,[16] compared BMDs of 28, clinically stable male COPD patients and 20 male volunteers with normal pulmonary function, as a control group. There was no statistically significant difference between the BMD values of the COPD and control groups. The TORCH study[11] demonstrated a higher prevalence of osteoporosis and osteopenia at baseline, in those patients with spirometrically confirmed COPD, but there was no association between FEV1 impairment and BMD when adjusted by age and gender. The lack of association between the severity of COPD and osteoporosis in this study could be due to less number of patients with mild stages of COPD. Majority of patients who seek consultation for COPD are having advanced disease in India. Additionally, methodological differences in other studies may account for the differences in results, with epidemiological studies using data obtained from population based survey and relying on clinical information and not on spirometry to define COPD severity. Thus, adjustments for confounding factors are usually not performed in the epidemiological database studies. These studies also conclude that the risk of osteoporosis is not increased in appropriately treated patients with moderate degree COPD[17] .
A correlation of BMI to the development of osteoporosis was also done in the study. It was observed that patients with lower BMI had higher prevalence of osteoporosis (37.3%) as compared to overweight patients. But this association was not statistically significant (P < 0.64). A study by Bisboking et al.,[18] observed that bone mass was directly correlated with BMI. Both men and women with high BMIs have higher BMD. This is thought to be partially due to the effect of the greater weight-bearing load on the bones. In addition, estrogen levels tend to be higher in obese people due to the increased aromatization of testosterone to estrogen in adipose tissue. The resulting higher estradiol levels may help to explain the higher BMD in obese persons, since estradiol levels in both men and women correlates with BMD. Many patients with end-stage COPD lose weight as the disease progresses due to decreased intake and increased energy requirements.[19] Furthermore, low bone mass was correlated with low fat free mass (FFM) in stage IV patients, and FFM could thus be used as a determinant of bone loss in this population. These findings were supported by a case-control study,[20] in which patients with COPD were found to have lower bone mass than controls, and decreasing BMD was found with increasing GOLD stage. Iqbal et al.,[21] reported that the lowest BMD was seen in a group of patients with BMI below the normal median and reported an independent correlation between BMI and BMD (r = 0.34; P < 0.05). Another recent study[22] of osteoporosis in COPD found that BMI was the strongest predictor of osteoporosis, with a BMI ≤ 22 having an odds ratio of 4.18 (95% CI: 1.19-14.71, P < 0.026).
Smoking has been shown to be an independent risk factor for osteoporosis in both men and women.[23] Slimed et al.,[24] reported that lumbar spine BMD was 12% lower in smokers who have smoked 20 pack years compared to nonsmokers. Several studies[24,25,26,27] have confirmed the finding of a significantly greater rate of bone loss in smokers. Both vertebral fractures and hip fractures are increased in smokers.[28] The pathophysiologic mechanism for the lower bone mass and increased fracture risk in smokers is unclear.[29] One study[25] has shown evidence of decreased calcium absorption in the gastrointestinal (GI) tract in smokers compared to nonsmokers. In the present study, all female patients were nonsmokers. Since female patients are already at high risk of osteoporosis the number of patients with osteoporosis in nonsmokers was higher and statistically also smoking showed no association with COPD (P < 0.11). But when compared among smoking group (male population), prevalence of osteoporosis was more in those with >10 pack years. Prevalence of osteoporosis in patients with smoking >10 pack years was 19.6% and while it was 10.8% in those with smoking of ≤10 pack years.
Other factor which has significant contribution to the development of osteoporosis in COPD is use of parenteral corticosteroids. The above findings can be explained clearly by higher intake of systemic steroids in the group with higher rate of exacerbations. The dose of steroids during exacerbation of COPD is much higher which may contribute in the long run to the higher incidence of reduced BMD. It is also likely that the group having more number of exacerbations is affected by prolonged immobilization. The rising prevalence of glucocorticoid-induced bone loss is so common that the National Osteoporosis Foundation[29] formulated the guidelines regarding these patients and recommended that all patients receiving chronic GC treatment (>1 month) with 7.5 mg/day of prednisone or equivalent should undergo screening for osteoporosis. In the present study, most of the patients with COPD were prescribed steroids during exacerbations and use of steroids may increase with number of exacerbations and hospitalizations. All the patients were categorized as those not on any steroids, those who used only inhaled steroids, those who used <1,000 mg of steroids (cumulative dose; equivalent of prednisolone) and those who used >1,000 mg (cumulative dose; equivalent of prednisolone). Osteoporosis was observed to be high in those using >1,000 mg (cumulative dose, prednisolone) and the association was statistically significant (P < 0.0001). COPD patients on high-dose glucocorticoid therapy exhibit a rapid loss of BMD within the first 6 months.[30] Normal bone metabolism is a result of the equilibrium between bone formation by osteoblasts and bone resorption by osteoclasts. The mechanism of bone loss induced by glucocorticoids is two-fold, with decreased bone formation and increased bone resorption.[31] Similar findings have been observed by Bhattacharya et al., in Indian patients.[12] COPD related risk facts for the development of osteoporosis and its functional consequences are summarized in Figure 1.
Figure 1.
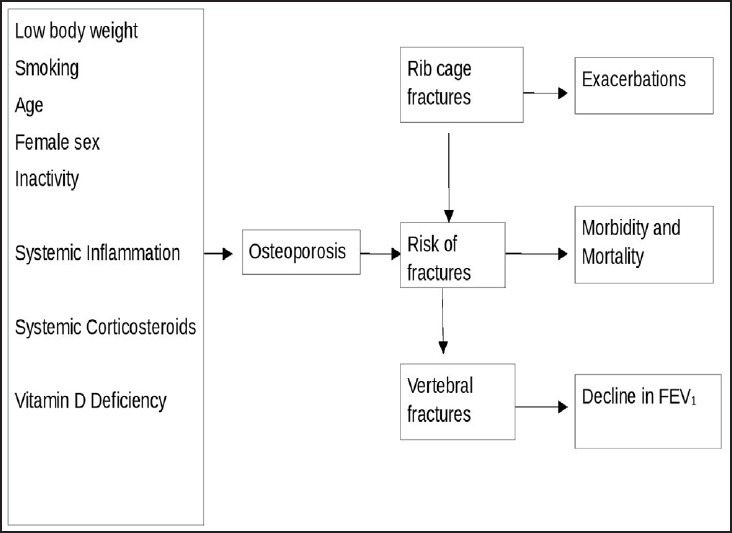
Chronic obstructive pulmonary disease (COPD) related factors for osteoporosis and its functional consequences
It was also observed in the present study that risk of developing osteoporosis in COPD patients using inhaled corticosteroids was almost same as those not using inhaled corticosteroids (3.9 versus 6.8% respectively). Similar results were observed in a study by de Vries et al.[14] They studied patients with more severe COPD and found that they had higher risks of fracture, and these risks were comparable between users and nonusers of inhaled corticosteroids. The adjusted OR for osteoporotic fracture was 1.47 (95% CI: 1.25–1.74) in nonusers and 1.48 (95% CI: 1.29–1.71) in users of inhaled corticosteroids with more severe COPD. TORCH study[11] investigated the long-term effects of therapy with inhaled corticosteroids fluticasone propionate (FP) alone, salmeterol (SAL) alone, and a SAL/FP combination (SFC) on BMD and bone fractures in patients with moderate-to-severe COPD. No significant differences were observed between treatment arms (adjusted mean percent change from baseline at hip was 3.1% for placebo, 1.7% for SAL, 2.9% for FP, and 3.2% for SFC therapy, respectively; while, the corresponding changes for the lumbar spine were 0, 1.5, 0.3, and 0.3% for placebo, respectively, SAL, FP, and SFC therapy). The incidence of fractures was low and was similar for all treatments (5.1–% to 6.3%). Thus even in the TORCH study,[11] no significant effect on BMD was detected for inhaled steroids therapy compared with placebo. Thus, it was concluded that inhaled corticosteroid (ICS) use does not cause thinning of the bone. However, it is more appropriate to think about the complexity of the situation. The administration of systemic corticosteroids (SCSs) at a larger than minimum dose, and particularly if they are administered over a long period of time, is generally thought to cause osteopenia and osteoporosis. Whether ICS therapy produces adequate blood levels that can adversely affect BMD is not certain, but even a small effect over an extended period of time may produce serious side effects.[32] Finally, in COPD the lungs are a source of proinflammatory molecules and a contributor to systemic inflammation. If ICS therapy can reduce the lung source of these molecules, it may actually be beneficial to the bones.[33,34]
In conclusion, it is now established that osteoporosis in COPD is multifactorial. Many risk factors have been involved in the development of osteoporosis in COPD. The clinical significance of these individual risk factors varies in different studies. It is evident from the present study that the patients who have moderate-to-severe COPD have advanced nature of the disease which predisposes them to osteoporosis, by virtue of being elderly or chronically disabled, and having chronic systemic inflammation. COPD is now increasingly being recognized as an inflammatory condition of the lung; and over the past decade, it has been recognized for its systemic inflammation and having extrapulmonary manifestations. Patients with COPD are often treated with oral or parenteral glucocorticoids, during exacerbations. Such oral or parenteral glucocorticoid therapy along with various other risk factors clearly increases the risk for the development of osteoporosis. Hence in an ideal set up, all patients with COPD should be screened for osteoporosis using BMD measurements made by DEXA, which is considered the GOLD standard method for the early diagnosis and proper therapy of this condition can be advised at the earliest. This will help in improving the quality of life in these patients and thus improving the morbidity of this condition.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Murray CJ, Lopez AD. Global mortality, disability and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–42. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 2.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 3.Murthy KJ, Sastry JG. Burden of Diseases In India. Published by National Commission on Macroeconomic and Health. New Delhi: Ministry of Health and Family Welfare; 2005. Economic burden of COPD; pp. 264–74. [Google Scholar]
- 4.Fabbri LM, Luppi F, Beghé B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31:204–12. doi: 10.1183/09031936.00114307. [DOI] [PubMed] [Google Scholar]
- 5.Gregg EW, Cauley JA, Seeley DG, Ensred KE, Bauer DC. Physical activity and osteoporotic fracture risk in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1998;129:81–8. doi: 10.7326/0003-4819-129-2-199807150-00002. [DOI] [PubMed] [Google Scholar]
- 6.Graat-Verboom L, Wouters EF, Smeenk FW, van den Borne BE, Lunde R, Spruit MA. Current status of research on osteoporosis in COPD: A systematic review. Eur Respir J. 2009;34:209–18. doi: 10.1183/09031936.50130408. [DOI] [PubMed] [Google Scholar]
- 7.Ionescu AA, Schoon E. Osteoporosis in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;46:64S–75S. doi: 10.1183/09031936.03.00004609. [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA. World Health Organization Collaborating Centre for Metabolic Bone Diseases. UK: University of Sheffield; 2008. On behalf of the World Health Organization Scientific Group. Assessment of osteoporosis at the primary health-care level: Technical report. [Google Scholar]
- 9.Ohara T, Hirai T, Muro S, Haruna A, Terada K, Kinos D, et al. Relationship between pulmonary emphysema and osteoporosis assessed by CT in patients with COPD. Chest. 2008;134:1244–9. doi: 10.1378/chest.07-3054. [DOI] [PubMed] [Google Scholar]
- 10.Graat-Verboom L, Spruit MA, van den Borne BE, Smeenk FW, Martens EJ, Lunde R, et al. Correlates of osteoporosis in chronic obstructive pulmonary disease: An underestimated systemic component. Respir Med. 2009;103:1143–51. doi: 10.1016/j.rmed.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson GT, Calverley PM, Anderson JA, Jenkins CR, Jones PW, Willits LR, et al. Prevalence and progression of osteoporosis in patients with COPD: Results from the towards a revolution in COPD Health Study. Chest. 2009;136:1456–65. doi: 10.1378/chest.08-3016. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya P, Paul R, Ghosh M, Dey R, Barooah N, et al. Prevalence of osteoporosis and osteopenia in dvanced chronic obstructive pulmonary disease patients. Lung India. 2011;28:184–6. doi: 10.4103/0970-2113.83974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jørgensen NR, Schwarz P, Holme I, Henriksen BM, Petersen LJ, Backer V. The prevalence of osteoporosis in patients with chronic obstructive pulmonary disease: A cross sectional study. Respir Med. 2007;101:177–85. doi: 10.1016/j.rmed.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 14.de Vries F, van Staa TP, Bracke MS, Cooper C, Leufkens HG, Lammers JW. Severity of obstructive airway disease and risk of osteoporotic fracture. Eur Respir J. 2005;25:879–84. doi: 10.1183/09031936.05.00058204. [DOI] [PubMed] [Google Scholar]
- 15.Graat-Verboom L, Spruit MA, van den Borne BE, Smeenk FW, Wouters EF. Whole-Body versus local DEXA-Scan for the Diagnosis of Osteoporosis in COPD Patients. J Osteoporos. 2010;10:640878. doi: 10.4061/2010/640878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karadag F, Orhan C, Yakup Y, Ozgur G. Should COPD patients be routinely evaluated for bone mineral density? J Bone Miner Metab. 2003;21:242–246. doi: 10.1007/s00774-002-0416-0. [DOI] [PubMed] [Google Scholar]
- 17.Daniell HW. Osteoporosis of the slender smoker. Vertebral compression fractures and loss of metacarpal cortex in relation to postmenopausal cigarette smoking and lack of obesity. Arch Intern Med. 1976;136:298–304. doi: 10.1001/archinte.136.3.298. [DOI] [PubMed] [Google Scholar]
- 18.Biskobing DM. COPD and osteoporosis. Chest. 2002;121:609–20. doi: 10.1378/chest.121.2.609. [DOI] [PubMed] [Google Scholar]
- 19.Engelen MP, Schols AM, Does JD, Wouters EF. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2000;71:733–8. doi: 10.1093/ajcn/71.3.733. [DOI] [PubMed] [Google Scholar]
- 20.Marquis K, Debigare R, Lacase Y, LeBlanc P, Jobin J, Carrier G, et al. Mid thigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002:166809–13. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 21.Aloia JF, Vaswani A, Ma R, Flaster E. To what extent is bone mass determined by fat-free or fat mass? Am J Clin Nutr. 1995;6:1110–4. doi: 10.1093/ajcn/61.4.1110. [DOI] [PubMed] [Google Scholar]
- 22.Seeman E. The effects of tobacco and alcohol use on bone. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. New York: Academic Press; 1996. pp. 577–97. [Google Scholar]
- 23.Iqbal F, Michaelson J, Thaler L, Rubin J, Roman J, Nanes MS. Declining bone mass in men with chronic pulmonary disease: Contribution of glucocorticoid treatment, body mass index, and gonadal function. Chest. 1999;116:1616–24. doi: 10.1378/chest.116.6.1616. [DOI] [PubMed] [Google Scholar]
- 24.Incalzi RA, Caradonna P, Ranieri P, Basso S, Fuso L, Pagano F, et al. Correlates of osteoporosis in chronic obstructive pulmonary disease. Respir Med. 2000;94:1079–84. doi: 10.1053/rmed.2000.0916. [DOI] [PubMed] [Google Scholar]
- 25.Baldock PA, Thomas GP, Hodge JM, Baker SU, Dressel U, O’Loughlin PD. Vitamin D action and regulation of bone remodeling: Suppression of osteoclastogenesis by the mature osteoblast. J Bone Miner Res. 2006;21:1618–26. doi: 10.1359/jbmr.060714. [DOI] [PubMed] [Google Scholar]
- 26.Slemenda CW, Hui SL, Longcope C, Johnston CC., Jr Cigarette smoking, obesity, and bone mass. J Bone Miner Res. 1989;4:737–41. doi: 10.1002/jbmr.5650040513. [DOI] [PubMed] [Google Scholar]
- 27.Krall EA, Dawson-Hughes B. Smoking and bone loss among postmenopausal women. J Bone Miner Res. 1991;6:331–8. doi: 10.1002/jbmr.5650060404. [DOI] [PubMed] [Google Scholar]
- 28.Slemenda CW, Christian JC, Reed T, Reistar TK, William CJ, Johnston CC., Jr Long-term bone loss in men: Effects of genetic and environmental factors. Ann Intern Med. 1992;117:286–91. doi: 10.7326/0003-4819-117-4-286. [DOI] [PubMed] [Google Scholar]
- 29.Seeman E, Melton LJ, 3rd, O’Fallon WM, Riggs BL. Risk factors for spinal osteoporosis in men. Am J Med. 1983;75:977–83. doi: 10.1016/0002-9343(83)90878-1. [DOI] [PubMed] [Google Scholar]
- 30.Cooper C, Barker DJ, Wickham C. Physical activity, muscle strength, and calcium intake in fracture of the proximal femur in Britain. BMJ. 1988;297:1443–6. doi: 10.1136/bmj.297.6661.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston CC, Melton LJ, Lindsay R. National osteoporosis foundation. Clinical indications for bone mass measurements. J Bone Miner Res. 1989:1–28. doi: 10.1002/jbmr.5650040803. [DOI] [PubMed] [Google Scholar]
- 32.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoidinduced osteoporosis: Pathophysiology and therapy. Osteoporos Int. 2007;18:1319–28. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 33.Man SF, Sin DD. Thinning Bone and Inhaled Corticosteroid in COPD: What to do until there is definitive proof? Chest. 2009;136:1448–9. doi: 10.1378/chest.09-1787. [DOI] [PubMed] [Google Scholar]
- 34.Robbins J, Aragaki AK, Kooperberg C, Watts N, Wactawski-Wende J, Jackson RD, et al. Factors associated with 5-year risk of hip fracture in post menopausal women. JAMA. 2007;298:2389–98. doi: 10.1001/jama.298.20.2389. [DOI] [PubMed] [Google Scholar]


