Abstract
Context:
There is conflicting evidence of effect of diabetes on treatment of tuberculosis (TB). There is a need to investigate effect of diabetes on outcomes of TB treatment under field conditions in India.
Aims:
To compare treatment outcomes among TB patients with diabetes with those without diabetes.
Setting and Design:
Study was conducted in Cuddalore, Tamil Nadu, among patients registered with Revised National TB Control Programme. Prospective observational study design was used.
Materials and Methods:
Registered TB patients aged 30 and above were invited to participate in the study. Those who were not aware of their diabetic status were diagnosed using oral glucose tolerance test. A total of 89 diabetic and 120 non-diabetic patients were recruited in the study. They were followed up till the end of treatment and outcomes were recorded.
Statistical Analysis Used:
Treatment outcomes in the two groups were compared using bi-variate and multi-variate analysis.
Results:
Bi-variate (unadjusted) analysis showed similar treatment success rates in the two groups. But, the adjusted odds ratios for successful treatment among diabetic patients were significantly lower (0.191, 95% CI 0.04-0.90) for pulmonary TB patients and for smear positive pulmonary TB patients (odds ration 0.099, 0.013-0.761). Diabetes was found to be predictor for sputum positivity at end of treatment.
Conclusions:
Diabetes increases risk of poor treatment outcomes among pulmonary TB patients. The study highlights need of screening of TB patients for diabetes. There is need to see the effect of glycemic control on treatment outcomes among diabetics.
Keywords: Diabetes, treatment outcomes, tuberculosis
INTRODUCTION
Tuberculosis (TB) still continues to be a major health problem in India. Although the National TB Control Program has helped reduce burden of disease, incidence of disease is unacceptably high. Among the 8.7 million new incident cases of TB in 2009, 2.2 million are said to have occurred in India accounting for a fifth of the global disease burden.[1] Although TB is an infectious disease, previous studies did show increased susceptibility to and increased incidence of TB among those with diabetes.[2] India currently experiences an epidemic of diabetes mellitus (DM) with an estimated 40.9 million diabetics in 2006 and an estimated 70 million in 2025.[3] An epidemiological modelling study reported increased incidence of TB among those with diabetes in India.[4] Thus, rising DM is expected to fuel incidence of TB even further.
Multiple studies of TB treatment have indicated that patients with diabetes mellitus may experience poor outcomes.[5] However, some others had contradictory evidence. A study in India under controlled environment inferred that neither diabetes nor HIV co-morbidity interfere with course or outcome of TB.[6] A systematic review of these studies suggested that DM increases the risk of failure and death combined, death, and relapse among patients with TB. However, most studies in this review were retrospective studies which relied on medical records for presence of DM. We conducted a prospective study to assess the influence of diabetes on the treatment outcomes of TB under field conditions, among those taking Directly Observed Treatment Short course (DOTS) under Revised National Tuberculosis Control Programme (RNTCP) in Cuddalore district of Tamil Nadu (TN).
MATERIALS AND METHODS
A prospective observational study design was used with two sub-groups of TB patients, one with diabetes and other without DM. Since DM has a long latent period and may remain undiagnosed in the absence of screening, an oral glucose tolerance test (OGTT) was chosen for diagnosing DM among patients on treatment of TB. TB patients registered in RNTCP were considered for recruitment in the study.
For estimating sample size for comparison of treatment outcomes among TB patients with and without co-morbid diabetic status, we used the following formula,
n = {z1-α√ [2P (1-P)] + z1-β√ [P1 (1-P1) +P2 (1-P2)]}2 /(P1 -P2)2
where, P = (P1 -P2)/2
The treatment success rate among the non-diabetic TB patients (P1) was assumed to be 90% (based on RNTCP accomplishments over the years). And the treatment success rate among the diabetic TB patients (P2) was assumed to be 75%. With confidence level (α) of 95% and the power (1 - β) of 90%, the sample size needed to find difference between the two groups was estimated to be 109 in each group.[7]
Cuddalore district in TN was chosen because the district had consistently achieved global targets of cure rates for TB patients. Two TB units, namely Virudhachalam and Orathur, were randomly chosen. Inclusion criteria included TB cases aged 30 years and above and registered between 1st November 2011 and 31st May 2012. Exclusion criteria included known HIV-positive status as well as those with impaired glucose test or impaired fasting glucose on OGTT. The study participants were recruited in the study while they were on treatment for TB. Some of them were in intensive phase and some in continuation phase of treatment when they underwent OGTT. OGTT results were interpreted based on WHO guidelines. Patients belonging only to the normal and diabetic category were included in the study and were assigned to the study and comparison group, respectively. TB patients who were diagnosed with diabetes before initiating the study were not subjected to OGTT for ethical considerations but were included in the study in the diabetics group.
A written informed consent was taken from participants prior to subjecting them to OGTT. The interview schedule was administered during time between fasting and post glucose test. Information about the demographics and category of the patient were noted from the TB register or the treatment card. Information regarding tobacco and alcohol use and history and details of medication in known diabetics were recorded in the interview schedule. All diabetic patients were prescribed hypoglycemic agents including insulin, were counseled for adhering to treatment and were followed monthly for monitoring of diabetic control. Data on follow-up sputum examinations during and at end of treatment and on completion of full course of anti-TB drugs was collected during the follow up period. All the study participants were followed up till declaration of their treatment outcomes. The criteria used in RNTCP were employed. Treatment success was defined as either ‘cure’ or ‘completed treatment’. Sputum smear-positive patients were declared cured only if they had negative sputum results at the end of full course of anti-tubercular treatment. The patients were declared to have completed treatment if they had completed full course of anti-tubercular drugs and had no symptoms at the end of treatment. SPSS version 20 was used for bi-variate and multi-variate analysis of data and appropriate tests of significance were employed. For subgroup analysis, patients were classified into pulmonary and extra-pulmonary. Pulmonary patients were classified further as sputum positive or negative and new or re-treatment patients. We did not specifically collect data on type of pulmonary lesions and analysis for specific pulmonary lesions.
RESULTS
Patient characteristics: Review of records from TB registers of the two TB units identified a total of 584 patients aged 30 years and above registered from 1st November 2011 to 31st May 2012. A total of 233 TB patients consented to participate in the study. Fifty of these 233 patients were known diabetics and their diabetes status was confirmed with review of medical records. Remaining 183 participants were screened for DM with OGTT test. Of these 183 patients, 120 had normal glucose levels, 24 showed impaired glucose tolerance and 39 were found to be diabetic. The patients with impaired glucose tolerance were excluded from study. Fifty patients who were known diabetics and 39 patients diagnosed by OGTT were included into the TB with the DM group (n = 89). The comparison group included 120 who had normal glucose levels on OGTT. Table 1 provides details of patient characteristics. Out of the total 209 patients in the study, three-fourths were males (76.1%) and one-fourth were females (23.9%) whereas 31.1% were illiterate. Majority (55%) were daily wage labourers and most (76.6%) had monthly income less than Rs. 5000/- per month. 44.5% (n = 93) were smokers and 55.5% (n = 116) were consuming alcohol among the study group and considerable overlap was seen. The diabetic and non-diabetic groups were not statistically different with respect to smoking or alcohol use. The average weight at treatment initiation was 51.09 kg for a diabetic and 45.01 kg for a non-diabetic. Among the known cases of diabetes (n = 50), two (4%) were not taking any treatment and one (2%) was diagnosed as a diabetic just 1 week prior to the interview. The rest (n = 47) were taking allopathic treatment from a registered allopathic practitioner either from the government sector or from private practitioners. Nearly one-third (32%) of these 50 known diabetics had missed at least one dose during 2 weeks prior to the interview. There was no evidence of diabetic nephropathy or renal failure in these patients.
Table 1.
Characteristics of tuberculosis patients who had diabetes mellitus and of TB patients who did not have DM
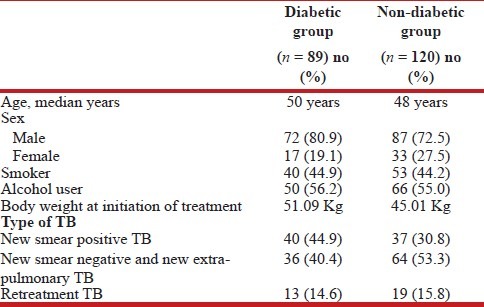
It is seen from Table 1 that among new TB patients, more diabetics had sputum smears positive before initiation of treatment compared to the non-diabetics. Combining the new and retreatment cases, pre-treatment sputum positivity among diabetics (59.6%) was higher (but not significantly higher with P = 0.5) than among non-diabetics (45.8%).
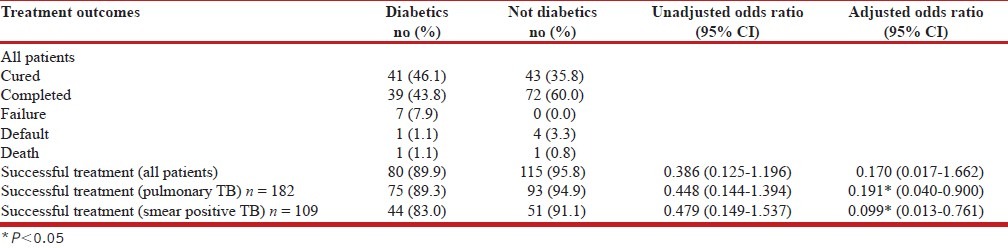
Diabetes and TB treatment Outcomes: Initial response to TB treatment was good. Out of 109 smear positive TB patients, 106 converted to smear negative status by end of intensive phase (IP). Sputum smears for the remaining three patients (all new smear positive patients) were positive at end of IP; of these three, two were diabetics and one was non-diabetic. It is seen from Table 2 that the success rate of TB treatment was 89.9% (n = 80) among those with diabetes and 95.8% (n = 115) among those without diabetes. The success rate was not associated with diabetes in univariate analysis or after adjustment with age, sex, smoking, alcohol use and adherence to treatment. Treatment success rates among pulmonary patients among non-diabetics were higher although not significantly higher in univariate analysis. After adjusting for age, sex, smoking, alcohol use and adherence to treatment and sputum smear result at end of IP, diabetics had less likelihood of successful treatment. Limiting analysis to sputum smear positive pulmonary TB patients also showed that diabetics had less likelihood to be successful compared with non-diabetics. However, the success rates in the two groups were very similar for new smear positive (NSP) patients, 90% for diabetics and 91.9% for non-diabetics. No relation was seen between the presence of diabetes and successful treatment among NSP in univariate or multivariate analysis. All sputum smear negative and extra-pulmonary TB patients successfully completed their treatment. Only one among the extra-pulmonary patients was a diabetic. The treatment outcomes of 14 patients were not successful. Among them nine (64.3%) were diabetics and five were non-diabetics. The adverse treatment outcomes included failure (7), default (5) and death (2) as a whole. Among the five patients who defaulted, four were new sputum positives and one was retreatment with history of default previously. Only one among the five defaulters was a diabetic. Overall, the adherence to treatment was found to be better in the diabetic group. Nearly 75% of non-diabetics were not regular in treatment missing at least three doses during treatment, thereby extending treatment duration by at least 1 week; this proportion was less for diabetics (57%). Mean delay in completing treatment was significantly smaller for diabetics (13.2 days) compared to non-diabetics (18.5 days). Two patients, one male and one female died during the course of the study.
Table 2.
Treatment outcomes of tuberculosis among patients with and without diabetes
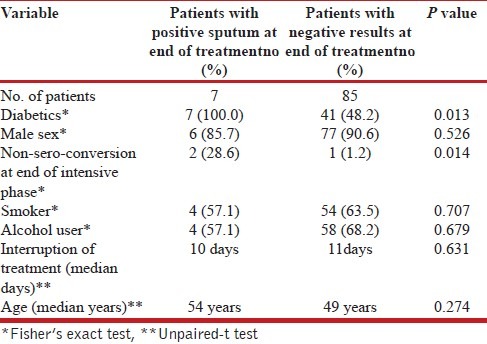
Determinants of failure: A total of seven patients continued to remain sputum positive in spite of taking full course of anti-tubercular drugs. At the beginning of therapy, they were registered as relapse (three cases), failure (two cases) and new smear positive (two cases). All the seven were cases of diabetes. Of the seven patients, six were lost to follow up and samples could not be sent for drug sensitivity testing. One patient could be followed up was diagnosed as a case of multiple-drug resistant TB and was initiated on category IV regimen under RNTCP. Table 3 shows univariate association between independent variables and sputum result at the end of treatment. Diabetes and positive sputum at the end of intensive phase were factors found to be associated with positive sputum at the end of treatment.
Table 3.
Predictors of sputum positivity at end of treatment
DISCUSSION
In the present study, only three new smear-positive patients failed to convert at the end of intensive phase. The smear conversion rate was lower among diabetics but data was not sufficient to draw meaningful conclusions. A previous Indian study[6] showed no difference in both smear and culture conversion results at the end of 2 months among new pulmonary TB patients. However, many studies in rest of the world showed that smear and culture conversion rates were poor among diabetics.[5] Two such studies did not find difference at the end of intensive phase but reported some delay in conversion in the diabetic group.[8,9] The previous Indian study did not report differences in smear or culture conversion at the end of treatment. In present study, at the end of treatment, seven patients had sputum-positive results, five of whom had negative sputum at the end of intensive phase. Does this mean that the response to treatment is better in intensive but not in continuation phase? A study done in Indonesia[10] to understand the influence of diabetes on the pharmacokinetics of anti-tubercular drugs reported no differences in the pharmacokinetics of rifampin, pyrazinamide and ethambutol in the intensive phase of TB treatment between diabetics and non-diabetics. The study found that DM reduces the exposure to rifampin in the continuous phase and was strongly associated with a positive sputum culture after the continuation phase, but not after the intensive phase of TB treatment. The differences in rifampin induction during the intensive and continuation phase of treatment might be the result of the differential effect of diabetes mellitus.
In this study, the treatment success rates were similar in the diabetic and non-diabetic groups. However, after adjusting for some confounders we found that diabetes is associated with poor outcomes among pulmonary TB patients. A systematic review on this issue also found that patients with DM receiving TB therapy are at risk for poor outcomes, but not controlling for confounders may underestimate the negative impact of DM in TB patients.[5] Better adherence to treatment among diabetics, as observed in the study was one such confounder.
The previous Indian study by Tuberculosis Research Institute found no difference in conversion at end of intensive phase and recommended that treatment regimen may not be different for TB-diabetes co-morbidity.[6] However, the study was conducted in experimental settings where diabetics were provided treatment and were monitored as well. In real life situation, many diabetics remain undiagnosed and not all of those who are diagnosed will be adherent to treatment. In the present study, diabetes was diagnosed while the patients were on treatment and the newly diagnosed diabetics were offered treatment for diabetes. However, they were not on hypoglycemic agents throughout the TB treatment period. Even those who were known diabetics, at least one-third mentioned about missing at least one dose during previous fortnight. Thus, the study participants included diabetics with varying levels of glycemic control. We did not measure glycemic control among the participants during or at the end of the study. Response to TB treatment may vary among diabetics depending upon glycemic control and may be a reason why we found poor outcomes among diabetics after adjustment for confounders. To what extent does the poor glycemic control affects TB treatment needs to be studied further.
This study in Tamil Nadu showed that out of 233 TB patients who consented to participate in the study, 89 (38.2%) were diabetic. However, about 60% of eligible TB patients did not participate in the study. It is likely that TB patients who felt that they are at risk of diabetes came forward for OGTT. Thus, possibility of participation bias cannot be ruled out. However, even after assuming that all of the TB patients who did not participate were non-diabetics, the prevalence of DM among TB patients would still be 15.2% (89 out of 584); much higher than 10.4% among general population in TN. This indicates that the prevalence of DM is much higher among TB patients. A recent study found prevalence of DM among TB patients to be 20% in South India[11] and recommended screening of TB patients for presence of DM. We also recommend that screening for DM among TB patients aged 30 and above, will be a good strategy, especially for state of TN as it will help detect more diabetic patients who could be provided necessary counselling and treatment. There is some evidence of transient hyperglycemia during TB[12] which cannot be ruled out in this study as diagnosis was during treatment and based on OGTT alone, glycosylated haemoglobin test was not employed for logistic reasons However, in the present study, less than half of diabetics were diagnosed during TB treatment indicating that high prevalence found in this study and in study by India TB-diabetes study group cannot be explained by transient hyperglycaemia.
The study had certain limitations. First, success of treatment was based upon sputum smear results and smear cultures were not obtained. Secondly, none of these patients were tested for the presence of drug resistance before beginning of the study and only one patient could be tested at the end of treatment. More than a third were diagnosed during course of TB treatment and were not on hypoglycemic agents throughout TB treatment and glycemic control was not measured among these patients. Serum levels of rifampicin or other drugs were not measured. Although we attempted adjusting for some confounders, we could not adjust for some like severity of chest radiograph findings, glycemic control and susceptibility to drugs.
To summarize, we found poor treatment outcomes among diabetic TB patients compared those without DM. There is need to review policy of similar treatment regimen for DM-TB co-morbid patients. Lack of sputum culture at the end of treatment and other limitations discussed earlier affects the strength of evidence in this study and similar studies are needed to provide evidence for the policy decision. Prevalence of DM is high among TB patients and there is need to screen adult TB patients for presence of DM, which is expected to result in better management of co-morbidity. This will also make more data available on treatment outcomes among DM-TB co-morbid patients that will help appropriate policy decision.
ACKNOWLEDGEMENT
We are thankful to Shri. Pankaj Kumar Bansal I.A.S., Project Director, Tamil Nadu Health System Project, Dr. Raja, Director of Medical Services (DMS), Tamil Nadu., Dr. Porkai Pandian, Director of Public Health and Preventive Medicine (DPH&PM), Tamil Nadu and Dr. Arunagiri, State Tuberculosis Officer (STO), Tamil Nadu who provided administrative support to the study. We also thank Dr. Arul Anand who provided both administrative support and gave valuable suggestions for the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Global Tuberculosis Report 2012. [Last accessed on 2013 July 18]. Available from: http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf .
- 2.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 4.Stevenson CR, Forouhi NG, Roglic G, Williams BG, Lauer JA, Dye C, et al. Diabetes and tuberculosis: The impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banu Rekha VV, Balasubramanian R, Swaminathan S, Ramachandran R, Rahman F, Sundaram V, et al. Sputum conversion at the end of intensive phase of Category-1 regimen in the treatment of pulmonary tuberculosis patients with diabetes mellitus or HIV infection: An analysis of risk factors. Indian J Med Res. 2007;126:452–8. [PubMed] [Google Scholar]
- 7.Lwanga SK, Lemeshow S. Sample size determination in health studies: A practical manual. Geneva: World Health Organization; 1991. [Google Scholar]
- 8.Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, Ottenhoff TH, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis. 2007;45:428–35. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 9.Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg. 2009;80:634–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Ruslami R, Nijland HM, Adhiarta IG, Kariadi SH, Alisjahbana B, Aarnoutse RE, et al. Pharmacokinetics of antituberculosis drugs in pulmonary tuberculosis patients with type 2 diabetes. Antimicrob Agents Chemother. 2010;54:1068–74. doi: 10.1128/AAC.00447-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.India Tuberculosis-Diabetes Study Group. Screening of patients with tuberculosis for diabetes mellitus in India. Trop Med Int Health. 2013;18:636–45. doi: 10.1111/tmi.12084. [DOI] [PubMed] [Google Scholar]
- 12.Oluboyo PO, Erasmus RT. The significance of glucose intolerance in pulmonary tuberculosis. Tubercle. 1990;71:135–8. doi: 10.1016/0041-3879(90)90010-6. [DOI] [PubMed] [Google Scholar]


