Abstract
To understand the contribution of animal- and human-derived fecal pollution sources in shaping integron prevalence and diversity in beach waters, 414 Escherichia coli strains were collected from beach waters (BW, n = 166), seagull feces (SF, n = 179), and wastewaters (WW, n = 69), on the World Biosphere Reserve of the Berlenga Island, Portugal. Statistical differences were found between the prevalence of integrons in BW (21%) and WW (10%), but not between BW and SF (19%). The majority of integrase-positive (intI+)-strains affiliated to commensal phylogroups B1 (37%), A0 (24%), and A1 (20%). Eighteen different gene cassette arrays were detected, most of them coding for resistances to aminoglycosides, trimethoprim, chloramphenicol, and quaternary ammonia compounds. Common arrays were found among strains from different sources. Multi-resistance to three or more different classes of antibiotics was observed in 89, 82, and 57% of intI+-strains from BW, SF and WW, respectively. Plasmids were detected in 79% of strains (60/76) revealing a high diversity of replicons in all sources, mostly belonging to IncF (Frep, FIA, and FIB subgroups), IncI1, IncN, IncY, and IncK incompatibility groups. In 20% (15/76) of strains, integrons were successfully mobilized through conjugation to E. coli CV601. Results obtained support the existence of a diverse integron pool in the E. coli strains from this coastal environment, associated with different resistance traits and plasmid incompatibility groups, mainly shaped by animal fecal pollution inputs. These findings underscore the role of wild life in dissemination of integrons and antibiotic resistance traits in natural environments.
Keywords: environmental reservoirs, microbial risk assessment, multi-resistance, integron diversity, replicon typing, Enterobacteriaceae
Introduction
Environmental antibiotic resistance reservoirs are known to represent the origins of the resistance determinants that nowadays constitute major clinical threats (Davies and Davies, 2010; Tacão et al., 2012, 2013; Perry and Wright, 2013). In the recent years much attention has been given to marine environments and migratory birds with increasing evidence of their role in the dissemination of antibiotic resistant Enterobacteriaceae, particularly Escherichia coli (Dolejska et al., 2007, 2009; Poeta et al., 2008; Radhouani et al., 2009; Poirel et al., 2012; Hernandez et al., 2013; Kmet et al., 2013; Santos et al., 2013; Veldman et al., 2013). E. coli is the predominant facultative anaerobe in gastrointestinal tract of humans and animals (Tenaillon et al., 2010). Although most E. coli are commensal, some can be pathogenic and may be transmitted through contaminated water or food, or through contact with animals and people. Pathogenic E. coli has been reported as a major cause of mortality as a result of infant diarrhea, extra-intestinal and urinary tract infections, thus constituting an important hospital- and community-acquired pathogen (Guentzel, 1996; Touchon et al., 2009). Due to their genetic flexibility and adaptability to diverse stress conditions, both commensal and pathogenic E. coli strains have the ability to persist in terrestrial and aquatic habitats (Van Elsas et al., 2011).
Integrons are bacterial site-specific recombination platforms of acquisition and expression of mobile genes, called gene cassettes (Stokes and Hall, 1989). It has been shown that the persistence of antibiotics in the environment at sub-therapeutic concentrations contributes to the acquisition of antibiotic resistance genes between different strains, mediated by integrons, as a result of the activation of bacterial SOS responses (Baharoglu et al., 2010; Andersson and Hughes, 2011). In addition, integrons are often associated with conjugative plasmids which contribute to their mobilization and wide dissemination (Moura et al., 2012a). The spread of such determinants can constitute serious environmental risks, compromising both ecosystem and human health.
In this study, we aimed to understand the involvement of animal- and human-derived fecal pollution sources in shaping integron prevalence and diversity in beach waters. Sampling was performed in the World Biosphere Reserve of Berlenga Island, located in the Atlantic Ocean, because here the sources of fecal pollution are limited and well-identified, consisting of both animal- and human-derived origins. The Berlenga Island constitutes an important nesting area of sea birds, in particular the yellow-legged gulls (Larus [cachinnans] michahellis), which are, by far, the dominant local fauna and a major source of fecal pollution in the island (Araújo et al., 2014). This island is only circumstantially inhabited by tourists in the summer season, and human-derived wastewaters are discharged near the coastline of the island without prior treatment (Araújo et al., 2014).
To address our aims, we examined the prevalence and diversity of integrons in E. coli strains collected from beach waters, as well as from seagull feces and raw wastewaters in the Berlenga Island. The association of integrons and plasmids was also assessed in order to determine the extent of the environmental risk at play.
Materials and methods
Sampling, E. coli isolation and molecular typing
In a previous study, a collection of 939 E. coli isolates was obtained from samples collected between May and September 2011 at the Berlenga Island (Latitude: 39° 24′ 52″ N; Longitude: 9° 30′ 22″ W), located 5.7 miles northwest of Cape Carvoeiro, Portugal. Samples consisted of: (i) beach waters; (ii) composite seagull (Larus [cachinnans] michahellis) fresh fecal samples; and (iii) human-derived raw wastewaters (Araújo et al., 2014). Isolates were selected in Chromocult Coliform Agar, confirmed by plating in MacConkey and mFC agar and 16S rRNA gene sequencing, as previously described (Araújo et al., 2014). Molecular typing was performed by BOX-PCR (Araújo et al., 2014), resulting in a total of 414 different E. coli strains that were used in this study.
Integron screening detection and characterization
E. coli strains were screened by PCR for the presence of class 1 and class 2 integrase genes (intI1 and intI2, respectively), as previously described (Moura et al., 2012b). Integrase-positive (intI+)-strains were further characterized. Class 1 and class 2 integron variable regions were amplified using primers targeting flanking regions of gene cassette arrays (class 1: intI1 or attI1 at 5′ region and tniC, qacE/sul1, or sul3 at 3′ region; class 2: intI2 or attI2 at 5′ region and ybeA at 3′ region), using the Extensor Long Range PCR Master Mix (Thermo Scientific, USA), as described before (Moura et al., 2012b). Specific primers for gene cassettes were also used in primer walking. All primer sequences are listed in Table 1. Sequences obtained were subjected to BLAST (Altschul et al., 1997) searches against the INTEGRALL database (http://integrall.bio.ua.pt; Moura et al., 2009). Insertion sequences were compared against ISFinder database (http://www-is.biotoul.fr; Siguier et al., 2006) to confirm identity. Gene cassette promoters were annotated according to Jové et al. (2010).
Table 1.
Primers used in this study in the characterization of integrons.
| Primer namea | Target | Sequence (5′–3′) | References |
|---|---|---|---|
| INTEGRASE GENES | |||
| intI1F | intI1 | CCTCCCGCACGATGATC | Kraft et al., 1986 |
| intI1_894F(ER.1.6F) | intI1 | CCCAGTGGACATAAGCCTG | Moura et al., 2012b |
| intI1R | intI1 | TCCACGCATCGTCAGGC | Kraft et al., 1986 |
| intI2F | intI2 | TTATTGCTGGGATTAGGC | Goldstein et al., 2001 |
| intI2R | intI2 | ACGGCTACCCTCTGTTATC | Goldstein et al., 2001 |
| FLANKING REGIONS | |||
| 5′-CS | attI1 | GGCATCCAAGCAGCAAG | Levesque et al., 1995 |
| 3′-CS | 3′ conserved segment | AAGCAGACTTGACCTGA | Levesque et al., 1995 |
| qacE-F | qacE/qacEdelta1 | ATCGCAATAGTTGGCGAAGT | Sandvang et al., 1997 |
| qacE-R | qacE/qacEdelta1 | CAAGCTTTTGCCCATGAAGC | Sandvang et al., 1997 |
| sul1F | sul1 | CTGAACGATATCCAAGGATTYCC | Heuer and Smalla, 2007 |
| sul1R | sul1 | AAAAATCCCATCCCCGGRTC | Heuer and Smalla, 2007 |
| sul3F | sul3 | AAGAAGCCCATACCCGGRTC | Heuer and Smalla, 2007 |
| sul3R | sul3 | ATTAATGATATTCAAGGTTTYCC | Heuer and Smalla, 2007 |
| RH506 | tniC | TTCAGCCGCATAAATGGAG | Post et al., 2007 |
| orf513_6F | ISCR1 | ATGGTTTCATGCGGGTT | Arduino et al., 2003 |
| orf513_7R | ISCR1 | CTGAGGGTGTGAGCGAG | Arduino et al., 2003 |
| qnrS_rev2 | qnrS | CAAATTGGCGCGTAGAGCGCC | This study |
| hep74 | attI2 | CGGGATCCCGGACGGCATGCACGATTTGTA | White et al., 2001 |
| hep51 | ybeA | GATGCCATCGCAAGTACGAG | White et al., 2001 |
| GENE CASSETTE PRIMER WALKING | |||
| aadA1_F | aadA1 | TATCAGAGGTAGTTGGCGTCAT | Randall et al., 2004 |
| aadA1_R | aadA1 | AATGAAACCTTAACGCTATGGAAC | Randall et al., 2004 |
| aacA4F (ER.1.17F) | aacA4 | CGAGCGAACACGCAGTG | Moura et al., 2012b |
| dfrA12_F | dfrA12 | CCCACTCCGTTTATGCGCG | This study |
| dfrA17_F | dfrA17 | CACGTTGAAGTCGAAGGTGA | This study |
| estXF (MM.2.11F) | sat/estx | GGCCGAGGATTATCCA | Moura et al., 2007 |
| cmlA_F | cmlA | GGACATGTACTTGCCAGCA | This study |
| cmlA_R | cmlA | GGGATTTGAYGTACTTTCCGC | This study |
| qacH_F | qacH | GAGGTCRTCGCAACTTCC | This study |
| qacH_R | qacH | GCGCTGACCTTGGATAGC | This study |
| linF_F | linF | CGCTTGAGGCGGCTGTTTTG | This study |
| psp_F | psp | CCGGATTTTGTGCGGCGGTC | This study |
| orfF_F | orfF | GGCGTTATTCAGTGCCTGTT | This study |
| IS1_F | IS1 | CGGTAACCTCGCGCATACAG | This study |
| ISUnCu_F | ISUnCu1 | GGACTCTCCCCACAAGTAGTG | This study |
F, forward; R, reverse.
Phylogrouping and antibiotic susceptibility profiles
E. coli phylogenetic groups (A0, A1, B1, B2, D1, D2) were determined by PCR using the NZYTaq Green Master Mix (NZYTech, Portugal) and primers and conditions described before (Clermont et al., 2000; Figueira et al., 2011). Antibiotic susceptibilities were tested by disc diffusion agar according to the Clinical and Laboratory Standards Institute recommendations (CLSI, 2012) and using E. coli ATCC 25922 as control strain. The following antibiotics were tested: ampicillin (AMP, 10 μg), amoxicillin (AML, 10 μg), amoxicillin + clavulanic acid (AMC, 30 μg), piperacillin (PRL, 100 μg), piperacillin + tazobactam (TZP, 110 μg), cefalothin (CEF, 30 μg), ceftazidime (CAZ, 30 μg), cefotaxime (CTX, 30 μg), gentamicin (GEN, 10 μg), streptomycin (STR, 10 μg), imipenem (IPM, 10 μg), nalidixic acid (NAL, 30 μg), ciprofloxacin (CIP, 5 μg), tetracycline (TET, 30 μg), chloramphenicol (CHL, 30 μg) and trimethoprim/sulfamethoxazole (STX, 25 μg) (Oxoid, Basingstoke, UK).
Genomic location of integrons and plasmid characterization
To determine the genomic location (plasmid/chromosomal) of integrons, genomic DNA and plasmid DNA were extracted and purified using the Silica Bead DNA Extraction Kit (Thermo Scientific, USA) and the E.Z.N.A. Plasmid Mini Kit II (Omega Bio-tek, GA, USA), respectively. Aliquots were loaded onto 0.9% agarose gels and separated by electrophoresis at 80 V for 80 min. Gels were then stained with ethidium bromide and documented with the Molecular Imager® Gel Doc™ XR System and Image Lab™ Software (Bio-Rad, Hercules, CA, USA). DNA was transferred under vacuum onto positively charged nylon membranes (Hybond N+; Amersham, Freiburg, Germany) and subsequently cross-linked under UV irradiation for 5 min. Hybridizations with intI1- and intI2-digoxigenin (DIG) labeled probes (Moura et al., 2007, 2012b) were performed overnight in 50% formamide hybridization buffer at 42°C. Detections were carried out using the DIG Nucleic Acid Detection Kit (Roche Diagnostics, Germany) following instructions provided by the manufacturer. Positive and negative controls were included in all experiments to confirm the specificity of detection.
In addition, intI+-strains were included as donors in mating assays using rifampicin-resistant E. coli CV601-GFP (Smalla et al., 2006) as recipient strain, using previously described procedures (Moura et al., 2012a). Briefly, liquid cultures of donor and recipient strains were prepared separately in 10 mL Luria–Bertani broth (LB) without antibiotics and grown overnight with gentle shaking at 28°C. Recipient and donor strains were mixed (ratio 1:1) and centrifuged for 5 min at 6700 g to precipitate cells. Supernatants were discarded and replaced by 1 mL fresh LB. Mixtures were incubated overnight at 28°C without shaking. Cells were then precipitated by centrifugation for 5 min at 6700 g and washed in 0.9% NaCl solution. Serial dilutions were prepared in 0.9% NaCl and aliquots of 100 μL were spread on Plate Count Agar plates supplemented with rifampicin (50 mg.L−1) and streptomycin (50 mg.L−1). Putative transconjugants were grown at 28°C for 48 h. Assays were run in duplicate. Donor and recipient were also placed on the selective plates for mutant detection. Putative transconjugants growing in plates were confirmed by BOX-PCR typing by comparison with donor and recipient banding profiles. BOX-PCR reaction mixtures of 25 μL consisted of 0.5 × NZYTaq Green Master Mix (NZYtech, Portugal), 0.8 μM of primer BOXAIR (5′-CTACGGCAAGGCGACGCTGACG-3′; Versalovic et al., 1991) and 1 μL of cell suspension prepared in 100 μL of distilled water (~1.0 McFarland turbidity standard). Amplification was carried out as follows: initial denaturation for 7 min at 94°C, then 30 cycles of denaturation at 94°C for 1 min, followed by annealing at 53°C for 1 min and extension at 65°C for 8 min, and a final extension at 65°C for 16 min. Generated profiles were separated in 1.5% agarose gels in TAE buffer 5× (50 mM Tris, 50 mM boric acid, 0.5 mM EDTA), at 50 V for 95 min, and stained with ethidium bromide. Plasmid DNA from transconjugants were extracted using E.Z.N.A. Plasmid Mini Kit II (Omega Bio-Tek, Georgia, USA), according to the manufacturer's instructions. Among transconjugants, diversity of plasmids was evaluated by PstI/Bst1770I restriction analyses and replicon typing, as previously described (Carattoli et al., 2005; Moura et al., 2012a). The antibiotic susceptibilities patterns of transconjugants were determined by the disc diffusion method as described above.
Statistical analyses
Pearson Chi-squared test (χ2) was used to test the statistical significance (P) of the distribution of integrons and replicons in the different sample sources. Associations were considered significant when P was <0.05.
Nucleotide sequence accession numbers
All integron sequences determined in this study were deposited in GenBank under the accession numbers KF921520 to KF921601.
Results and discussion
In this study, we investigated the occurrence of integrons and associated plasmids in E. coli strains (N = 414) from the World Biosphere Reserve of the Berlenga Island. Our goal was to understand whether the source of pollution, i.e., seagull feces (SF) and human-derived wastewaters (WW), influenced integron prevalence and diversity in E. coli from beach waters (BW).
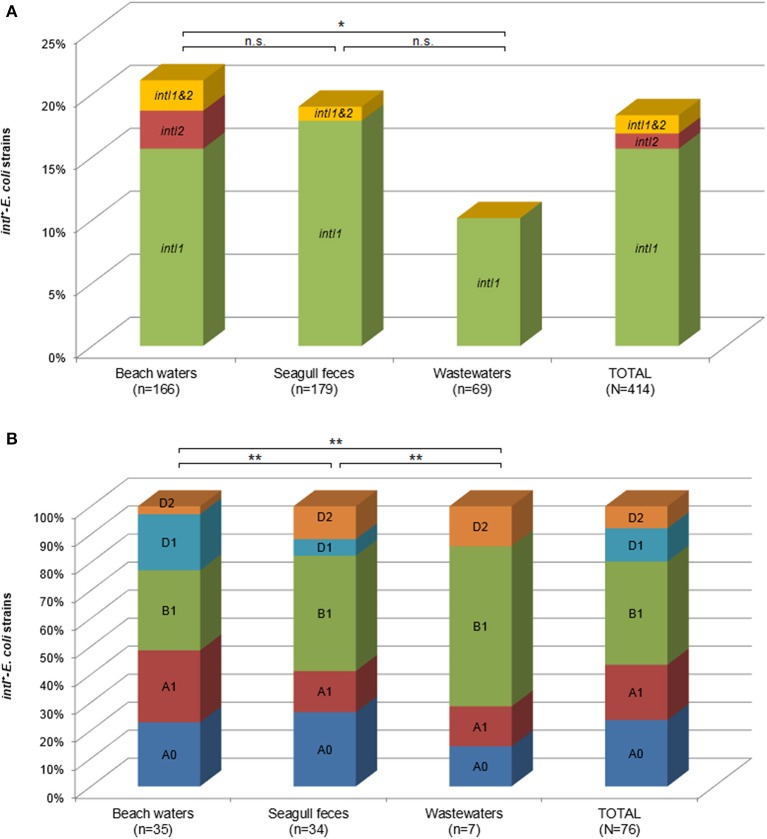
Overall, nearly 20% (76/414) of strains harbored intI genes (Figure 1A). Prevalence of class 1 and class 2 integron integrases was 18 and 2% in BW, 19 and 0.5% in SF and 10 and 0% in WW, respectively. Previous studies targeting antibiotic resistant bacteria in similar environments (Dolejska et al., 2009) have reported comparable prevalence of class 1 integrons in E. coli from surface waters (21%) and black-headed gulls (Larus ridibundus) nesting nearby (15%), although with higher prevalence of intI2 in gulls (11%). Prevalence found at the untreated effluent of Berlengas was also similar to those found in raw human- and animal-derived wastewaters (Moura et al., 2007, 2012b). In this study, differences between prevalence of intI genes in BW and WW were statistically different (χ21 = 3.98; P < 0.05), but not between BW and SF (χ21 = 0.261; P > 0.05). These results confirm the significant contribution of seagull microbiota in shaping the prevalence of integrons in this ecosystem.
Figure 1.
Prevalence of intI+-E. coli detected in the Berlenga Island among different sources (A) and phylogroups (B). Statistical significance: *P < 0.05; **P < 0.01; n.s., not significant.
Phylotyping showed a wide intraspecific diversity among integron carrying (intI+)-E. coli. As shown in Figure 1B, the majority of intI+-strains affiliated to commensal phylogroups B1 (37%), A0 (24%), and A1 (20%). The prevalence of intI genes among phylogroups was not statistically significant (χ25 = 4.70; P > 0.05), being more constraint by the association of the different E. coli phylogroups to the different ecological niches (Figure 1B).
Table 2 provides the detailed characterization of the 76 intI+-E. coli strains obtained in this study. Up to 18 different gene cassettes were found organized into 18 distinct arrays (summarized in Table 3). Common arrays were found among strains from different sources. Gene cassettes detected coded for resistance to aminoglycosides (aadA1, ΔaadA1, aadA2, aadA5, aadB, aacA4, sat2), trimethoprim (dfrA1, dfrA12, dfrA14, dfrA17), chloramphenicol (cmlA1, catB3), lincosamides (linF) and quaternary ammonia compounds (qacH). In addition, gene cassettes coding for putative esterases (estX) and phosphoserine phosphatases (psp), as well gene cassettes of unknown function (orfF) were also present. Though not as part of gene cassettes, genes coding for quinolone resistance (qnrS1), quaternary ammonia compounds (qacEdelta1) and sulfonamides (sul1, sul3) were also associated with the integrons found.
Table 2.
Characteristics of E. coli intI+-strains detected in this study.
| Straina | Phylogroup | Antibiotic resistance (and Intermediary) phenotypeb | pDNA repliconsc | intI1 | intI2 | Pc promoterd | Class 1 integron | Pc2 promotere | Class 2 integron | Conjugationf | Locationg | Accession no(s). |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F38 | A1 | STR, TET | n.d. | + | – | PcW-P2 | intI1-aadA1-3CS | – | C | KF921520 | ||
| F109 | B1 | AMP, AML, PRL, NAL, CIP, TET, STX | Frep, FIB | + | – | PcW | intI1-aadA1-3CS | – | C | KF921521 | ||
| F120 | B1 | STR, TET, STX | Frep, K | + | – | PcW-P2 | intI1-aadA1-3CS | – | C | KF921522 | ||
| F202 | D2 | GEN, STR, TET | Frep, FIB, I1 | + | – | PcW-P2 | intI1-aadA1-3CS | – | C | KF921523 | ||
| A4 | A0 | AMP, (AMC), AML, (CEF), PRL, STR, TET, CHL, STX | Frep | + | + | PcS | intI1-dfrA12-tniC | n.d. | intI2-estX-sat2-aadA1-yebA | + [Frep] | P | KF921524; KF921591 |
| A9 | A1 | AMP, (AMC), AML, PRL, (CAZ), CEF, CTX, STR, NAL, CIP, TET, (CHL), STX | n.d. | + | – | PcWTGN-10 | intI1-dfrA12-tniC | – | P | KF921525 | ||
| A85 | A1 | AMP, AML, PRL, (CEF), GEN, STR, NAL, CIP, TET, STX | Frep, FIB, I1 | + | – | PcWTGN-10 | intI1-dfrA12-tniC | – | C | KF921526 | ||
| A237 | A0 | AMP, (AMC), AML, PRL, CEF, CTX, (STR), NAL, TET, CHL, STX | Frep | + | – | PcWTGN-10 | intI1-dfrA12-tniC | – | C | KF921527 | ||
| A300 | A1 | AMP, AML, PRL, (CEF), STR, CHL, STX | Frep, FIB | + | – | PcS | intI1-dfrA12-tniC | – | C | KF921528 | ||
| F31 | A0 | (AMP), (AML), (CEF), STR, TET, STX | n.d. | + | – | PcW-P2 | intI1-dfrA12-tniC | – | C | KF921529 | ||
| F33 | A0 | NAL, (STR), TET, STX | n.d. | + | – | PcW-P2 | intI1-dfrA12-tniC | – | C | KF921530 | ||
| F63 | A0 | AMP, AML, (AMC), PRL, CEF, STR, TET, CHL, STX | n.d. | + | – | PcWTGN-10 | intI1-dfrA12-tniC | – | P | KF921531 | ||
| F180 | B1 | STR, CHL, STX | Frep, N | + | – | PcH1 | intI1-dfrA12-tniC | – | P | KF921532 | ||
| E109 | B1 | AMP, AML, AMC, PRL, (CEF), (STR), TET, (CHL), STX | n.d. | + | – | PcWTGN-10 | intI1-dfrA12-tniC | – | P | KF921533 | ||
| E137 | A1 | AMP, AML, AMC, PRL, IPM, STR, TET, CHL, STX | I1 | + | – | PcWTGN-10 | intI1-dfrA12-tniC | – | C | KF921534 | ||
| F123 | B1 | AMP, AML, PRL, TET, STX | Y | + | – | PcH1 | intI1-dfrA14-IS26 | – | C | KF921535 | ||
| F192 | B1 | STR, STX | n.d. | + | – | PcW | intI1-dfrA17-3CS | – | C | KF921536 | ||
| A57 | A0 | AMP, AML, (AMC), (PRL), CEF, GEN, STR, NAL, CIP, TET, STX | FIA, FIB | + | – | PcH1 | intI1-dfrA17-IS1 | – | P | KF921537 | ||
| F29 | B1 | AMP, AML, AMC, PRL, (CEF), NAL, CIP, (STR), TET, CHL, (STX) | Frep, FIB | + | – | PcH1 | intI1-dfrA17-IS26 | – | C | KF921538 | ||
| A7 | A1 | AMP, (AMC), AML, (CAZ), PRL, CEF, CTX, STR, NAL, CIP, TET, (CHL), STX | Frep, FIB, I1 | + | – | PcW | intI1-dfrA1-aadA1-3CS | – | + [Frep, FIB] | P | KF921539 | |
| A25 | A1 | AMP, AML, (PRL), (CEF), STR, TET, STX | n.d. | + | – | PcW | intI1-dfrA1-aadA1-3CS | – | C | KF921540 | ||
| A47 | A1 | AMP, AML, (PRL), STR, TET, STX | Frep | + | – | PcW | intI1-dfrA1-aadA1-3CS | – | C | KF921541 | ||
| A62 | A1 | AMP, AML, PRL, (CEF), STR, NAL, TET, CHL, STX | Frep | + | – | PcW | intI1-dfrA1-aadA1-3CS | – | C | KF921542 | ||
| A94 | A1 | AMP, AML, (PRL), (CEF), STR, TET, CHL, STX | Frep | + | – | PcW | intI1-dfrA1-aadA1-3CS | – | C | KF921543 | ||
| A102 | D1 | AMP, AML, (PRL), STR, CIP, TET, CHL | Frep, FIB | + | – | PcW | intI1-dfrA1-aadA1-3CS | – | + [Frep, FIB] | P | KF921544 | |
| A154 | B1 | AMP, AML, PRL, (CEF), STR, TET, STX | n.d. | + | – | PcW | intI1-dfrA1-aadA1-3CS | – | C | KF921545 | ||
| F11 | D1 | AMP, AML, PRL, STR, TET, CHL, STX | Frep, FIB | + | – | PcW | intI1-dfrA1-aadA1-3CS | – | C | KF921546 | ||
| F17 | D1 | AMP, AML, PRL, (CEF), STR, TET, STX | Frep, FIB | + | – | PcW | intI1-dfrA1-aadA1-3CS | – | C | KF921547 | ||
| F65 | B1 | AMP, (AMC), AML, PRL, (CEF), STR, NAL, CIP, TET, STX | Frep, FIB | + | – | PcW | intI1-dfrA1-aadA1-3CS | – | + [Frep, FIB] | P | KF921548 | |
| F351 | B1 | AMP, (AMC), AML, (PRL), STR, NAL, CIP, TET, CHL, STX | Frep, FIB, I1 | + | – | PcW | intI1-dfrA1-aadA1-3CS | – | + [Frep, FIB] | P | KF921549 | |
| F358 | B1 | AMP, ([AMC]), AML, PRL, (CEF), STR, TET, STX | Frep, FIB, I1 | + | – | PcW | intI1-dfrA1-aadA1-3CS | – | + [Frep, FIB, I1] | P | KF921550 | |
| A108 | B1 | AMP, AML, STR, NAL, CIP, TET, CHL, STX | Frep | + | – | PcH1 | intI1-dfrA17-aadA5-3CS | – | P | KF921551 | ||
| A180 | B1 | AMP, AML, (PRL), STR, NAL, TET, CHL, STX | Frep, FIB | + | – | PcH1 | intI1-dfrA17-aadA5-3CS | – | + [Frep, FIB] | P | KF921552 | |
| E108 | B1 | AMP, AML, AMC, PRL, ([CEF]), IPM, STR, NAL, CIP, TET, CHL, STX | Frep, FIB | + | – | PcS | intI1-aacA4-catB3-dfrA1-3CS | – | + [Frep, FIB] | P | KF921553 | |
| F255 | A1 | AMP, AML, PRL, GEN, STR, NAL, TET, CHL, STX | Frep, FIB | + | – | PcW-P2 | intI1-aadB-aadA1-ISUnCu1-3CS | – | C | KF921554 | ||
| E80 | B1 | STR, (NAL), (CIP), CHL | I1 | + | – | PcH1 | intI1-aadA2-linF-IS26-ISKp19(trunc.)-qnrS1 | – | + [I1] | P | KF921555 | |
| A172 | D1 | AMP, (AMC), AML, PRL, CEF, GEN, STR, NAL, CIP, TET, CHL, STX | Frep | + | – | PcH1 | intI1-estX-psp-aadA2-qacH-IS440-sul3 | – | C | KF921556 | ||
| A33 | A0 | AMP, (AMC), AML, (PRL), (IPM), STR, TET, CHL, STX | I1 | + | + | PcH1 | intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | Pc2A | intI2-dfrA1-sat2-aadA1-yebA | C | KF921557; KF921594 | |
| A107 | D1 | AMP, AML, PRL, STR, TET, CHL | Frep, FIB | + | – | PcH1 | intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | – | C | KF921558 | ||
| A110 | A0 | (STR), (NAL), (CIP) | Frep | + | + | PcW | intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | n.d. | intI2-dfrA1-sat2-aadA1-yebA | C | KF921559; KF921597 | |
| A127 | D1 | AMP, AML, PRL, STR, CIP, TET, CHL | Frep, FIB | + | – | PcH1 | intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | – | C | KF921560 | ||
| A128 | D1 | AMP, AML, PRL, (CEF), STR, NAL, CIP, TET, STX | FIB | + | – | PcH1 | intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | – | P | KF921561 | ||
| A148 | D1 | AMP, AML, PRL, STR, TET, CHL | Frep, FIB | + | – | PcH1 | intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | – | + [Frep, FIB] | P | KF921562 | |
| A176 | D2 | STR, CHL | Frep, I1 | + | – | PcW | intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | – | C | KF921563 | ||
| A182 | B1 | AMP, AML, PRL, (CEF), STR, TET, (CHL) | Frep, I1 | + | – | PcH1 | intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | n.d. | – | C | KF921564 | |
| F18 | A0 | AMP, AML, PRL, (CEF), STR, TET, CHL, STX | n.d. | + | + | PcH1 | intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | n.d. | intI2-dfrA1-sat2-aadA1-yebA | C | KF921565; KF921600 | |
| F317 | A0 | AMP, AML, PRL, (NAL), TET, CHL | Frep | + | – | PcW | intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | – | C | KF921566 | ||
| F368 | A0 | AMP, AML, (PRL), (NAL), (CIP), STR, TET, (CHL), STX | n.d. | + | – | PcH1 | intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | – | C | KF921567 | ||
| F380 | B1 | AMP, (AMC), AML, PRL, CEF, NAL, CIP, (STR), TET, CHL, STX | Frep | + | – | PcW | intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | – | C | KF921568 | ||
| E3 | B1 | (STR), TET | Frep | + | – | PcH1 | intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | – | C | KF921569 | ||
| A30 | A0 | AMP, AML, (IPM), GEN, STR, TET, CHL, STX | Frep, I1 | + | + | PcS | intI1-dfrA12-orfF-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | n.d. | intI2-estX-sat2-aadA1-yebA | C | KF921570; KF921593 | |
| A49 | A1 | CEF, STR, TET, CHL, STX | Frep | + | – | PcWTGN-10 | intI1-dfrA12-orfF-aadA2-cmlA1-aadA1-qacH-IS440-sul3 | – | C | KF921571 | ||
| F278 | A1 | AMP, AML, PRL, STR, TET, CHL, STX | Frep, FIB, I1, N | + | + | PcWTGN-10 | intI1-dfrA12-orfF-aadA2-cmlA-1aadA1-qacH-IS440-IS10-sul3 | Pc2A-Pc2B | intI2-dfrA1-sat2-aadA1-yebA | C | KF921572; KF921601 | |
| A115 | D1 | (STR), TET | Frep, I1 | + | – | PcW | intI1 | – | C | KF921573 | ||
| A222 | B1 | AMP, AML, PRL, (CEF), STR, NAL, CIP, STX | Frep, I1 | + | – | PcH1 | intI1 | – | P | KF921574 | ||
| A270 | B1 | AMP, AML, PRL, (CEF), STR, NAL, CIP, TET, CHL, STX | Frep, FIB | + | – | PcH1 | intI1 | – | + [Frep, FIB] | P | KF921575 | |
| A280 | B1 | STR, TET, STX | Frep, I1 | + | – | PcH1 | intI1 | – | C | KF921576 | ||
| F20 | B1 | AMP, AML, PRL, (CEF), STR, (CIP), TET, CHL, STX | Frep, FIB | + | – | PcW | intI1 | – | C | KF921577 | ||
| F42 | A1 | AMP, AML, PRL, (CEF), STR, NAL, CIP, TET, CHL (STX) | Frep, I1 | + | – | PcH1 | intI1 | – | C | KF921578 | ||
| F59 | B1 | AMP, [AMC], AML, PRL, ([CEF]), (IPM), STR, TET, STX | Frep, FIB | + | – | PcW | intI1 | – | + [Frep, FIB] | P | KF921579 | |
| F84 | A1 | AMP, AML, (PRL), (CEF), (STR), (NAL), TET | n.d. | + | – | PcW | intI1 | – | C | KF921580 | ||
| F140 | B1 | STR, NAL, CIP, STX | Frep, FIB | + | – | PcH1 | intI1 | – | C | KF921581 | ||
| F153 | A0 | AMP, AML, PRL, (CEF), (STR), TET, STX | Frep | + | – | PcH1 | intI1 | – | C | KF921582 | ||
| F154 | B1 | AMP, AML, PRL, STR, NAL, CIP, STX | Frep | + | – | PcH1 | intI1 | – | + [Frep] | P | KF921583 | |
| F158 | D2 | AMP, AML, PRL, (CEF), STR, TET, STX | FIB | + | – | PcW | intI1 | – | + [FIB] | P | KF921584 | |
| F217 | D2 | GEN, STR, TET | Frep, I1 | + | – | PcW-P2 | intI1 | – | C | KF921585 | ||
| F354 | A0 | TET, STX | n.d. | + | – | PcH1 | intI1 | – | C | KF921586 | ||
| F355 | A0 | ([AMP]), ([AML]), [CEF], GEN, STR, TET, CHL | I1 | + | – | PcWTGN-10 | intI1 | – | + [I1] | P | KF921587 | |
| F357 | D2 | AMP, AML, (PRL), STR, TET, STX | Frep | + | – | PcW | intI1 | – | C | KF921588 | ||
| E18 | D2 | AMP, AML, PRL, (IPM), STR, CIP, TET, STX | FIB | + | – | PcW | intI1 | – | C | KF921589 | ||
| E77 | A0 | (STR) | n.d. | + | – | PcH1 | intI1 | – | C | KF921590 | ||
| A6 | A0 | AMP, AML, AMC, PRL, (CEF), STR, TET, CHL | n.d. | – | + | – | – | n.d. | intI2-estX-sat2-aadA1-yebA | C | KF921592 | |
| A93 | A0 | TET, (STR), STX | n.d. | – | + | – | – | n.d. | intI2-dfrA1-sat2-ΔaadA1-yebA | C | KF921595 | |
| A109 | B1 | STR, NAL, TET | Frep, FIB | – | + | – | – | n.d. | intI2-dfrA1-sat2-aadA1-yebA | C | KF921596 | |
| A113 | B1 | (IPM), STR, NAL, TET | Frep | – | + | – | – | n.d. | intI2-dfrA1-sat2-aadA1-yebA | C | KF921598 | |
| A142 | B1 | STR, NAL, TET | Frep | – | + | – | – | n.d. | intI2-dfrA1-sat2-aadA1-yebA | C | KF921599 |
Strains A#, F#, and E# were obtained from beach waters, seagull feces and raw wastewaters, respectively.
AMP, ampicillin; AML, amoxicillin; AMC, amoxicillin + clavulanic acid; PRL, piperacillin; CEF, cefalothin; CAZ, ceftazidime; CTX, cefotaxime; GEN, gentamicin; STR, streptomycin; IPM, imipenem; NAL, nalidixic acid; CIP, ciprofloxacin; TET, tetracycline; CHL, chloramphenicol; and STX, trimethoprim/sulfamethoxazole.Intermediary resistance phenotypes are shown in brackets. Phenotypes shown by donors and transconjugants are highlighted in bold. Phenotypes observed in transconjugants but not in donors are shown in square brackets.
Plasmid incompatibility groups determined by replicon typing in E. coli donor strains; n.d., not detected.
Class 1 promoter variants: PcS, TTGACA-N17-TAAACT; PcW, TGGACA-N17-TAAGCT; PcH1, TGGACA-N17-TAAACT; PcH2, TTGACA-N17-TAAGCT; P2, TTGTTA-N17-TACAGT (Jové et al., 2010).
Class 2 promoter variants: Pc2A, TTTTAA-N17-TAAAAT; Pc2B, TTGTAT-N16-TTTAAT (Jové et al., 2010).
Transfer of intI-conjugative plasmids in mating assays; replicon types detected in intI-transconjugants are shown in square brackets.
Genomic location, as determined by mating assays and hybridization of plasmid and genomic DNA using intI probes: P, plasmid; C, chromosome.
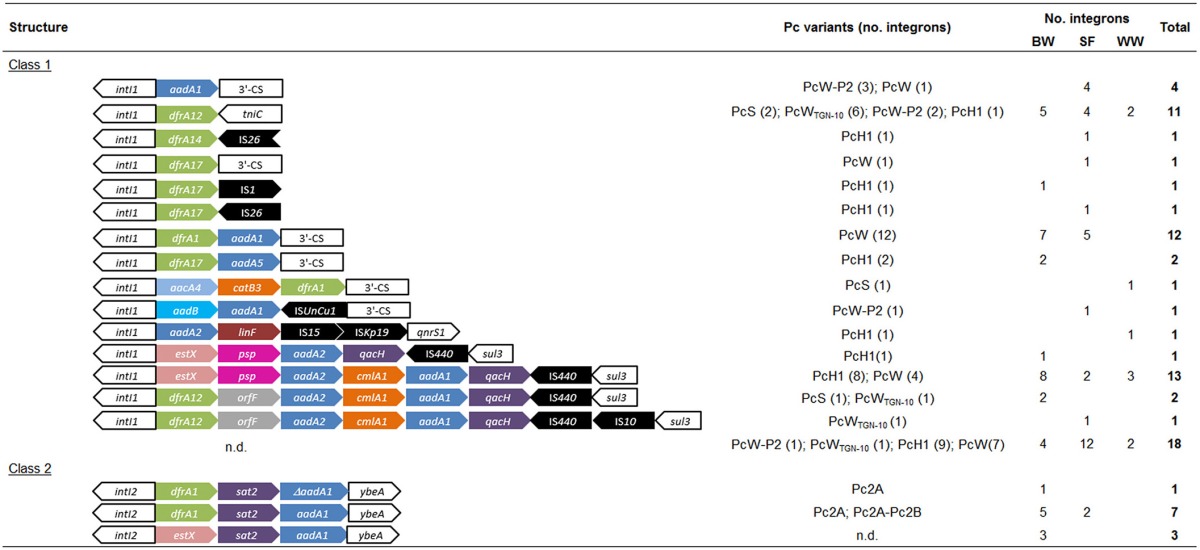
Table 3.
Overview of the gene cassette arrays and Pc promoter variants present in the 82 integron structures detected in this study among intI+-E. coli strains isolated from beach waters (BW), seagull feces (SF) and wastewaters (WW).
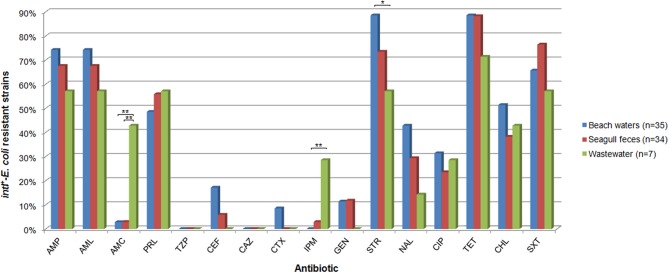
Thus, integron structures detected contained genes involved in diverse resistance mechanisms, including enzymatic antibiotic modification (aadA, aadB, aacA, catB, sat, sul), efflux pumps (qacH, qacE) and target protection proteins (qnrS). This diversity of resistance mechanisms largely contributed to the high prevalence of multiresistant intI+-E. coli (64/76, 83%; considering simultaneous resistance to 3 or more different classes of antibiotics), although the presence of additional mechanisms of resistance besides those within integrons cannot be excluded. Prevalence of multi-resistant strains in BW (89%) was statistically different from that observed in WW (57%), but not to the one observed in SF (82%). Overall, the most frequently resistances detected were against tetracycline (87%), streptomycin (79%), ampicillin (70%), amoxicillin (70%), trimethoprim-sulfamethoxazole (70%), piperacillin (53%), and chloramphenicol (45%) (Figure 2). Differences among sources were not statistically significant, except for resistances against amoxicillin+clavulanic acid and imipenem, that were more prevalent in wastewaters (P < 0.01). The prevalence and risk of dissemination of resistant strains to last-resort antibiotics, such as imipenem, is nowadays a matter of great concern, reducing treatment options for infectious diseases. Imipenem resistance if often associated to the presence of integron-borne carbapenemase gene cassetes, such as blaVIM, blaIMP, and blaGES (INTEGRALL database, Moura et al., 2009) and/or plasmid-borne carbapenemases, such as blaKPC, blaOXA−48, and blaNDM−1 (Carattoli, 2013). Nevertheless, none of these mechanisms have been detected in these strains (Alves et al., 2014). Further investigations will allow to elucidate the mechanisms of carbapenem resistance present in these strains as well their potential risk of dissemination into natural environments.
Figure 2.
Prevalence of antimicrobial resistance in intI+-E. coli strains. Antibiotic abbreviations: AMP, ampicillin; AML, amoxicillin; AMC, amoxicillin + clavulanic acid; PRL, piperacillin; TZP, piperacillin + tazobactam; CEF, cefalothin; CAZ, ceftazidime; CTX, cefotaxime; GEN, gentamicin; STR, streptomycin; IPM, imipenem; NAL, nalidixic acid; CIP, ciprofloxacin; TET, tetracycline; CHL, chloramphenicol; STX, trimethoprim/sulfamethoxazole. Only statistical significant differences are shown: *P < 0.05; **P < 0.01.
Different insertion sequences (IS1, IS10, IS15, IS26, IS440, ISKp19, ISUnCu1) were also found within 50% (9/18) of the different arrays (Tables 2–3). Comparative analyses of 20 E. coli genomes have also shown the presence of a large number of IS-like elements, constituting 21% of all genes annotated (Touchon et al., 2009) and likely to contribute to the high genome dynamics and adaptation seen in E. coli.
Class 1 integrons lacking the 3′-conserved segment (qacEdelta1/sul1) represented nearly half of int1I+-E. coli strains (33/71; 46.5%). These included sul3-type (n = 17) integrons and Tn402-derivative integrons containing tniC (n = 11). Dissemination of sul3-containing elements linked to class 1 integrons with an unusual 3′CS region has been reported among clinical and meat-associated Salmonella and E. coli isolates, including in poultry, often as large platforms with the structures intI1-dfrA12-orfF-aadA2-cmlA1-aadA1-qacH-IS440-sul3 or intI1-estX-psp-aadA2-cmlA1-aadA1-qacH-IS440-sul3 (Antunes et al., 2007; Sáenz et al., 2010; Curiao et al., 2011; Pérez-Moreno et al., 2013), as observed in this study. Although some variations in these array structures may occur, such as additional IS insertions (e.g., IS10 in intI1-dfrA12-orfF-aadA2-cmlA1-aadA1-qacH-IS440-IS10-sul3, Tables 2–3) or gene cassette deletions (e.g., cmlA1-aadA1 in the structure intI1-estX-psp-aadA2-qacH-IS440-sul3, Tables 2–3), the apparent conservation and dissemination of these arrays among isolates from different sources and countries, suggest their mobilization through horizontal gene transfer or specific clone dissemination and diversification, rather than cumulative gene cassette acquisition.
Tn402-derivative integrons are thought to be the progenitors of classical class 1 integrons that contain the 3′-conserved segment (Post et al., 2007). Integrons derived from Tn402 are flanked by the tniC gene (also called tniR) that makes part of the transposition tniABQC module. Reports of tniC-like integrons are scarce likely because gene cassette characterization usually relies only on the amplification of 3′-CS conservative region (Post et al., 2007). At INTEGRALL database, tniC-integrons have been identified in few Pseudomonas putida, Pseudomonas aeruginosa, Aeromonas caviae and IncP-1 plasmids, many of those containing gene cassettes coding resistance against beta-lactams (blaVIM, blaOXA, blaNPS−1), aminoglycosides (aacA4, aacA7, aacC5, aadA1, aadA11) and trimetophrim (dfrB5). In this study, all tniC-integrons carried the dihydrofolate reductase dfrA12 gene cassette, coding for resistance to trimethoprim, and it constitutes the first report on tniC-like integrons in E. coli.
No significant differences were found on promoter distribution accordingly to sample origin (χ210 = 16.25; P > 0.05), contrarily to what has been observed in animal- and human-derived wastewaters (Moura et al., 2012c). The majority of integrons detected possessed weak Pc promoter variants (PcW and PcH), which are known to be associated to weak expression of gene cassette arrays (Jové et al., 2010). PcH1 and PcW variants were more prevalent among A0 and B1 phylogroups (χ220 = 36.56; P < 0.01). Previous studies concerning aquatic environments have also reported higher prevalence of weaker promoters among environmental strains (Moura et al., 2012c; Tacão et al., 2014), as well as studies concerning commensal microbiota (Soufi et al., 2009). Weaker Pc variants are associated to a higher capacity for gene cassette rearrangements, leading to more dynamic arrays (Jové et al., 2010). Interestingly, among tniC-type integrons stronger Pc variants were identified: PcS (n = 2), PcWTGG−10 (n = 6), PcW-P2 (n = 1). These results corroborate that integron platforms had probably evolved to favor high rate of gene cassette recombination compensating low expression levels and contributing to genome plasticity, as discussed before (Moura et al., 2012c).
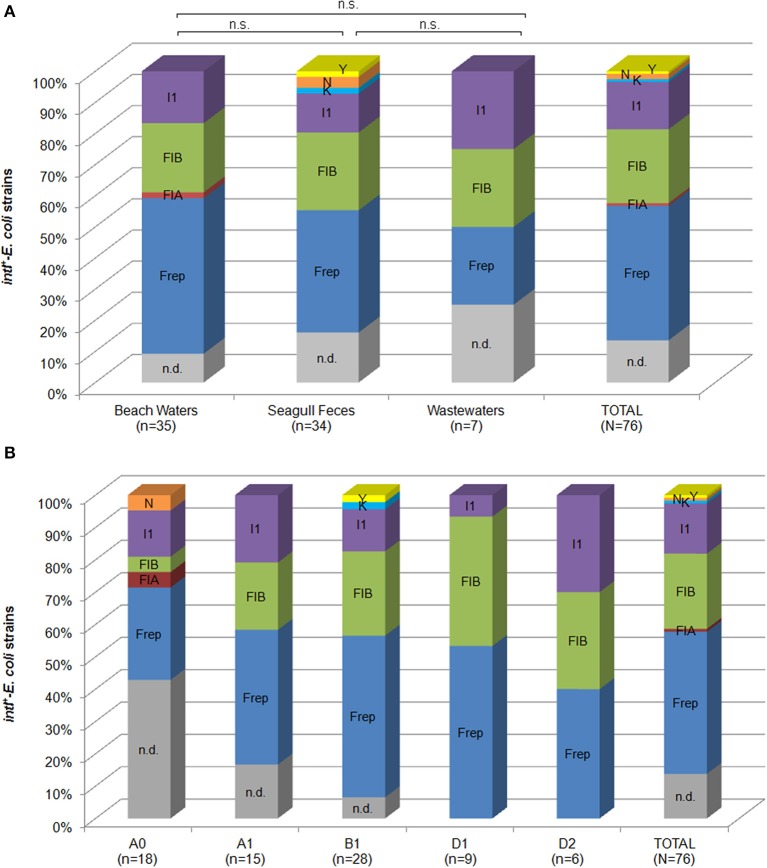
Similar to previous reports on plasmid diversity among intI+-strains (Moura et al., 2012a), a wide and diverse plasmid pool was present in these E. coli (Figure 3A). Replicons were detected in 80% (60/76) of strains (Figure 3A; Table 2), though differences among BW, SF, and WW were not statistically significant. Replicons detected belonged to IncF (Frep, FIA, and FIB subgroups), IncI1, IncN, IncY, and IncK incompatibility groups. More than one replicon type was detected in 41% (31/76) of strains. In strains from phylogroups A0 and B1, up to 5 different replicon types were detected (Figure 3B). Integrons were successfully mobilized through IncF (Frep and FIB subgroups) and IncI1 conjugative plasmids into E. coli CV601 in 20% (15/76) of strains, using streptomycin as selective marker. The majority of intI-transconjugants displayed the resistance patterns observed in donor strains (Table 2), highlighting the importance of co-selection in the spread of multi-resistance traits through horizontal gene transfer. Plasmid DNA from transconjugants showed different restriction patterns (data not shown), including in transconjugants from donors that shared identical integron structures. These results may be explained by the presence of identical integron platforms in different plasmids. Nevertheless, the co-mobilization of multiple plasmids and/or the occurrence of genetic rearrangements in transconjugants resulting in different restriction patterns cannot be excluded. It is also noteworthy that plasmid prevalence and diversity, as well as their transfer ability may be, however, under-estimated due to biases introduced by the technical approaches. Alkaline extraction of plasmid DNA may affect the efficiency to recover larger plasmids, and the mating conditions used may favor the transfer the plasmids of IncF and IncI complexes, which are liquid maters.
Figure 3.
Prevalence of replicon types detected in intI+-E. coli among different sources (A) and phylogroups (B). Abbreviations: n.d., not detected; n.s., not statistically significant.
In conclusion, results obtained confirmed the existence of a diverse integron pool in this coastal environment, associated with different resistance traits and plasmid incompatibility groups. The prevalence and diversity of integrons, as well as of multi-drug resistance phenotypes, found in beach waters were more influenced by animal-derived fecal inputs rather human-derived wastewaters. Results obtained thus reinforce the important input of commensal E. coli from wild animals in this ecosystem, largely dominated by seagulls. These findings underscore the role of wild life in dissemination of integrons and antibiotic resistance traits in natural environments.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank Alessandra Carattoli (Department of Infectious, Parasitic and Immune-Mediated Diseases, Istituto Superiore di Sanità, Italy) for providing replicon typing control strains. This work was supported by European Funds through COMPETE and by National Funds through FCT—Fundação para a Ciência e a Tecnologia within the projects PEst-C/MAR/LA0017/2013 and SEAGULL (FCOMP-01-0124-FEDER-008640, PTDC/AAC-AMB/109155/2008). FCT also financed the grants SFRH/BPD/72256/2010 (Alexandra Moura) and SFRH/BPD/26685/2006 (Anabela Pereira). Isabel Henriques received an individual Research Assistant contract within the project Sustainable Use of Marine Resources MARES (CENTRO-07-ST24-FEDER-002033), co-financed by QREN (Mais Centro-Programa Operacional Regional do Centro) and FEDER.
References
- Altschul S. F., Madden T. L., Schaffer A. A., Zhang J., Zhang Z., Miller W., et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves M. S., Pereira A., Araujo S. M., Castro B. B., Correia A. C., Henriques I. (2014). Seawater is a reservoir of multi-resistant Escherichia coli, including strains hosting plasmid-mediated quinolones resistance and extended-spectrum beta-lactamases genes. Front. Microbiol. 5:426 10.3389/fmicb.2014.00426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson D. I., Hughes D. (2011). Persistence of antibiotic resistance in bacterial populations. FEMS Microbiol. Rev. 35, 901–911 10.1111/j.1574-6976.2011.00289.x [DOI] [PubMed] [Google Scholar]
- Antunes P., Machado J., Peixe L. (2007). Dissemination of sul3-containing elements linked to class 1 integrons with an unusual 3′ conserved sequence region among Salmonella isolates. Antimicrob. Agents Chemother. 51, 1545–1548 10.1128/AAC.01275-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo S., Henriques I. S., Leando S. M., Alves A., Pereira A., Correia A. (2014). Gulls identified as major source of fecal pollution in coastal waters: a microbial source tracking study. Sci. Total Environ. 470–471, 84–91 10.1016/j.scitotenv.2013.09.075 [DOI] [PubMed] [Google Scholar]
- Arduino S. M., Catalano M., Orman B. E., Roy P. H., Centron D. (2003). Molecular epidemiology of orf513-bearing class 1 integrons in multiresistant clinical isolates from Argentinean hospitals. Antimicrob. Agents Chemother. 47, 3945–3949 10.1128/AAC.47.12.3945-3949.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharoglu Z., Bikard D., Mazel D. (2010). Conjugative DNA transfer induces the bacterial SOS response and promotes antibiotic resistance development through integron activation. PLoS Genet. 6:e1001165 10.1371/journal.pgen.1001165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattoli A. (2013). Plasmids and the spread of resistance. Int. J. Med. Microbiol. 303, 298–304 10.1016/j.ijmm.2013.02.001 [DOI] [PubMed] [Google Scholar]
- Carattoli A., Bertini A., Villa L., Falbo V., Hopkins K. L., Threlfall E. J. (2005). Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63, 219–228 10.1016/j.mimet.2005.03.018 [DOI] [PubMed] [Google Scholar]
- Clermont O., Bonacorsi S., Bingen E. (2000). Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66, 4555–4558 10.1128/AEM.66.10.4555-4558.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI, (2012). Performance Standars for Antimicrobial Susceptability Testing. Document M100-S22. Wayne, PA: CLSI [Google Scholar]
- Curiao T., Cantón R., Garcillán-Barcia M. P., De La Cruz F., Baquero F., Coque T. M. (2011). Association of composite IS26-sul3 elements with highly transmissible IncI1 plasmids in extended-spectrum-beta-lactamase-producing Escherichia coli clones from humans. Antimicrob. Agents Chemother. 55, 2451–2457 10.1128/AAC.01448-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Davies D. (2010). Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 74, 417–433 10.1128/MMBR.00016-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolejska M., Bierosova B., Kohoutová L., Literak I., Cizek A. (2009). Antibiotic-resistant Salmonella and Escherichia coli isolates with integrons and extended-spectrum beta-lactamases in surface water and sympatric black-headed gulls. J. Appl. Microbiol. 106, 1941–1950 10.1111/j.1365-2672.2009.04155.x [DOI] [PubMed] [Google Scholar]
- Dolejska M., Cizek A., Literak I. (2007). High prevalence of antimicrobial-resistant genes and integrons in Escherichia coli isolates from Black-headed Gulls in the Czech Republic. J. Appl. Microbiol. 103, 11–19 10.1111/j.1365-2672.2006.03241.x [DOI] [PubMed] [Google Scholar]
- Figueira V., Serra E., Manaia C. M. (2011). Differential patterns of antimicrobial resistance in population subsets of Escherichia coli isolated from waste- and surface waters. Sci. Total Environ. 409, 1017–1023 10.1016/j.scitotenv.2010.12.011 [DOI] [PubMed] [Google Scholar]
- Goldstein C., Lee M. D., Sanchez S., Hudson C., Phillips B., Register B., et al. (2001). Incidence of class 1 and 2 integrases in clinical and commensal bacteria from livestock, companion animals, and exotics. Antimicrob. Agents Chemother. 45, 723–726 10.1128/AAC.45.3.723-726.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N. (1996). Escherichia, klebsiella, enterobacter, serratia, citrobacter, and proteus, in Medical Microbiology, 4th Edn., ed Baron S. (Galveston, TX: University of Texas Medical; ). [PubMed] [Google Scholar]
- Hernandez J., Johansson A., Stedt J., Bengtsson S., Porczak A., Granholm S., et al. (2013). Characterization and comparison of extended-spectrum beta-lactamase (ESBL) resistance genotypes and population structure of Escherichia coli isolated from Franklin's gulls (Leucophaeus pipixcan) and humans in Chile. PLoS ONE 8: e76150 10.1371/journal.pone.0076150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer H., Smalla K. (2007). Manure and sulfadiazine synergistically increased bacterial antibiotic resistance in soil over at least two months. Environ. Microbiol. 9, 657–666 10.1111/j.1462-2920.2006.01185.x [DOI] [PubMed] [Google Scholar]
- Jové T., Da Re S., Denis F., Mazel D., Ploy M. C. (2010). Inverse correlation between promoter strength and excision activity in class 1 integrons. PLoS Genet. 6: e1000793 10.1371/journal.pgen.1000793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmet V., Drugdova Z., Kmetova M., Stanko M. (2013). Virulence and antibiotic resistance of Escherichia coli isolated from rooks. Ann. Agric. Environ. Med. 20, 273–275 [PubMed] [Google Scholar]
- Kraft C. A., Timbury M. C., Platt D. J. (1986). Distribution and genetic location of Tn7 in trimethoprim-resistant Escherichia coli. J. Med. Microbiol. 22, 125–131 10.1099/00222615-22-2-125 [DOI] [PubMed] [Google Scholar]
- Levesque C., Piche L., Larose C., Roy P. H. (1995). PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39, 185–191 10.1128/AAC.39.1.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura A., Henriques I., Ribeiro R., Correia A. (2007). Prevalence and characterization of integrons from bacteria isolated from a slaughterhouse wastewater treatment plant. J. Antimicrob. Chemother. 60, 1243–1250 10.1093/jac/dkm340 [DOI] [PubMed] [Google Scholar]
- Moura A., Jové T., Ploy M. C., Henriques I., Correia A. (2012c). Diversity of gene cassette promoters in class 1 integrons from wastewater environments. Appl. Environ. Microbiol. 78, 5413–5416 10.1128/AEM.00042-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura A., Oliveira C., Henriques I., Smalla K., Correia A. (2012a). Broad diversity of conjugative plasmids in integron-carrying bacteria from wastewater environments. FEMS Microbiol. Lett. 330, 157–164 10.1111/j.1574-6968.2012.02544.x [DOI] [PubMed] [Google Scholar]
- Moura A., Pereira P., Henriques I., Correia A. (2012b). Novel gene cassettes and integrons in antibiotic resistant bacteria isolated from urban wastewaters. Res. Microbiol. 163, 92–100 10.1016/j.resmic.2011.10.010 [DOI] [PubMed] [Google Scholar]
- Moura A., Soares M., Pereira C., Leitão N., Henriques I., Correia A. (2009). INTEGRALL: a database and search engine for integrons, integrases and gene cassettes. Bioinformatics 25, 1096–1098 10.1093/bioinformatics/btp105 [DOI] [PubMed] [Google Scholar]
- Pérez-Moreno M. O., Picó-Plana E., De Toro M., Grande-Armas J., Quiles-Fortuny V., Pons M. J., et al. (2013). Beta-lactamases, transferable quinolone resistance determinants, and class 1 integron-mediated antimicrobial resistance in human clinical Salmonella enterica isolates of non-Typhimurium serotypes. Int. J. Med. Microbiol. 303, 25–31 10.1016/j.ijmm.2012.11.003 [DOI] [PubMed] [Google Scholar]
- Perry J. A., Wright G. D. (2013). The antibiotic resistance “mobilome”: searching for the link between environment and clinic. Front. Microbiol. 4: 138 10.3389/fmicb.2013.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeta P., Radhouani H., Igrejas G., Goncalves A., Carvalho C., Rodrigues J., et al. (2008). Seagulls of the Berlengas natural reserve of Portugal as carriers of fecal Escherichia coli harboring CTX-M and TEM extended-spectrum beta-lactamases. Appl. Environ. Microbiol. 74, 7439–7441 10.1128/AEM.00949-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L., Potron A., De La Cuesta C., Cleary T., Nordmann P., Munoz-Price L. S. (2012). Wild coastline birds as reservoirs of broad-spectrum-beta-lactamase-producing Enterobacteriaceae in Miami Beach, Florida. Antimicrob. Agents Chemother. 56, 2756–2758 10.1128/AAC.05982-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post V., Recchia G. D., Hall R. M. (2007). Detection of gene cassettes in Tn402-like class 1 integrons. Antimicrob. Agents Chemother. 51, 3467–3468 10.1128/AAC.00220-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhouani H., Poeta P., Igrejas G., Goncalves A., Vinue L., Torres C. (2009). Antimicrobial resistance and phylogenetic groups in isolates of Escherichia coli from seagulls at the Berlengas nature reserve. Vet. Rec. 165, 138–142 10.1136/vr.165.5.138 [DOI] [PubMed] [Google Scholar]
- Randall L. P., Cooles S. W., Osborn M. K., Piddock L. J., Woodward M. J. (2004). Antibiotic resistance genes, integrons and multiple antibiotic resistance in thirty-five serotypes of Salmonella enterica isolated from humans and animals in the UK. J. Antimicrob. Chemother. 53, 208–216 10.1093/jac/dkh070 [DOI] [PubMed] [Google Scholar]
- Sáenz Y., Vinué L., Ruiz E., Somalo S., Martínez S., Rojo-Bezares B., et al. (2010). Class 1 integrons lacking qacEdelta1 and sul1 genes in Escherichia coli isolates of food, animal and human origins. Vet. Microbiol. 144, 493–497 10.1016/j.vetmic.2010.01.026 [DOI] [PubMed] [Google Scholar]
- Sandvang D., Aarestrup F. M., Jensen L. B. (1997). Characterisation of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica Typhimurium DT104. FEMS Microbiol. Lett. 157, 177–181 10.1111/j.1574-6968.1998.tb12887.x [DOI] [PubMed] [Google Scholar]
- Santos T., Silva N., Igrejas G., Rodrigues P., Micael J., Rodrigues T., et al. (2013). Dissemination of antibiotic resistant Enterococcus spp. and Escherichia coli from wild birds of Azores Archipelago. Anaerobe 24, 25–31 10.1016/j.anaerobe.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Siguier P., Perochon J., Lestrade L., Mahillon J., Chandler M. (2006). ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 34, D32–D36 10.1093/nar/gkj014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalla K., Haines A. S., Jones K., Krögerrecklenfort E., Heuer H., Schloter M., et al. (2006). Increased abundance of IncP-1beta plasmids and mercury resistance genes in mercury-polluted river sediments: first discovery of IncP-1beta plasmids with a complex mer transposon as the sole accessory element. Appl. Environ. Microbiol. 72, 7253–7259 10.1128/AEM.00922-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi L., Abbassi M. S., Saenz Y., Vinue L., Somalo S., Zarazaga M., et al. (2009). Prevalence and diversity of integrons and associated resistance genes in Escherichia coli isolates from poultry meat in Tunisia. Foodborne Pathog. Dis. 6, 1067–1073 10.1089/fpd.2009.0284 [DOI] [PubMed] [Google Scholar]
- Stokes H. W., Hall R. M. (1989). A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol. Microbiol. 3, 1669–1683 10.1111/j.1365-2958.1989.tb00153.x [DOI] [PubMed] [Google Scholar]
- Tacão M., Correia A., Henriques I. (2012). Resistance to broad-spectrum antibiotics in aquatic systems: anthropogenic activities modulate the dissemination of blaCTX-M-like genes. Appl. Environ. Microbiol. 78, 4134–4140 10.1128/AEM.00359-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacão M., Correia A., Henriques I. (2013). Environmental Shewanella xiamenensis strains that carry blaOXA-48 or blaOXA-204 genes: additional proof for blaOXA-48-like gene origin. Antimicrob. Agents Chemother. 57, 6399–6400 10.1128/AAC.00771-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacão M., Moura A., Correia A., Henriques I. (2014). Co-resistance to different classes of antibiotics among ESBL-producers from aquatic systems. Water Res. 48, 100–107 10.1016/j.watres.2013.09.021 [DOI] [PubMed] [Google Scholar]
- Tenaillon O., Skurnik D., Picard B., Denamur E. (2010). The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 8, 207–217 10.1038/nrmicro2298 [DOI] [PubMed] [Google Scholar]
- Touchon M., Hoede C., Tenaillon O., Barbe V., Baeriswyl S., Bidet P., et al. (2009). Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 5:e1000344 10.1371/journal.pgen.1000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elsas J. D., Semenov A. V., Costa R., Trevors J. T. (2011). Survival of Escherichia coli in the environment: fundamental and public health aspects. ISME J. 5, 173–183 10.1038/ismej.2010.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman K., Van Tulden P., Kant A., Testerink J., Mevius D. (2013). Characteristics of cefotaxime resistant E. coli from wild birds in The Netherlands. Appl. Environ. Microbio. 79, 7556–7561 10.1128/AEM.01880-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versalovic J., Koeuth T., Lupski J. R. (1991). Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19, 6823–6831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. A., Mciver C. J., Rawlinson W. D. (2001). Integrons and gene cassettes in the enterobacteriaceae. Antimicrob. Agents Chemother. 45, 2658–2661 10.1128/AAC.45.9.2658-2661.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]