Abstract
Few studies have focused on sepsis in patients with preexisting immunosuppression. Since their numbers and the incidence of sepsis are increasing, sepsis in immunosuppressed patients will increase in importance. We studied the epidemiology of sepsis and risk factors for 28-day mortality in patients immunosuppressed prior to the onset of sepsis using data from the Academic Medical Center Consortium’s (AMCC) prospective observational cohort study of sepsis. We compared characteristics of immunosuppressed (N=412) and immunocompetent (N=754) patients. Immunosuppressed patients were younger and more likely to have underlying liver or lung disease, and nosocomial infection or blood stream infection of unknown source when presenting with sepsis. They were also more likely to die within 28 days compared to immunocompetent patients (adjusted relative risk 1.62, 95% CI 1.38–1.91). Septic shock, hypothermia, cancer and invasive fungal infections were associated with increased mortality in immunosuppressed patients. Black race and the presence of rigors were independent predictors of survival in immunosuppressed patients. We conclude that sepsis among patients immunosuppressed prior to the onset of sepsis was associated with higher mortality than in immunocompetent patients. As the numbers of immunosuppressed patients continues to grow, more studies on the epidemiology of sepsis in this group will become increasingly important.
INTRODUCTION
The incidence of sepsis has almost tripled over the past several decades [1, 2]. Over this same time period, the number of immunosuppressed persons has also risen [3]. These combined trends are likely to lead to increased public health importance of sepsis in patients with preexisting immunosuppression. However, there is little information in the literature regarding the presentation and outcomes of sepsis in this population.
Outcomes after sepsis are influenced by numerous host and pathogen factors [4] such as comorbidities, development of shock, multiple organ failure, blood stream infections with specific organisms, the site of infection, nosocomial versus community acquisition of infection and the onset of immune suppression resulting from a dysregulation of the immune system in response to the inciting infection [4–10]. Specific gene polymorphisms and variations of cytokine production have also been associated with mortality after sepsis [11, 12] The role of immunosuppression predating the onset of sepsis in modifying risk factors has not been adequately investigated.
We studied the epidemiology of sepsis in patients immunosuppressed prior to the onset of sepsis by (1) comparing the presentation and mortality of critically ill sepsis patients who were and were not immunocompetent, and (2) examining potential factors associated with mortality in immunosuppressed patients.
METHODS
We used data from the AMCC observational cohort study of the sepsis syndrome conducted between January 1993 and April 1994 in eight academic medical centers. Detailed methodology on the construction of the cohort, definitions and data collection has been described [13].
Study Population
The study population consisted of patients meeting the definition of severe sepsis syndrome with or without septic shock as defined by the AMCC (Table I) [13], which is slightly modified from the definition of others [2]. Patients with systemic inflammatory response syndrome with or without evidence of clinical infection or with blood stream infection, who did not have evidence of organ dysfunction or hypoperfusion as shown in the confirmatory criteria in Table I, were excluded. In this study, we evaluated outcomes in patients after their first episode of severe sepsis. From here on in, the term sepsis will denote severe sepsis. There was no uniform administration of steroids or tight glucose control as supportive care in this study. The study was approved by the institutional review boards of the original AMCC study centers and was conducted in accordance with the 1983 modification of the Declaration of Helsinki.
Table I.
Criteria for Sepsis Syndrome
| Screening Criteria | Confirmatory Criteria |
|---|---|
| Presence of either (1) or (2): | Presence of any 1 of the following, without an alternative explanation: |
| 1. All 4 of the following: | 1. PaO2/FiO2 < 280 (intubated) or when using a 40% face mask (not intubated) |
| Rectal temperature >38.3°C or <35.6°C | 2. Arterial pH < 7.30 |
| Respiratory rate of >20/min or mechanical ventilation |
3. Urine output < 30 ml/hour |
| Heart rate > 90 beats/min | 4. Systolic blood pressure < 90 mm Hg or fall in systolic blood pressure > 40 mm Hg sustained for 2 hours despite fluid challenge |
| Clinical evidence of infection | 5. Systemic vascular resistance < 800 dynes.s.cm2 |
| 2. One or more blood cultures positive for a pathogen at 48 hours after the onset of sepsis |
6. Prothrombin time or partial thromboplastin time greater than normal or platelets > 100×106/l or platelets decreased to <50% of most recent measurement before current day |
| 7. Deterioration in mental status within 24 hours | |
Definitions
Definitions for acute respiratory distress syndrome and acute renal failure have been described [13]. Blood stream infection was defined as the isolation of a known pathogen such as Staphylococcus aureus, a Gram negative bacillus or yeast from one or more blood cultures. If common skin colonizers, such as coagulase-negative Staphylococcus or viridans group of Streptococcus were isolated, two positive blood culture sets were required to designate the episode as a blood stream infection. Preexisting mmunosuppression was broadly defined as patients with human immunodeficiency virus, hematological or solid cancer, solid organ or hematopoietic stem cell transplantation, neutropenia (total neutrophil count <500/mm3), or receiving immunosuppressive medications (corticosteroids within the prior week, myelosuppressive agents including chemotherapy, and/or immunosuppressive agents active against T lymphocytes within the prior month). Hypothermia was defined as a rectal temperature less than 35.6°C. Rigors were defined as shaking chills observed by a physician and recorded in the medical record within 48 hours prior to the onset of sepsis.
Data Collection
The original prespecified demographic, historical, clinical and laboratory data elements were abstracted from the medical record in the 24 hours before until 6 hours after onset of sepsis. Results of blood cultures available 24 hours prior to 48 hours after the onset of sepsis were collected. Vital status was recorded at hospital discharge if prior to 28 days, and determined by telephone contact at 28 days. Study personnel were trained on standardized data collection procedures prior to the start of the study. Regular telephone conferences were used to ensure the consistency of the data collection process.
Study Variables
Prior to model construction, we selected the following epidemiologically and clinically relevant variables from the original dataset for analysis: age, gender, race, insurance status, vital signs, and laboratory values such as total white blood cell count, creatinine, and hematocrit. APACHE II scores were derived by the original AMCC study group using variables in the original dataset [14]. We also derived prespecified variables from the original data for this analysis. These included the presence or absence of comorbid conditions, or events in the 24 hours prior to or at the onset of sepsis. Self-described race data was collected because of racial differences in the incidence and outcomes of sepsis [1, 15]. Race was categorized as a binary variable (black, non-black). We reviewed positive blood cultures to determine if they arose from an identified source (same organism isolated from blood and another site, or an organism isolated from blood and a clinically consistent but not microbiologically documented site of infection). If no source could be identified, the blood stream infection was said to have an unknown source. Infections were classified according to the microorganism isolated (Gram positive, Gram negative or fungal) as well as if due to Staphylococcus aureus or Pseudomonas aeruginosa.
Statistics
Continuous variables were summarized as medians and interquartile ranges. We used t-tests for continuous variables with normally distributed observations and Wilcoxon rank sum tests for continuous variables with nonparametric distributions. Chi square tests were used to compare categorical variables between groups. For Kaplan-Meier analysis, the log-rank test was used to compare strata.
For model construction, the primary outcome was mortality within 28 days after the onset of sepsis. Using univariate logistic regression, we examined the following potential risk factors for association with mortality: demographic data, past or current comorbidities, laboratory data collected from the 24 hours prior to the 6 hours after the onset of sepsis, and information on the presenting infection (blood and other culture data, site(s) of infection, organism(s), nosocomial infection). In this initial step in the construction of the statistical models, unadjusted odds ratios and 95% confidence intervals were reported.
Variables were entered into the multivariable logistic regression models using forward selection processes, where the criterion for entry was a coefficient with p<0.10, and for staying in the model, p=0.05. From the resulting models, terms were created to test for interactions. Tests of goodness of fit, comparing predicted with observed mortality, were performed using the Lemeshow Hosmer method [16]. Collinearity of variables was assessed by calculating the tolerance and variable inflation factor [17, 18]. Model performance was tested by the construction of receiver-operator characteristic (ROC) curves [19]. After logistic regression model diagnostics, variables with coefficients with p<0.10 in the multivariable logistic regression models were analyzed via generalized estimating equations using a Poisson distribution to control for clustering within center and obtain adjusted relative risks, rather than odds ratios, since the outcome of interest, death at 28 days, was common. [20, 21;22]. In the final models, coefficients with p<0.05 were considered statistically significant. There was no attempt to correct for multiple testing.
Missing data were assessed for association with medical centers and risk factors for mortality. Imputation of missing data was not performed.
All analysis was done utilizing SAS, Version 9.1, (Cary, NC).
RESULTS
Of the 1166 patients included in the original AMCC Sepsis cohort, there were 412 (35%) patients with preexisting immunosuppression. Thirty four patients in the entire cohort had missing 28 day vital status. Of the 694 patients who survived, 287 had been discharged prior to day 28 and were contacted by telephone to verify vital status. Of the 438 patients who died by day 28, 18 died after being discharged alive from the hospital prior to day 28. Nine patients in the immunosuppressed group had missing 28 day vital status and were excluded from mortality analyses. By day 28, 205 of 403 (51%) of patients had died. Of these, 5 had been discharged alive and died by day 28. Of those who survived to day 28, 119 were still hospitalized by day 28. An additional 19 patients had incomplete data records for explanatory variables. The final 28 day mortality analyses included 384 patients with complete data records.
Comparisons between Patients With and Without Preexisting Immunosuppression
Persons with preexisting immunosuppression were younger, had more underlying liver and lung disease, and more likely to receive mechanical ventilation and nutritional supplementation (Table II). They had an increased severity of disease, as indicated by higher APACHE II scores. Immunosuppressed patients were less likely to have undergone surgery or to have had a myocardial infarction in the 24 hours prior to the onset of sepsis and more likely to have do not resuscitate orders in effect. At the presentation of sepsis, they had higher heart rates and lower white blood cell counts and hematocrits than did patients without preexisting immunosuppression. Nosocomial infection and blood stream infection with unknown source were more common in immunosuppressed patients (Table II). There was a trend toward an increased incidence of septic shock in immunosuppressed patients. The two groups did not differ with respect to gender, renal disease, the use of intravascular catheters, the occurrence of cardiopulmonary arrest or ARDS, minimal systolic blood pressure or the use of vasopressor agents, or the presence of any Staphylococcus aureus, Pseudomonas aeruginosa or invasive fungal infection (data not shown). A higher proportion of patients with preexisting immunosuppression died within the 28 days after the onset of sepsis compared to those who were immunocompetent (p<0.0001, Figure 1). The adjusted relative risk of dying in the immunosuppressed group was 1.62 (95% CI 1.38–1.91, p<0.0001). When adjusted only for do not resuscitate and do not treat status, the likelihood of dying within 28 days of the onset of sepsis was significantly higher in the immunosuppressed group (RR 1.51, 95% CI 1.32–1.72, p<0.0001).
Table II.
Characteristics of Immunocompetent Patients and Patients Immunosuppressed Prior to the Onset of Sepsis
| CHARACTERISTIC | # missing |
STATUS | |
|---|---|---|---|
| Immunocompetent N= 754 (%) |
Immunosuppressed N=412 (%) |
||
| Age (years, median and IQR)a | 0 | 64 (50, 74) | 55 (41, 68)**** |
| Death within 4 days | 0 | 108 (14.3) | 100 (24.3)**** |
| Death within 28 days | 34 | 233 (32.0) | 205 (50.9)**** |
| Discharged alive within 28 days and survived | 327 (44.9) | 122 (30.3)**** | |
| Discharged alive but died within 28 days | 10 (1.4) | 4 (1.0) | |
| Do Not Resuscitate Status | 7 | 67 (8.9) | 56 (13.7)** |
| Do Not Treat Status | 2 | 3 (0.4) | 4 (1.0) |
| Black race | 8 | 98 (13.1) | 71 (17.2) |
| Insurance status | |||
| None/self-pay | 116 | 70 (10.4) | 24 (6.4)** |
| Government source | 364 (54.1) | 181 (48.0) | |
| HMO/private | 239 (35.5) | 172 (45.6) | |
| Septic shock | 73 | 463 (65.7) | 276 (71.1) |
| Liver disease | 8 | 47 (6.3) | 52 (12.7)** |
| Lung Disease | 23 | 36 (4.9) | 41 (10.3)*** |
| Diabetes mellitus | 4 | 132 (17.6) | 55 (13.4) |
| Surgery within 24 hours of onset | 0 | 199 (23.4) | 68 (16.5)**** |
| Trauma | 4 | 51 (6.8) | 8 (2.0)*** |
| Use of Nutritional Supplements | 2 | 344 (45.7) | 234 (56.8)*** |
| Myocardial infarction | 5 | 177 (23.6) | 51 (12.4)**** |
| Cardiogenic shock | 41 | 25 (3.4) | 6 (1.5) |
| Ventilation before or at onset | 13 | 437 (58.9) | 200 (48.7)*** |
| Maximual heart rate (beats/min, median, IQR) | 18 | 122 (108, 140 ) | 130 (116, 148)**** |
| Maximum temperature (degrees Centigrade) | 20 | 38.9 (38.5, 39.3) | 39.0 (38.4, 39.6)* |
| Maximum WBC (x10,000/mm3) | 33 | 14.2 (9.8, 20.1) | 10.2 (3.5, 16.7)**** |
| Minimum hematocrit (%) | 28 | 30.0 (26.7), 35.5) | 26.0 (25.0, 31.7)**** |
| Apache II Score | 0 | 19.0 (15.0, 25.0) | 23.0 (19.0, 29.0)**** |
| Nosocomial infection | 1 | 434 (57.6) | 275 (66.7)** |
| Site of presenting infection* | |||
| unknown | 36 (4.8) | 7 (1.7) | |
| skin | 44 (5.8) | 18 (4.4) | |
| device | 34 (4.5) | 22 (5.3) | |
| respiratory tract | 315 (41.8) | 179 (43.5) | |
| urinary tract | 0 | 76 (10.1) | 36 (8.7) |
| central nervous system | 16 (2.1) | 12 (2.9) | |
| abdominal | 85 (11.3) | 34 (8.3) | |
| blood stream, unknown source | 113 (15.0) | 81 (19.7) | |
| other | 35 (4.6) | 23 (5.6) | |
| BSI of unknown source | 0 | 89 (11.8) | 71 (17.2)** |
| Any GPC infection | 0 | 294 (39.0) | 139 (33.7) |
Abbreviations: IQR, interquartile range; HMO, health maintenance organization; WBC, white blood cell count; BSI, blood stream infection; GPC, Gram positive coccus.
T-tests for continuous variables and chi square tests for categorical variables.
Levels of significance:
<0.05,
≤0.01,
≤0.001,
≤0.0001.
Figure 1. 28 Day Survival of Immunocompetent Patients and Patients with Preexisting Immunosuppression.
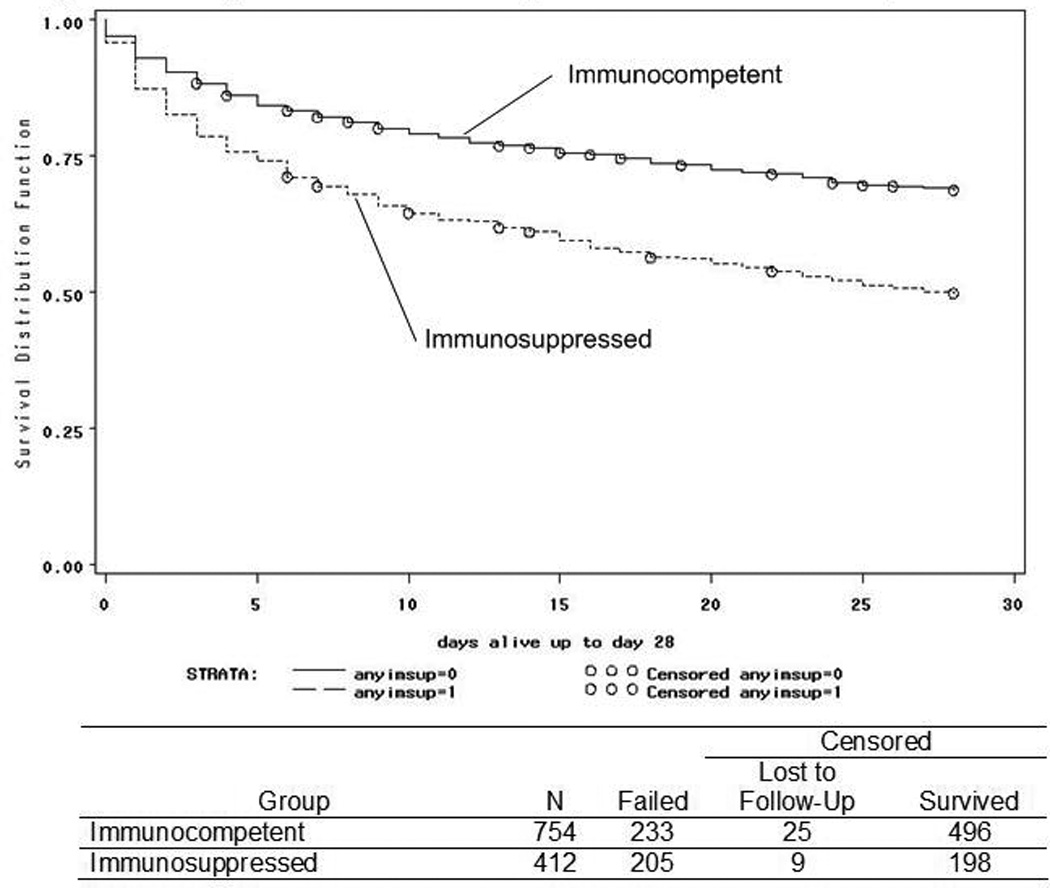
Solid line -- immunocompetent; broken line -- immunosuppressed (p<0.0001, log-rank test). X-axis -- days after the onset of sepsis; Y-axis -- proportion surviving. Open circles -- censoring events (loss to follow-up before day 28 or survival beyond day 28).
Univariate Analysis of Risk Factors for 28 Day Mortality in Immunosuppressed Patients
The type of preexisting immunosuppression in the immunosuppressed patients is displayed in Table III. In some instances, there are fewer than 403 subjects analyzed for a given variable because of missing data. There were no statistically significant differences in the types of preexisting immunosuppression between immunosuppressed patients who lived or died.
Table III.
Characteristics of Immunosuppression at the Onset of Sepsis
| Characteristic | # missing |
Outcome at 28 Days | Odds Ratio (95% CI) |
|
|---|---|---|---|---|
| Alive (%) N=198 |
Dead (%) N=205 |
|||
| Cancera | 0 | 74 (37.4) | 99 (48.3) | 1.57 (1.05. 2.33)* |
| HIV infectionb | 0 | 30 (15.2) | 24 (11.7) | 0.74 (0.42, 1.32) |
| Transplantationc | ||||
| Liverd | 9 (4.5) | 12 (5.9) | 1.33 (0.55, 3.25) | |
| Kidney | 0 | 5 (2.5) | 7 (3.4) | 1.40 (0.44, 4.50) |
| Heart | 4 (2.0) | 0 (0) | -- | |
| HSCTe | 16 (8.1) | 24 (11.7) | 1.50 (0.77, 2.93) | |
| Other | 4 (2.0) | 2 (1.0) | 0.50 (0.09, 2.77) | |
| Any immunosuppressive therapyf | 0 | 158 (79.8) | 168 (82.0) | 1.15 (0.70, 1.89) |
| Any corticosteroid agentsg | 2 | 123 (62.1) | 135 (65.9) | 1.18 (0.78, 1.77) |
| Any myelosuppressive agentsh | 1 | 60 (30.3) | 75 (36.6) | 1.32 (0.87, 2.00) |
| Any T lymphocyte suppressioni | 2 | 16 (8.1) | 29 (14.1) | 1.85 (0.97, 3.53) |
| Neutropeniaj | 0 | 6 (3.0) | 4 (2.0) | 0.64 (0.18, 2.29) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency; HSCT, hematopoietic stem cell transplantation.
Nine patients did not have mortality data and are excluded from this analysis.
Reference group is no cancer.
Includes patients with and without acquired immunodeficiency syndrome (AIDS). Of these, 51 had AIDS, 24 (47%) of whom died.
Reference group is no transplantation.
One patient who had a combined liver-kidney transplantation is included in the liver transplantation group for analysis.
Includes allogeneic (n=19, 3 who lived and 16 who died) and autologous (n=21, 18 who lived and 3 who died) hematopoeitic stem cell transplantation (HSCT).
Within the month prior to onset of sepsis.
Within the week prior to the onset of sepsis.
Patients frequently received more than one agent. The most common agents utilized, followed by the number of patients receiving each agent in parentheses, were: cyclophosphamide (34), cytosine arabinoside (26), azathiaprine (15), methotrexate (14), cisplatinum (9), daunorubicin (8), vincristine (8), hydroxyurea (6), 5-fluorouracil (5) and VP-16 (5). Not all agents administered are listed.
Thirty-seven patients received cyclosporine A, 25 of whom died; 3 received tacrolimus, 1 of whom died; and 5 received antithymocyte globulin, 3 of whom died.
Absolute neutrophil count of ≤ 500 cells/mm3.
Levels of significance:
<0.05.
In the unadjusted analysis, there were several factors that predicted 28 day mortality in patients immunosuppressed prior to the onset of sepsis (Table IV). Factors that were not associated with death at 28 days included gender, insurance status, lung disease, diabetes mellitus, myocardial infarction, adult respiratory distress syndrome, surgery or trauma with the 24 hours prior to the onset of sepsis, and the use of intravascular catheters or nutritional supplements (data not shown). Features of the presenting infection, except for fungal infection, were not associated with death at 28 days (data not shown).
Table IV.
Univariate Analysis of Factors Associated with Death at 28 Days after the Onset of Sepsis in Patients with Preexisting Immunosuppression
| # missing |
Outcome at 28 Days | ODDS RATIO (95% CI) |
||
|---|---|---|---|---|
| Alive (%) N=198 |
Dead (%) N=205 |
|||
| Age (median years, IQR) | 0 | 52.5 (39.0, 66.0) | 56.0 (42.0, 70.0) | 1.02 (0.99, 1.02) |
| Black race | 1 | 43.0 (21.7) | 26 (12.7) | 0.53 (0.31, 0.90)* |
| Septic shock | 23 | 115 (62.1) | 153 (78.5) | 2.22 (1.41, 3.49)*** |
| Preexisting Liver disease | 2 | 16 (8.1) | 35 (17.1) | 2.37 (1.27, 4.44)** |
| Preexisting Kidney disease | 1 | 13 (6.6) | 25 (12.2) | 1.99 (0.99, 4.01)* |
| Cardiopulmonary arrest | 0 | 3 (1.5) | 26 (12.7) | 9.44 (2.81, 31.7)*** |
| Acute renal failure | 4 | 14 (7.1) | 29 (14.1) | 2.17 (1.11, 4.24)* |
| Rigors | 1 | 54 (27.3) | 26 (12.7) | 0.39 (0.23, 0.65)*** |
| Use of vasopressors | 0 | 42 (21.2) | 70 (34.1) | 1.93 (1.23, 3.01)** |
| Ventilation before or at onset |
1 | 77 (38.9) | 119 (58.0) | 2.20 (1.48, 3.28)**** |
| Refractory hemorrhage | 0 | 1 (0.5) | 7 (3.4) | 6.96 (0.85, 57.0) |
| Invasive fungal infection | 0 | 21 (10.6) | 42 (20.5) | 2.17 (1.23, 3.82)** |
| Maximal HR (beats/min)a | 4 | 129 (108, 141) | 135 (120, 151) | 1.02 (1.01, 1.02)*** |
| Minimal SBPb (mm Hg) | 4 | 88 (76, 106) | 80 ( 70, 100) | 0.84 (0.77, 0.93)*** |
| Maximal temperature (°C) | 4 | 39.2 (38.6, 39.7) | 38.8 (38.2, 39.5) | 0.71 (0.58, 0.86)*** |
| Maximal creatinine (mg/dl) | 9 | 1.2 (0.9, 1.8) | 1.4 (1.0, 2.4) | 1.13 (0.99, 1.30) |
| Maximal WBCc (x103/mm3) | 8 | 9.7 (3.2, 15.6) | 10.4 (3.7, 19.1) | 1.02 (1.01, 1.04) |
| Minimal hematocrit (%) | 6 | 28.2 (25.3, 32.8) | 27.0 (24.2, 30.9) | 0.96 (0.93, 0.99) |
Abbreviations: CI, confidence interval; IQR, interquartile range; HR, heart rate; SBP, systolic blood pressure; WBC, white blood cell count; OR, odds ratio.
OR for death for every 10 beat/minute increase in maximal heart rate.
OR for death for every 10 mm Hg rise in minimal systolic blood pressure.
OR for death for every 1000 cells/mm3 increase in maximal white blood cell count.
Levels of significance:
<0.05,
≤0.01,
≤0.001,
≤0.0001.
Multivariable Analysis of Risk Factors for 28 Day Mortality in Patients with Preexisting Immunosuppression
The following variables were entered into the multivariable logistic regression model: age, race, the presence of preexisting liver disease, rigors, mechanical ventilation at the onset of sepsis, cardiopulmonary arrest, septic shock, vital signs at the onset of sepsis (except for minimal systolic blood pressure, which was a component of the definition of septic shock), maximal creatinine, maximal white blood cell count, minimal hematocrit, the presence of hematologic or solid cancer, and the presence of a fungal infection. There were no statistically significant interactions between variables entered into the model. There was no collinearity between any combination of variables. The Receiver-Operator Characteristic (ROC) curve for 28 day mortality had an area under the curve of 0.77. There was no statistically significant difference between the observed and predicted mortalities (p=0.93).
Variables entered into the final model utilizing the generalized estimating equation that adjusted for clustering within centers were race, liver disease, cardiopulmonary arrest, rigors, maximal heart rate, maximal temperature, maximal creatinine, maximal white blood cell count, septic shock, the presence of cancer and the presence of an invasive fungal infection.
Independent predictors of death within 28 days for patients with preexisting immunosuppression and sepsis were cardiopulmonary arrest, maximal heart rate, maximal creatinine, maximal white blood cell count, the presence of cancer, septic shock and the presence of an invasive fungal infection (Figure 2). A moderate effect of clustering within centers was noted since liver disease no longer was significantly predictive of death as it had been in the multivariable logistic regression model (data not shown). Factors independently predicting survival were black race (adjusted RR, 0.63; 95% CI 0.52–0.76), rigors and an increase in each degree Celsius of the maximal temperature.
Figure 2. Multivariable Analysis of Predictors of Death at 28 days in Patients with Preexisting Immunosuppression.
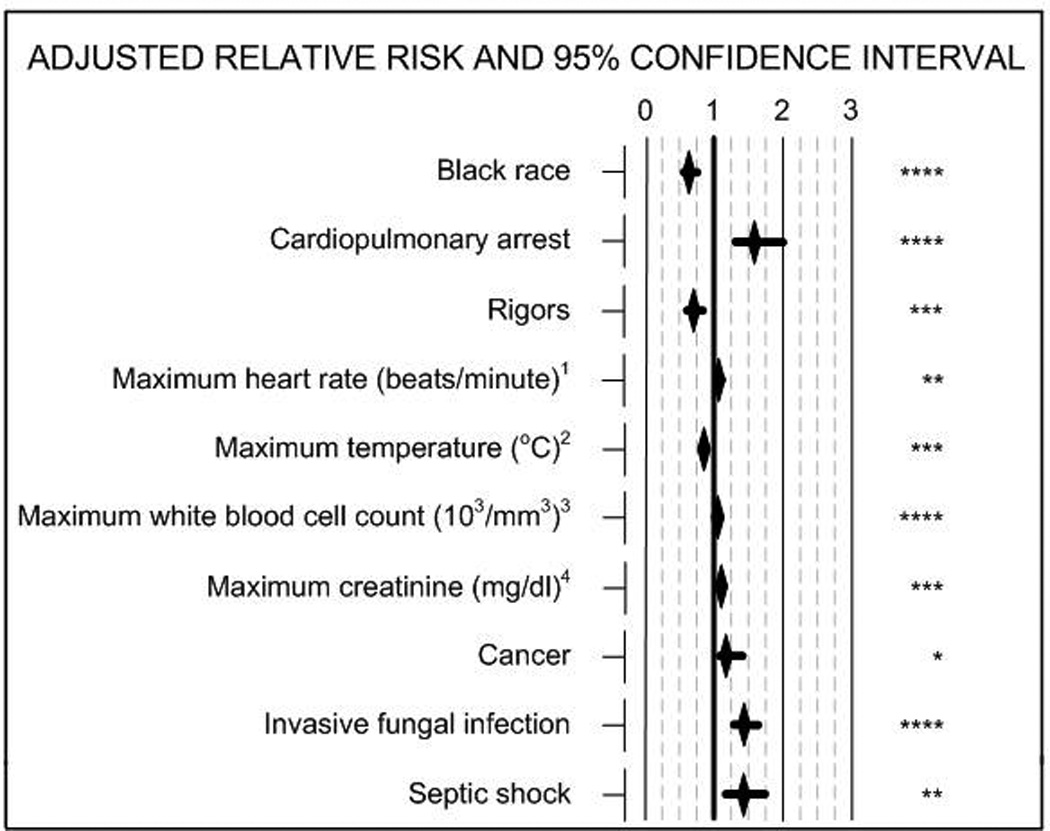
1Adjusted relative risk (RR) for death for every increase in heart rate of 10 beats/minute; 2RR for death for every increase in one degree Centigrade; 3RR for death for every increase of 1000 cell/mm3; 4RR for death for every increase in creatinine of 1 mg/dl. Asterisks indicate levels of significance: * <0.05, ** ≤0.01, *** ≤0.001, **** ≤0.0001.
To further investigate the association between the presence of rigors and increased survival, we examined the relationship between rigors and hypothermia. In the immunosuppressed group, 51 patients were hypothermic and of them, 4 (7.8%) experienced rigors, compared to 78 of the 360 patients who were not hypothermic (21.7%, p=0.02). We created a second model that substituted the binary variable hypothermia for the continuous variable, maximal temperature. Hypothermia was a strong independent predictor of death (adjusted RR 5.7, 95% CI 2.2–14.3, p=0.0002). In this secondary model, the presence of rigors was no longer a predictor of survival whereas the need for mechanical ventilation predicted death (adjusted RR 1.9, 95% CI 1.2–3.0, p=0.009). Otherwise, the two models were very similar (data not shown).
Race as an Independent Predictor of 28 Day Mortality
The finding of the association between black race and a decreased risk of death in patients with preexisting immunosuppression was unexpected since this association was not observed in the total cohort. Therefore, we examined this further in secondary analyses. Of the 384 patients with complete data records, 274 were white (71.4%), 64 were black (16.7%), 28 were Latinos (7.3%) and 18 were from other ancestral groups (4.7%). The percentage of black patients within the immunosuppressed group varied from 0% to 53% among the 8 study centers. Of 320 non-black patients, 170 (53.1%) died by 28 days, compared to 22 of 64 (34.4%) black patients. Blacks and non-blacks in the immunosuppressed cohort differed in many characteristics (Table V). Each of the potential confounders that was or trended toward a statistically significant difference between blacks and non blacks was forced into the final model that adjusted for clustering within center. The effect of T lymphocyte suppression was not tested as this applied to only 2 black patients. There was no substantial impact of the forcing of any of these variables on the association of race and reduced mortality (data not shown).
Table V.
Comparison between Black and Non-Black Patients Immunosuppressed Prior to the Onset of Sepsis
| Characteristic | # missinga |
Racial Group | |
| Black (%) N=71 |
Non-black (%) N=340 |
||
| Age | 0 | 47 (36,61)b | 56 (42, 69)*** |
| Male | 0 | 38 (53.5) | 186 (54.7) |
| Insurance Status | |||
| None/self pay | 5 (7.9) | 19 (6.0) | |
| Government source | 35 | 34 (54.0) | 147 (47.0) |
| HMO/private | 24 (38.1) | 147 (47.0) | |
| Liver disease | 2 | 5 (7.1) | 47 (13.9) |
| Lung disease | 12 | 8 (11.6) | 33 (9.7) |
| Renal disease | 1 | 13 (18.6) | 27 (7.9)** |
| Diabetes mellitus | 1 | 14 (19.7) | 40 (11.8) |
| Cancerc | 0 | 17 (23.9) | 159 (46.8)**** |
| HIV infectiond | 0 | 27 (38.0) | 29 (8.5)**** |
| Any transplantatione | 1 | 7 (9.9) | 77 (22.7)** |
| Any immunosuppressive therapy | 0 | 47 (66.2) | 224 (84.1)*** |
| Any corticosteroid agents | 2 | 40 (57.1) | 224 (66.1) |
| Any myelosuppressive agents | 1 | 12 (17.4) | 127 (37.4)**** |
| Any T lymphocyte suppression | 2 | 2 (2.9) | 33 (13.0)** |
| Neutropenia | 0 | 1 (1.4) | 8 (2.4) |
Abbreviations: HMO, health maintenance organization; HIV, human immunodeficiency virus.
One patient out of 412 did not have data on race. Therefore, the number missing refers to the number of subjects out of 411 with complete race data.
Median (interquartile range)
Of black patients, 9 (12.7%) had hematological and 8 (11.3%) had nonhematological malignancies. Of non-black patients, 87 (25.6%) had hematological and 72 (21.2%) had nonhematological malignancies.
Twenty five black patients and twenty eight non-black patients had AIDS (p=0.60).
For black patients, the types of transplantations were liver 1, kidney 5, heart 0, hematopoietic stem cell transplantation 1, other 0. For nonblack patients, the types of transplantations were liver 21 (including one patient who also had another transplantation), kidney 8, heart 5, hematopoietic stem cell transplantation 39, other 6.
Levels of significance:
<0.05,
≤0.01,
≤0.001,
≤0.0001.
Sensitivity Analysis of Race as a Predictor of 28 Day Mortality
To determine the robustness of the effect of race on 28 day mortality, a sensitivity dataset was created by randomly reassigning the vital status of varying proportions of black patients who survived to 28 days to having died. The effect of black race on 28 day mortality was then reassessed with the sensitivity dataset. Of the 64 black immunosuppressed patients in the analytical set, 22 died within 28 days. If as few as 3 of 42 (7%) patients who survived were reassigned to having died within 28 days, the effect of black race on mortality in the multivariable model adjusted for clustering within centers was not statistically significant although the coefficient indicated reduced mortality (adjusted RR 0.61, 95% CI 0.53,1.06, p=0.11).
DISCUSSION
In this study, persons immunosuppressed prior to the onset of sepsis differed from immunocompetent patients with sepsis in that they were younger, had underlying liver and lung disease, experienced septic shock and more often required nutritional supplementation and mechanical ventilation. In addition, they had a higher incidence of nosocomial infection as the presenting cause of sepsis and blood stream infection without a known source. Finally, they were less likely to survive within the 28 days after the onset of sepsis.
Factors with strong independent associations with death within 28 days after the onset of sepsis in patients with preexisting immunosuppression included an elevation in heart rate, white blood cell count and creatinine, septic shock, hypothermia and invasive fungal infection. Factors independently associated with survival at 28 days included black race, the presence of rigors and higher maximal temperatures.
An unanticipated finding was the association between black race and survival after sepsis in patients with preexisting immunosuppression. This effect was preserved when adjusting for potential confounders including HIV infection, which was present in proportionately more black patients than non-black patients. In both groups, most patients with HIV infection had AIDS. This study was performed before the availability of highly active anti-retroviral therapy therefore the association between black race and survival cannot be ascribed to effective treatment of the underlying immunosuppressive condition (AIDS) in a higher proportion of blacks compared to non-blacks. The effect of race on 28 day mortality was not robust in that the reassignment of the vital status of as few as 3 black patients who lived to having died at 28 days eliminated the statistical significance of the finding. However, the number of black immunosuppressed patients in the analytical sample who died by 28 days (22 of 384, 5.7%) was relatively small, limiting the utility of the sensitivity analysis.
Differences in the epidemiology of sepsis according to race have been noted before. Blacks had a higher incidence of sepsis, with a mean annual relative risk of sepsis almost twice that of whites [1, 15]. However, others have not found differences in mortality associated with sepsis by racial group [1, 2, 17–19].
Explanations for racial disparities in health and illness have been elusive but may be due to variations in comorbidities and educational level [23, 24]. In this study, insurance status, the only surrogate for socioeconomic status, was not related to mortality after sepsis. A similar conclusion was reported in a study of all patients admitted to the ICU, not just those with sepsis [25]. Other evidence for racial disparities in health care delivery have been noted in a study of short and long-term survival of elderly black and white patients after admission for 6 specific acute illnesses, and not specifically including sepsis. Mortality at 30 days was lower for black compared to white patients but 2-year mortality for blacks was higher than for whites except for the primary diagnosis of congestive heart failure [26]. The authors suggest that disparities in care occur after the initial hospitalization for an acute illness.
Certain genetic polymorphisms are associated with the incidence of and mortality associated with sepsis [11]. Racial differences in the distribution of cytokine and other gene polymorphisms involved in immunity have been demonstrated [24–26]. Finally, polymorphisms in the promoter for CD14 are found at a higher frequency in white patients with septic shock than those without septic shock [27]. Patients with sepsis who possessed the homozygous genotype for the T allele in the CD14 promoter region had a greater than 5 fold increased odds of death. Interestingly, the T allele appears to be present in 50% of European Americans but in only 28% of African Americans [28]. This raises the question of whether the observed effect of black race against mortality in our patients with preexisting immunosuppression and sepsis might be related to a decreased frequency of the T allele in blacks. However, to date, no specific gene polymorphisms that vary by racial group have been associated with mortality after sepsis.
Another interesting finding is the association between the presence of rigors and survival of patients with preexisting immunosuppression and severe sepsis syndrome. One potential explanation for this is that patients who experienced rigors were recognized by their physicians as having sepsis sooner than were patients without rigors, with more prompt institution of antimicrobial therapy and supportive care. Another explanation is that patients without rigors had an impaired thermal response to infection, as is suggested by the increased likelihood that patients who were hypothermic were less likely to experience rigors. Hypothermia and the absence of rigors have been associated with decreased survival after sepsis by others [29, 30].
Preexisting immunosuppression was an independent predictor of early (3 day) mortality but not 28 day mortality in septic patients [31]. In another study, preexisting immunosuppression was a risk factor for sepsis and death (with or without sepsis) after trauma, or for in-hospital death in patients admitted to the ICU, without regard to whether or not they had sepsis [32]. However, these studies did not examine risk factors for death in septic immunosuppressed patients. A number of other studies on the epidemiology of and risk factors for sepsis do not address preexisting immunosuppression at all or as a discreet variable outside of a severity of illness score [31–35]. Finally, studies on interventions in sepsis or on the construction of models predicting mortality after sepsis frequently include no or very few patients with immunosuppression prior to the onset of sepsis [36–39]. Thus, there is little known about the epidemiology of sepsis in these patients.
This study has several limitations. It does not account for the occurrence of the endogenous immunosuppressive state observed to occur during the response to sepsis [6–10]. Soon after the onset of sepsis and in response to the resultant pro-inflammatory immune response, there is a compensatory anti-inflammatory response, characterized by soluble mediators such as interleukin-10, interleukin-1 receptor antagonist and soluble tumor necrosis factor receptor, suppressive regulatory T cell networks, the involvement of intracellular signaling pathways and the dampening of monocyte activation [6–8, 33]. In some, dysregulation of the feedback loop results in a deepening of immune suppression translating into an increased propensity for nosocomial infection and perhaps increased late mortality [6, 8, 33]. In the future, monitoring for an endogenous immunosuppressive state associated with sepsis such as measuring HLA-DR expression on monocytes, levels of anti-inflammatory cytokines, and type 1 T cell responses, might aid in the characterization of the immune state and allow targeted immunotherapy [9] . Potential immunotherapies under investigation include granulocyte/monocyte-colony stimulating factor, the removal of pro-inflammatory mediators and intravenous gamma globulin [34–36]. This study was designed to investigate factors that were preexisting or present in the period immediately surrounding the onset of sepsis and therefore likely before the onset of endogenous immunosuppression. Nonetheless, endogenous immunosuppression is a potential factor in predicting 28 day mortality after the onset of sepsis study, and was not assessed in this study.
The cohort is more than 10 years old and since it was assembled, there have been changes in intensive care, transplantation, HIV and general infectious disease epidemiology and medicine. For instance, certain elements of management of the critically ill patient that are currently recommended [37] were not part of ICU care at the time data collection for this study occurred. These include the use of activated protein C, use of high dose steroids in some patients with septic shock, low tidal volume ventilation, tight glycemic control, and the early administration of antibiotics for community acquired pneumonia, although several of these are controversial [38–41]. The effects of early goal directed therapy in sepsis have not been studied in the population with preexisting immunosuppression and therefore, it is difficult to conjecture the impact this therapy would have. For instance, the use of high dose steroids might have little impact since many immunosuppressed patients, such as transplantation recipients, are already receiving steroid therapy upon presentation. However, since the early interventions noted above do not reverse the immunosuppression that predated the onset of sepsis, we suspect that their impact would be no greater, and perhaps less, than the impact in immunocompetent patients. Therefore, validation of early goal directed therapy in this population would be important.
We conclude that sepsis in patients with preexisting immunosuppression was associated with a higher mortality than in immunocompetent patients. As the immunosuppressed population is likely to grow, this group merits further investigation. Furthermore, in this study, black immunosuppressed patients fared much better than whites. This finding was unexpected and should be explored further to determine whether this is a true finding or due to an unexamined confounding factor.
ACKNOWLEDGEMENTS
The authors thank Richard Platt, MD, for his insight and helpful suggestions.
Financial Support: National Institutes of Health/National Center for Research Resources (5K23 RR020042-04 to DDP.); Tufts Clinical and Translational Science Institute (1UL1RR025752/1KL2RR025751, DDP); National Institutes of Health/National Center for Complementary and Alternative Medicine (5 K24 AT003683 to PLH).
The study was approved by the institutional review boards of the original AMCC study centers and was conducted in accord with the 1983 modification of the Declaration of Helsinki. Informed consent was obtained from all subjects or their representative.
Footnotes
Potential financial conflicts of interest: No conflicts.
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New England Journal of Medicine. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest; The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine; 1992. Jun, pp. 1644–1655. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease C, Prevention. Epidemiology of HIV/AIDS--United States, 1981–2005. MMWR Morbidity & Mortality Weekly Report. 2006;55(21):589–592. [PubMed] [Google Scholar]
- 4.Angus DC, Wax RS. Epidemiology of sepsis: an update. Critical Care Medicine. 2001;29(7 Suppl) doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 5.Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. erratum appears in Intensive Care Med. 2002 Apr;28(4):525–526. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]; Intensive Care Medicine. 2002 Feb;28(2):108–121. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- 6.Annane D, Bellissant E, Cavaillon JM, Annane D, Bellissant E, Cavaillon J-M. Septic shock. Lancet. 2005 Jan 1–7;365(9453):63–78. doi: 10.1016/S0140-6736(04)17667-8. [Review] [DOI] [PubMed] [Google Scholar]
- 7.Cinel I, Opal SM, Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Critical Care Medicine. 2009 Jan;37(1):291–304. doi: 10.1097/CCM.0b013e31819267fb. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J, Cohen J. The immunopathogenesis of sepsis. Nature. 2002 Dec 19–26;420(6917):885–891. doi: 10.1038/nature01326. [Review] [DOI] [PubMed] [Google Scholar]
- 9.Kox WJ, Volk T, Kox SN, Volk HD. Immunomodulatory therapies in sepsis. Intensive Care Medicine. 2000;26(Suppl 1):S124–S128. doi: 10.1007/s001340051129. [Review] [DOI] [PubMed] [Google Scholar]
- 10.Monneret G, Venet F, Pachot A, Lepape A, Monneret G, Venet F, et al. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Molecular Medicine. 2008 Jan-Feb;14(1–2):64–78. doi: 10.2119/2007-00102.Monneret. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mira JP, Cariou A, Grall F, Delclaux C, Losser MR, Heshmati F, et al. Association of TNF2, a TNF-alpha promoter polymorphism, with septic shock susceptibility and mortality: a multicenter study. Jama. 1999;282(6):561–568. doi: 10.1001/jama.282.6.561. [DOI] [PubMed] [Google Scholar]
- 12.Novotny AR, Emmanuel K, Ulm K, Bartels H, Siewert JR, Weighardt H, et al. Blood interleukin 12 as preoperative predictor of fatal postoperative sepsis after neoadjuvant radiochemotherapy. British Journal of Surgery. 2006 Oct;93(10):1283–1289. doi: 10.1002/bjs.5404. [DOI] [PubMed] [Google Scholar]
- 13.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. Jama. 1997;278(3):234–240. [PubMed] [Google Scholar]
- 14.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical Care Medicine. 1985 Oct;13(10):818–829. [PubMed] [Google Scholar]
- 15.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. American Journal of Respiratory & Critical Care Medicine. 2008;177(3):279–284. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosmer D, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons, Inc; 1989. [Google Scholar]
- 17.Hamilton L. Regression with Graphics. Belmont: Wadsworth, Inc; 1992. Symptoms of Multicollinearity; pp. 133–136. [Google Scholar]
- 18.Kleinbaum D, Kupper L, Muller K, Nizam A. Collinearity. In: Kugushev A, editor. Applied Regression Analysis and Other Multivariable Methods. 3rd ed. Pacific Grove: Duxbury Press; 1998. pp. 237–245. [Google Scholar]
- 19.Centor RM. Signal detectability: the use of ROC curves and their analyses. Medical Decision Making. 1991;11(2):102–106. doi: 10.1177/0272989X9101100205. [DOI] [PubMed] [Google Scholar]
- 20.Horton N, Lipsitz S. Review of software to fit generalized estimating equations. American Statistician. 1999 May;53:160–169. [Google Scholar]
- 21.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 22.McNutt L, Wu C, Xue X, Hafner J. Estimating the relative risk in cohort studies and clinical trials of common outcomes. American Journal of Epidemiology. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 23.Wong DT, Knaus WA. Predicting outcome in critical care: the current status of the APACHE prognostic scoring system. Canadian Journal of Anaesthesia. 1991 Apr;38(3):374–383. doi: 10.1007/BF03007629. [DOI] [PubMed] [Google Scholar]
- 24.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: Factors that influence disparities in sepsis. Critical Care Medicine. 2006;34(10):2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams JF, Zimmerman JE, Wagner DP, Hawkins M, Knaus WA. African-American and white patients admitted to the intensive care unit: is there a difference in therapy and outcome? Critical Care Medicine. 1995 Apr;23(4):626–636. doi: 10.1097/00003246-199504000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Polsky D, Jha AK, Lave J, Pauly MV, Cen L, Klusaritz H, et al. Short- and long-term mortality after an acute illness for elderly whites and blacks. Health Services Research. 2008 Aug;43(4):1388–1402. doi: 10.1111/j.1475-6773.2008.00837.x. [Comparative Study Research Support, Non-U.S Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibot S, Cariou A, Drouet L, Rossignol M, Ripoll L. Association between a genomic polymorphism within the CD14 locus and septic shock susceptibility and mortality rate. Critical Care Medicine. 2002;30(5):969–973. doi: 10.1097/00003246-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Barnes KC. Genetic determinants and ethnic disparities in sepsis-associated acute lung injury. Proceedings of the American Thoracic Society. 2005;2(3):195–201. doi: 10.1513/pats.200502-013AC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. Jama. 1995;274(12):968–974. [PubMed] [Google Scholar]
- 30.Pittet D, Thievent B, Wenzel RP, Li N, Auckenthaler R, Suter PM. Bedside prediction of mortality from bacteremic sepsis. A dynamic analysis of ICU patients. A dynamic analysis of ICU patients. American Journal of Respiratory & Critical Care Medicine. 1996;153(2):684–693. doi: 10.1164/ajrccm.153.2.8564118. [DOI] [PubMed] [Google Scholar]
- 31.Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez FJ, Perez-Paredes C, Ortiz-Leyba C. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Critical Care Medicine. 2003;31(12):2742–2751. doi: 10.1097/01.CCM.0000098031.24329.10. [DOI] [PubMed] [Google Scholar]
- 32.Osborn TM, Tracy JK, Dunne JR, Pasquale M, Napolitano LM. Epidemiology of sepsis in patients with traumatic injury. Critical Care Medicine. 2004 Nov;32(11):2234–2240. doi: 10.1097/01.ccm.0000145586.23276.0f. [DOI] [PubMed] [Google Scholar]
- 33.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nature Medicine. 1997 Jun;3(6):678–681. doi: 10.1038/nm0697-678. [Clinical Trial Research Support, Non-U.S Gov't] [DOI] [PubMed] [Google Scholar]
- 34.Kreymann KG, de Heer G, Nierhaus A, Kluge S, Kreymann KG, de Heer G, et al. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Critical Care Medicine. 2007 Dec;35(12):2677–2685. [Comment Meta-Analysis Review] [PubMed] [Google Scholar]
- 35.Nierhaus A, Montag B, Timmler N, Frings DP, Gutensohn K, Jung R, et al. Reversal of immunoparalysis by recombinant human granulocyte-macrophage colony-stimulating factor in patients with severe sepsis. Intensive Care Medicine. 2003 Apr;29(4):646–651. doi: 10.1007/s00134-003-1666-6. [Research Support, Non-U.S Gov't] [DOI] [PubMed] [Google Scholar]
- 36.Schefold JC, von Haehling S, Corsepius M, Pohle C, Kruschke P, Zuckermann H, et al. A novel selective extracorporeal intervention in sepsis: immunoadsorption of endotoxin, interleukin 6, and complement-activating product 5a. Shock. 2007 Oct;28(4):418–425. doi: 10.1097/shk.0b013e31804f5921. [Clinical Trial Research Support, Non-U.S Gov't] [DOI] [PubMed] [Google Scholar]
- 37.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. erratum appears in Crit Care Med. Critical Care Medicine. 2008 2008 Apr;Jan;3636(4)(1):1394–1396. 296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [Consensus Development Conference Research Support, Non-U.S Gov't] [DOI] [PubMed] [Google Scholar]
- 38.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. New England Journal of Medicine. 2008 Jan 10;358(2):125–139. doi: 10.1056/NEJMoa070716. [Comparative Study Multicenter Study Randomized Controlled Trial Research Support, Non-U.S Gov't] [DOI] [PubMed] [Google Scholar]
- 39.NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. New England Journal of Medicine. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 40.Wachter RM, Flanders SA, Fee C, Pronovost PJ, Wachter RM, Flanders SA, et al. Public reporting of antibiotic timing in patients with pneumonia: lessons from a flawed performance measure. Annals of Internal Medicine. 2008 Jul 1;149(1):29–32. doi: 10.7326/0003-4819-149-1-200807010-00007. [DOI] [PubMed] [Google Scholar]
- 41.Yu KT, Wyer PC, Yu KT, Wyer PC. Evidence-based emergency medicine/critically appraised topic. Evidence behind the 4-hour rule for initiation of antibiotic therapy in community-acquired pneumonia. Annals of Emergency Medicine. 2008 May;51(5):651–662. doi: 10.1016/j.annemergmed.2007.10.022. 2008 [Review] [DOI] [PubMed] [Google Scholar]


