Abstract
Orthopedic surgery often requires bone grafts to correct large defects resulting from congenital defects, surgery or trauma. Great improvements have been made in tissue engineering of bone grafts. However, these grafts lack the vascularized component that is critical for their survival and function. From a clinical perspective, it would be ideal to engineer vascularized bone grafts starting from one single cell harvest obtained from the patient. To this end, we explored the potential of human adipose derived mesenchymal stem cells (hASC) as a single cell source for osteogenic and endothelial differentiation and the assembly of bone and vascular compartments within the same scaffold. hASC were encapsulated in fibrin hydrogel as a angioinductive material for vascular formation, combined with a porous silk fibroin sponge to support osteogenesis, and subjected to sequential application of growth factors. Three strategies were evaluated by changing spatio-temporal cues: 1) induction of osteogenesis prior to vasculogenesis, 2) induction of vasculogenesis prior to osteogenesis, or 3) simultaneous induction of osteogenesis and vasculogenesis. By 5 weeks of culture, bone-like tissue development was evidenced by the deposition of bone matrix proteins, alkaline phosphatase activity and calcium deposition, along with the formation of vascular networks evidenced by endothelial cell surface markers, such as CD31 and von Willebrand factor, and morphometric analysis. Most robust development of the two tissue compartments was achieved by sequential induction of osteogenesis followed by the induction of vasculogenesis. Taken together, the collected data strongly support the utility of hASC as a single cell source for the formation of vascularized bone tissue.
Keywords: Tissue engineering, bone, vasculogenesis, osteogenesis, silk scaffolds, human adipose stem cells
1. Introduction
Bone vascularization has been a major hurdle for engineering viable and functional bone grafts. Several approaches have been proposed to reach this goal. The co-operation of endothelial cells (either EPCs - endothelial progenitor cells or HUVECs - human umbilical cord endothelial cells) and osteoblasts / osteo-progenitors have been the most studied (Usami et al. 2009; Tsigkou et al. 2010; Unger et al. 2010; Koob et al. 2011). Previous work of our own group (Correia, Grayson et al. 2011), where HUVECs and hMSCs (human bone marrow mesenchymal stem cells) were co-cultured in decellularized bone scaffolds has demonstrated that vascular development was increased when vasculogenesis is induced prior to osteogenesis, and that the addition of fresh hMSCs (human bone marrow mesenchymal stem cells) at the osteogenic induction stage improved both tissue outcomes. The in vitro developed networks successfully anastomosed with the host vessels when implanted sub-cutaneously in nude mice. In an alternate in vivo approach, Tsigkou et al. 2010 used a mouse model to develop a two-stage protocol for generating vascularized bone grafts using HUVECs and hMSC. Endothelial cells formed networks throughout the bone scaffold 4-7 days after implantation, which anastomosed with nude mice vasculature after 11 days. Vasculature was mature upon 4 weeks, at which time evidence of mineral deposition was also revealed (Tsigkou et al. 2010). Koob and co-workers 2011, also developed a completely in vivo approach, where HUVECs formed complex three-dimensional networks of perfused human neovessels in critical-sized calvarial defects in SCID mice. However, this neovasculature did not seem to improve MSC-triggered bone regeneration (Koob et al. 2011).
All these approaches combined osteogenic and vasculogenic cells from different sources, which limits their clinical utility. An ideal tissue engineering solution would use a complete autologous procedure (minimizing host rejection), and one single cell source (reducing therapy complexity and patient discomfort caused by multiple biopsy procedures) for the development of both independent structures – bone and vasculature.
In the present study, we propose the use of human adipose derived stem cells (hASC) as single cell source to generate both the bone tissue matrix and the associated vascular network. Adipose tissue represents an attractive cell source for tissue engineering for several reasons. These cells can be used in an autologous fashion, as tissue is harvested from the patient at a dedicated or non-dedicated liposuction surgery. Adipose tissue can be obtained repeatedly in large quantities under local anesthesia and with a minimum of patient discomfort. Additionally, the simple enzyme-based isolation procedure results in very high cell yield, ~404 000 cells/mL of lipoaspirate (Aust et al. 2004). Moreover, hASC are fairly well characterized, including their intrinsic potential to differentiate into osteogenic lineages – and thereby develop bone tissue (Gimble,Guilak 2003; Malafaya et al. 2005; Frohlich et al. 2010) – and endothelial lineages, to form capillaries (Miranville et al. 2004; Planat-Benard et al. 2004; Rehman et al. 2004; Cao et al. 2005; Mitchell et al. 2006; Fischer et al. 2009; Scherberich et al. 2010).
Herein, we hypothesize that a sequential application of osteogenic and endothelial growth factors to hASC cultured on biomaterial scaffolds, with different timing of addition of fresh cells can support the development of bone-like tissue containing an integrated vascular network. The experiments were performed by culturing hASC in porous silk fibroin scaffolds designed for bone tissue development in combination with fibrin-encapsulated hASC, to provide an environment conducive to the formation of capillary-like networks.
2. Materials and Methods
2.1 Preparation of silk fibroin scaffolds
Silk fibroin from silkworm (Bombix mori) cocoons was extracted with 0.02 M sodium carbonate (Na2CO3) solution, rinsed in distilled water, dissolved in a 9.3 M lithium bromide (LiBr) solution and dialyzed for 48h against distilled water in benzoylated dialysis tubing (Sigma D7884). Dissolved silk fibroin was centrifuged for 20 min at 9000 rpm (4°C). The resulting solution was determined by weighing the remaining solid after drying, yielding a 6-wt % aqueous silk fibroin solution. HFIP-derived silk fibroin scaffolds were prepared as previously described (Kim et al. 2005). Silk fibroin aqueous solution was lyophilized and further dissolved with HFIP (hexafluoro-2-propanol), resulting in a 17-wt % HFIP-derived silk fibroin solution. 4 g of granular NaCl, particle size 400–600 μm, were added to 2 mL of silk fibroin in HFIP. The containers were covered overnight to reduce evaporation of HFIP and to provide sufficient time for homogeneous distribution of the solution. Subsequently the solvent was evaporated at RT for 3 days. The matrices were then treated in 90% (v/v) methanol for 30 min, to induce the formation of the β-sheet structure, followed by immersion in water for 2 days to remove the NaCl. The porous silk scaffolds were then freeze-dried.
2.2 Isolation, characterization and expansion of hASC
hASC were isolated according to previously described methods (McIntosh et al. 2006) from liposuction aspirates of subcutaneous adipose tissue, at the Pennington Biomedical Research Center under protocols approved by the Institutional Review Board with informed patient consent. hASC were expanded to the third passage in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO 11965) supplemented with 10% fetal bovine serum (FBS) (GIBCO 26140), penicillin–streptomycin (1%) (GIBCO 15140) and 1 ng/mL basic fibroblast growth factor (bFGF) (Peprotech 100-18B). Passage 0 (P0) cells were examined for surface marker expression using flow cytometry. The presence of specific antigens such as CD29, CD105, CD45, CD34, CD44, CD73 and CD90 were analyzed, and reproducibility of cells was confirmed from various donors (McIntosh et al. 2006; Mitchell et al. 2006). hASC were confirmed for their differentiation capacity into the adipogenic and osteogenic lineages in monolayer cultures following induction with adipogenic and osteogenic inductive medium for up to 14 days and histochemical analysis of neutral lipid (Oil Red O) or mineralization (Alizarin Red) staining as published (Yu et al. 2010).
2.3 Cell encapsulation and scaffold seeding
Silk scaffolds were cut into 2mm thick, 4mm diameter cylinders, sterilized in 70% (v/v) ethanol overnight, washed in PBS and incubated in culture media prior to seeding, as in our previous studies (Correia, Bhumiratana et al. 2011). Expanded hASC were suspended at 20×106 cells/mL and seeded into silk scaffolds according to a well-established procedure (Frohlich et al. 2010; Grayson et al. 2010; Bhumiratana et al. 2011): 20 μL aliquot of cell suspension was pipetted to the top of blot-dried scaffods, pipetted up and down to ensure even distribution of cells. After 15 minutes in the incubator, scaffolds were rotated 180°, and 10 μL of cell-free medium was added to prevent drying. This process was repeated four times to allow uniform cell distribution, after which, constructs were incubated overnight to allow cell attachment (Fig.1). hASC attached to silk fibroin scaffolds were intended for bone formation. At day 1, fibrinogen (Sigma F8630) was prepared at a concentration of 5 mg/mL and thrombin (Sigma T6200-1KU) was used at 10 Units/mL. Fibrin has been extensively used to create vascularized tissue constructs (bone, adipose tissue, skin, cardiovascular tissue) due to its excellent pro-angiogenic properties (Tian,George 2011). Moreover, in a skin regeneration context, fibrin has proven to control ASC differentiation toward vascular cell types (Natesan et al. 2011). In our study, expanded hASC intended to form vasculature networks within the engineered bone tissue, were encapsulated in fibrin at a density of 20×106 cells/mL. Thrombin was added to cross-link the gel, giving a final fibrin concentration of 4 mg/mL. Before cross-linking occurred, 20 μl of cell/gel suspension was pipetted into blot-dried scaffolds to allow uniform cell seeding throughout the scaffolds. Before the gels became cross-linked fully, they were aspirated under a light vacuum so that they coated the walls of the scaffolds but did not fill the pore spaces. This step was performed on groups 1 to 5 at day 1, and on groups 6 and 7 only after 3 weeks of osteogenic culture (Fig.1).
Figure 1. Experimental design.
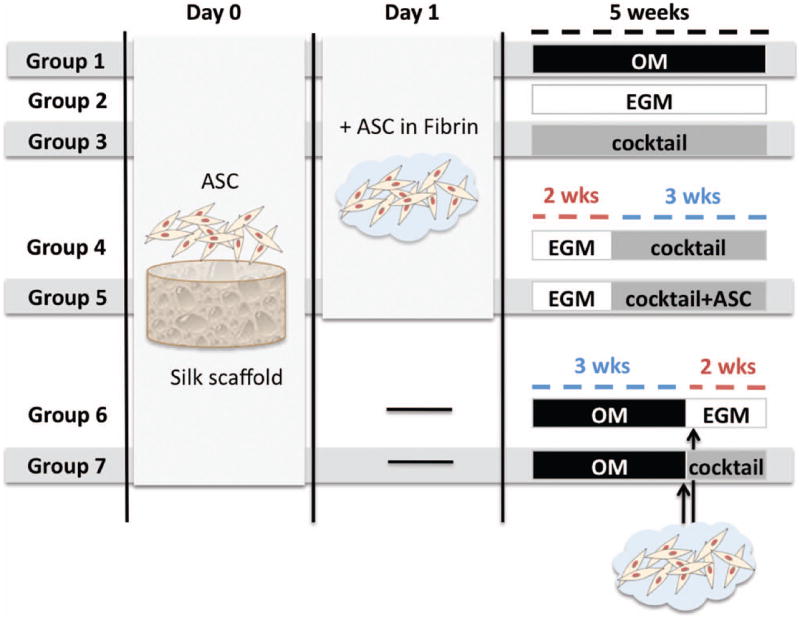
Groups 1 & 2 are controls, where constructs were provided osteogenic supplements (OM) or endothelial factors (EGM) for 5 weeks. Group 3 was cultured with a cocktail of both OM & EGM (1:1) for 5 weeks. In Groups 4 & 5, vascular differentiation was induced for 2 weeks before adding osteogenic factors in a cocktail medium (EGM∣cocktail). Additional hASC were added into the pore spaces at this point in Group 5 (EGM∣cocktail+ASC). Groups 6 & 7 were seeded with hASC to silk scaffold and osteogenic differentiation was induced for 3 weeks; only at this time point, hASC in fibrin were added to the scaffold for vascular development. Constructs were cultured in either EGM (Group 6 – OM∣EGM) or cocktail media (Group 7 – OM∣cocktail) for the remaining 2 weeks.
2.4 In vitro cultivation
Seven experimental groups of cell–biomaterial constructs were established as follows (Fig. 1), and the constructs were cultured for 5 weeks.
Group 1 served as osteogenic control, with constructs cultured in osteogenic medium (OM), which consisted of low glucose DMEM, 10% FBS, 1% Pen–Strep supplemented with osteogenic factors (all purchased from Sigma-Aldrich): 100 nM dexamethasone, 10 mM sodium-β-glycerophosphate, 10 mM HEPES and 50 μg/mL ascorbic acid-2-phosphate,
Group 2 served as vasculogenic control, where endothelial growth media-2 was used (EGM-2; Lonza CC-3162) to induce endothelial differentiation of hASCs as in prior studies (Heydarkhan-Hagvall et al. 2008). This endothelial cell basal medium contains, among other important growth factors, VEGF (Vascular endothelial growth factor) and FGF-b (basic fibroblast growth factor), known to stimulate endothelial differentiation, vasculogenesis and angiogenesis. Five further groups were chosen for growing vascularized bone constructs.
In Group 3, both osteogenic and vasculogenic supplements were provided simultaneously throughout the total period of culture by using cocktail medium composed by EGM and OM at 1:1 ratio (cocktail group).
Further, two sequential approaches were established: a) induction of vasculogenesis prior to osteogenesis (groups 4 and 5), and b) the reverse: induction of osteogenesis prior to vasculogenesis (groups 6 and 7). In both approaches, 2–week differentiation periods were chosen for vasculogenic induction, and 3 weeks for osteogenic differentiation. These time frames were based on prior studies: hASC cultured for 2 weeks in endothelial cell growth medium-2 (EGM-2) have demonstrated network formation of branched tube-like structures positive for CD31, CD144, and von Willebrand factor (Heydarkhan-Hagvall et al. 2008). With respect to bone-like tissue development, previous studies of our group have demonstrated osteogenic differentiation of hASCs and secretion of mineralized bone matrix as early as 2 weeks after osteogenic induction in this silk scaffold system (Correia, Bhumiratana et al. 2011), although 3 weeks was allowed for increased tissue development.
In Group 4, constructs were cultured in EGM for 2 weeks to induce endothelial differentiation, and in cocktail medium for the remaining 3 weeks (EGM∣cocktail) for maintenance and augmentation of endothelial phenotype, while simultaneously inducing osteogenesis. In Group 5, the constructs were cultured exactly as in Group 4 except that fresh hASC were added into scaffold pore spaces at the 2-week time point, intended to respond more promptly to osteogenic cues. This is indicated as EGM∣cocktail+ASC.
In Group 6 and Group 7, we ‘reversed’ the process and induced osteogenic differentiation first. hASC were seeded into the scaffolds and cultured in osteogenic media for 3 weeks. Subsequently, hASC in fibrin were added to the constructs and cultured either in EGM media (Group 6 - OM∣EGM), or in cocktail medium (Group 7 - OM∣cocktail).
2.5 Live/Dead assay
LIVE/DEAD Viability/Citotoxicity kit (Molecular Probes) was used to evaluate cell viability at the end of culture. Live cells (indicated by calcein AM) and dead cells (indicated by ethidium homodimer-1) were observed and imaged through a confocal microscope. Optical surfaces were taken from the surface up until 160 μm deep, in 10 μm intervals. All images are presented as vertical projections.
2.6 Biochemical characterization
Constructs were harvested, washed in PBS, cut in half and weighed. For DNA assay, samples were added to 1 mL of digestion buffer (10 mM Tris, 1 mM EDTA, 0.1% Triton X-100, 0.1 mg/mL proteinase K) and incubated overnight at 56°C. After centrifugation at 3000g for 10 minutes, the supernatants were removed, diluted and pippeted in duplicate into a 96-well plate and a 1:1 ratio of picogreen solution (Quant-iT™ PicoGreen ® dsDNA Kit, Invitrogen) was added. Sample fluorescence was measured with fluorescent plate reader at excitation ~480 nm, emission ~520 nm. Lambda DNA was used to prepare the standard curve.
For calcium quantification, samples were incubated in 1 mL TCA 5% (trichloroacetic acid 5% v/v) and calcium was extracted by disintegrating the construct using steel balls and MinibeadBeater™. The supernatant were transferred in duplicate into 96-well plate and calcium binding reagent was added at 1:10 ratio (StanbioTotal Calcium Liquicolor®). Sample optical density was measured using a microplate reader set to 575 nm. Calcium standard was used to prepare the standard curve.
Alkaline Phosphatase (AP) activity was determined by adding cell lysis solution to one-half of each scaffold, and these were disintegrated using steel balls and MinibeadBeater™. After centrifugation, 50 μL of supernatant was incubated with 50 μL pNPP (p-nitrophenyl-phosphate) substrate solution, at 37°C for 20 min. The reaction was stopped with 50 μL of stop solution and absorbance of was read at 405 nm. p-Nitrophenol at known concentrations was used to prepare the standard curve. All solutions were components of SensoLyte® pNPP Alkaline Phosphatase Complete Kit (Cell Biolabs, CBA-302).
2.7 Histology and Immunohistochemistry
Samples were fixed in 10% formalin for 1 day, dehydrated in graded ethanol washes, embedded in paraffin, sectioned to 5 μm and mounted on glass slides. Sections were deparaffinized with CitriSolv and rehydrated with a graded series of ethanol washes. For immunohistochemistry, sections were blocked with normal horse serum (NHS), stained sequentially with primary antibody (rabbit anti-human CD31 monoclonal antibody, Millipore 04-1074; rabbit anti-human von Willebrand factor (vWF), Sigma-Aldrich F3520; rabbit anti-human Osteopontin (OPN) polyclonal antibody, Chemicon ab1870; rabbit anti-bone sialoprotein (BSP) polyclonal antibody, Millipore ab1854; NHS for negative control), secondary antibody (Vectastain Universal Elite ABC Kit, PK-6200 Vector Laboratories) and developed with biotin-avidin system (DAB substrate kit SK-4100 Vector Laboratories).
2.8 Real-Time RT-PCR
For RNA extraction, samples were added to 800 μL of Trizol (Invitrogen 15596-026) and disintegrated by using steel balls and MinibeadBeater™. Suspensions were centrifuged at 12,000 g for 10 min at 4°C to remove tissue debris and extracted with chloroform (Sigma C2432). Colorless aqueous phase, containing RNA was removed and mixed with equal volume of isopropanol (Sigma I9516). Suspensions were again centrifuged at 12,000 g for 8 min at 4°C, supernatant discarded, and RNA pellet washed with 75% ethanol. Samples were centrifuged at 7,500 g for 5 min at 4°C, supernatant removed, pellet air-dried, and dissolved with DEPC-water (Applied Biosystems AM 9920). RNA was quantified using Nanodrop® ND 1000. Approximately 1 μg of RNA was reverse-transcribed with random hexamers using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems 4368814). CD31, von Willebrand factor (vWF) and the housekeeping gene glyceraldehyde-3-phosphatedehydrogenase (GAPDH) expression were quantified using the 7500 Fast Real-Time PCR System (Applied Biosystems). Primer sequences, probe, amplicon size and gene expression assay ID are described on table I. All TaqMan® Gene Expression Assay were purchased from Applied Biosystems. The expression data of each gene were normalized to GAPDH and relative gene expression was determined according to the 2-∆∆Ct method (Livak,Schmittgen 2001) and presented as average values for each group (n=3 ± standard deviation).
Table I. Information about gene expression assays used for real time RT-PCR.
Primer sequences, probe, amplicon size and gene expression assays
| Gene | GEA ID | Probe | Amplicon size (bp) |
|---|---|---|---|
| CD31 | Hs00169777_m1 | FAM | 65 |
| vWF | Hs00169795_m1 | FAM | 79 |
| GAPDH | Hs99999905_m1 | FAM | 122 |
bp, base pairs; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GEA ID, gene expression assay identification.
2.9 Statistical analysis
Data are presented as mean ± standard deviation (n=3). Statistical significance was determined using analysis of variance (ANOVA) followed by Tukey’s HSD (honestly significant difference) test using Prism software (Prism 4.0c, GraphPad Software Inc.). p< 0.05 were considered statistically significant.
3. Results and discussion
Cell viability and proliferation
The DNA content of constructs (Fig. 2A) revealed that cells within constructs cultured in EGM for the total 5 weeks proliferated significantly more than any other group, even osteogenic control group (p<0.05). This result is coherent with studies performed by Suga and colleagues, where ASC cultured with EGM-2 proliferated 105 times more in 2 weeks than those cultured with DMEM (Suga et al. 2007). It is interesting to notice that, in a study performed by our group, where HUVECs and hMSC (bone marrow derived) were used to develop a vascularized bone model, the opposite outcomes were obtained: these co-cultured cells proliferated better in OM rather than EGM media (Correia, Grayson et al. 2011). The mixture of OM and EGM to 1:1 ratio (group 3 - cocktail) resulted in significantly lower cell proliferation (p<0.05) than EGM alone. DNA contents obtained in group 6 - OM∣EGM and group 7 - OM∣cocktail support this proliferative effect of EGM, once cells cultured with EGM after osteoinduction (OM for 3 weeks) proliferated 1.84× more than the corresponding constructs cultured in cocktail medium. Clearly, growth factors present in EGM enhance proliferation, and their effects are diminished after dilution to 1:1 ratio with osteogenic media. Accordingly, group 4 - EGM∣cocktail show less DNA content than constructs of group 2 (EGM alone), showing that replacement of EGM by cocktail media at week 3, resulted in less cell proliferation. Unexpectedly, the addition of fresh cells after 3 weeks of culture, when culture medium was switched from EGM to cocktail, did not increase DNA contents (group 5 - EGM∣cocktail+ASC, Fig. 2A). Live/Dead imaging (Fig. 2B) show considerable cell viability in all experimental groups, with some cell debris in group 7.
Figure 2. Cell proliferation and viability after 5 weeks of culture.
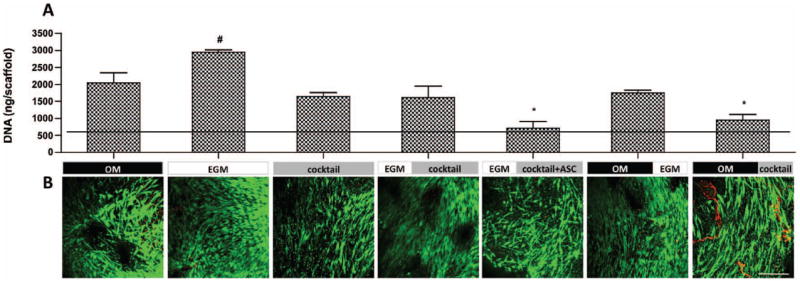
A) DNA quantification. Horizontal line indicates day 1 values. Data are shown as Ave ± SD (n=3), *p<0.001 to EGM group, #p<0.05 to all other groups; B) Live/Dead assay. Scale bar = 200 μm.
Vascular development
Endothelial cell markers - CD31 adhesion molecule and von Willebrand factor (vWF) - were used to identify and characterize specific endothelial cell types and evaluate vascular development in cultured tissues.
Both gene (Fig. 3) and protein expression (Fig. 4) of endothelial cell markers were assessed. CD31 relative gene expression (Fig. 3A) in groups 1 through 4 - OM, EGM, cocktail and EGM∣cocktail respectively, was comparable to that observed at day 1 (horizontal line). In contrast, statistically significant increases in CD31 gene expression was shown by group 5 - EGM∣cocktail+ASC (p<0.01), group 6 – OM∣EGM (p<0.05) and group 7 – OM∣cocktail (p<0.01), relatively to day 1. The addition of fresh hASCs in all these groups at later stages of culture appears crucial for enhanced endothelial cell differentiation.
Figure 3. Gene expression of endothelial markers after culture.
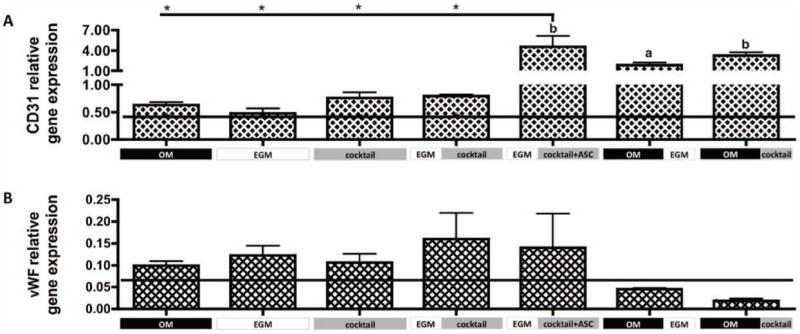
A) CD31 and B) vWF gene expression relative to GAPDH. Horizontal line indicates day 1 values. Data are shown as Ave ± SD (n=3), *p<0.01 to EGM∣cocktail+ASC group.
Figure 4. Immunohistological analysis of constructs after 5 weeks.
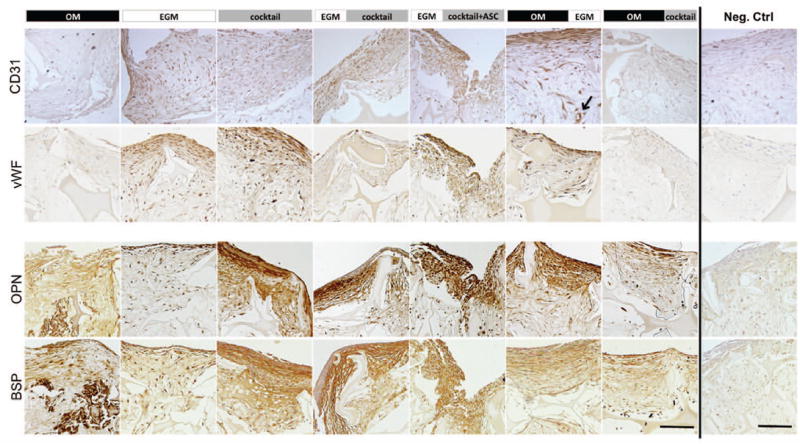
The expression of vascular and osteogenic proteins was evaluated. CD31 and von Willebrand Factor (vWF) were expressed in endothelial control group (EGM) as well as in both groups where endothelial differentiation was firstly induced (EGM∣cocktail & EGM∣cocktail+ASC). These proteins were also detected in the group where EGM was supplemented after osteogenic differentiation (OM∣EGM). Osteopontin (OPN) and bone sialoprotein (BSP) were expressed in the matrix of all groups with exception to EGM group, where these proteins were detected only at a cellular level. Scale bar = 100 μm.
After 5 weeks of culture, group 5 - EGM∣cocktail+ASC expressed significantly higher CD31 (p<0.01) than groups 1 through 4. CD31 protein expression (Fig. 4) correlates with the expression profile of the CD31 gene, with the groups 1 through 4 showing weak staining and groups 5 and 6 showing strong protein expression. Additionally, we observe that culture conditions at group 6 – OM∣EGM promoted the development of elongated and circumferential structures, corresponding to vascular lumen (indicated by arrow). In contrast, protein staining in group 7 – OM∣cocktail, was relatively weak, although CD31 gene expression was relatively high. Though, it is not uncommon for protein and mRNA levels to differ (Ghazalpour et al. 2011). vWF protein secretion was consistent with CD31 protein expression, with very weak staining in group 7, and good staining in groups 5 and 6. Again, elongated structures were stained for vWF at group 6 – OM∣EGM.
Groups 1 through 5 had comparable vWF gene expression (GE), which was upregulated relatively to day 1, although not significantly different. The same was not observed for groups 6 and 7, where vWF GE was downregulated relative to day 1. No significant differences were obtained between groups. Regarding vWF protein detection by immunohistochemistry (Fig.4), positive staining was obtained for groups 2 (EGM control), 3, 5 and 6. Among these, more elongated stained structures are observed in group 6, what is not observed in other experimental groups.
Overall, the cultivation system comprised of hASC adhered to silk scaffold surface, and encapsulated in fibrin hydrogel, was not sufficient to support the formation of robust tube like structures in the vasculogenic control group (group 2 – EGM), as no lumens were detected despite positive staining for CD31 and vWF. Previous work developed by Heydarkhan-Hagvall and colleagues demonstrated formation, by hASC, of branched tube-like structures positive for these endothelial markers as early as 2 weeks cultured in same endothelial media (Heydarkhan-Hagvall et al. 2008). Similar observations were reported for cultures in endothelial growth media supplemented with known concentrations of VEGF and IGF (0.5 and 20 ng/mL, respectively) (Miranville et al. 2004), in methylcellulose medium (Planat-Benard et al. 2004) and EGM-2MV medium (Rehman et al. 2004).
In the current study, we observed that sequential nourishment of growth factors was most beneficial to vascular development, specifically when osteogenesis was induced before vasculogenesis, consisting of the addition of hASC with EGM media at the 3 week time point. Elongated structures were identified positive for both CD31 and vWF, in association with what appeared to be small lumen in histological sections (Fig.4, black arrow). It is known that osteoblasts secrete not only VEGF and IGF but also BMPs, which induce endothelial migration, filopodia and tube formation (via activation of the transcription factor Id1 through SMADs or ERK signaling) (Valdimarsdottir et al. 2002; Suzuki et al. 2008). The osteogenic differentiation of hASC at a first stage may lead to the secretion of these growth factors (GF) as the BMPs have been detected at the mRNA level in previous studies (Halvorsen et al. 2001). Furthermore, hASC added to the system after 3 weeks are immediately in close proximity to the GF-secreting osteogenic induced cells. Consequently, these paracrine relationships, in combination with GF provided by endothelial growth media, may be playing a synergistic effect in stimulating the freshly delivered hASC into the endothelial lineage. Given the concurrent outcomes evaluated, we propose that culturing conditions provided in group 6 – OM∣EGM are the most promising for vascular development in this synergistic approach to engineer vascularized bone.
Bone tissue development
Bone tissue markers were assessed in order to evaluate bone-like tissue development in the proposed vascularized bone tissue culture. Bone Sialoprotein II (BSP) and Osteopontin were detected in cultured grafts through immunolocalization on histological slides (Fig. 4). As expected, the EGM group was the least stained, as this group was not supplemented with osteogenic growth factors. The group that showed the strongest osteogenesis was the cocktail group. It seems that the simultaneous presence of osteogenic and vasculogenic growth factors over 5 weeks of culture improved bone protein deposition. We hypothesized that hASC cultured in endothelial media, triggered by angiogenic cues for 2 weeks, would commit to the endothelial cell lineage, and consequently be less able to respond to osteogenic cues provided at the second induction stage. The addition of fresh hASC at this time-point would potentially respond to the osteogenic growth factors present in cocktail media and develop bone tissue, but differences in staining intensity were not that evident: groups 4 and 5 - EGM∣cocktail and EGM∣cocktail+ASC respectively, demonstrated comparable bone protein deposition. From groups 6 – OM∣EGM and 7 – OM∣cocktail, that were subjected to osteogenic cues before vasculogenic induction, superior staining of bone proteins was observed in group 6.
Alkaline phosphatase (AP) activity was quantified as an early marker of osteogenic differentiation (Beck et al. 2000) (Fig. 5). Constructs cultured in OM exhibited a typical progression of osteogenic differentiation, as AP activity peaked at 3 weeks of osteogenic induction, and decreased by the end culture in all groups. Lower AP activity was detected in groups 6 and 7 (OM∣EGM and OM∣cocktail) as endothelial factors were provided at the last two weeks of culture. AP activity of cells cultured in EGM media the complete experimental period (EGM group) decreased throughout the 5-week culture period, while constructs cultured in cocktail media after endothelial media (groups 4 and 5), showed characteristic increases in AP activity, suggesting that osteogenesis still occur in these groups, but with delay. Constructs cultured during total 5 weeks in cocktail media demonstrated a decrease of AP activity after the first 2 weeks, increasing by the end of the culture period.
Figure 5. Alkaline phosphatase activity.
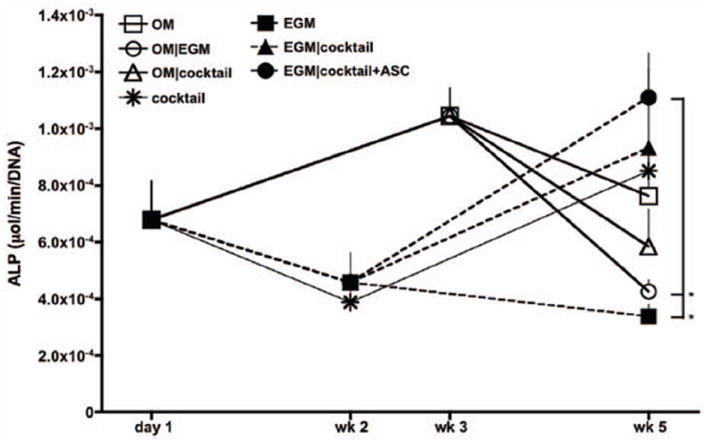
n=3, *p<0.01 to EGM∣cocktail+ASC.
Mineralization of engineered tissue was evaluated by calcium quantification (Fig. 6). After 5 weeks of culture, the osteogenic control group (OM) presented the highest amount of calcium, significantly different from all other groups (p<0.001), while the vasculogenic control presented the least. Among groups where sequential induction of both lineages were evaluated, group 7 - OM∣cocktail presented the highest amount of calcium deposition, followed by group 6 – OM∣EGM. Significant differences (p<0.05) were observed between these groups, suggesting that osteogenic growth factors present in cocktail media are essential to maintain bone development. Constructs from group 5 – EGM∣cocktail+ASC demonstrated 1.7× higher calcium content than group 4, suggesting that the addition of hASC at the osteogenic induction stage improved calcification of the overall system.
Figure 6. Mineralization of tissue engineered grafts after 5 weeks.
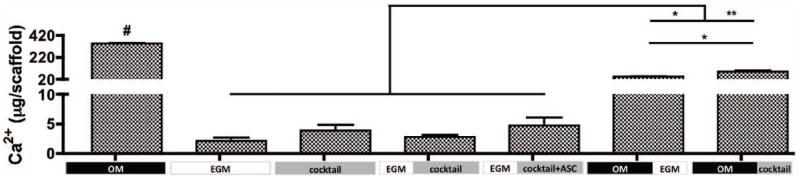
Calcium content. n=3, #p<0.001 to all other groups, *p<0.01, **p<0.001
Taken altogether, in accordance to collected data for bone-like tissue development in the culturing conditions evaluated, we suggest that the combination tested in Group 6 – OM∣EGM promoted the most promising synergistic deposition of calcium and bone proteins.
Synergistic development of vascularized bone
The establishment of an environment suitable for the in vitro development of vascularized bone from a single cell source presents a considerable challenge. In this study we proposed changing spatio-temporal cues by manipulating delivery of cells to scaffolds and/or timing of vasculogenic and osteogenic cues leading to the concomitant formation of the bone and vascular compartments in the same scaffold. The collected experimental data are consistent with the model presented in Fig. 7.
Figure 7. Model of in vitro coordination of bone and vascular tissue development.
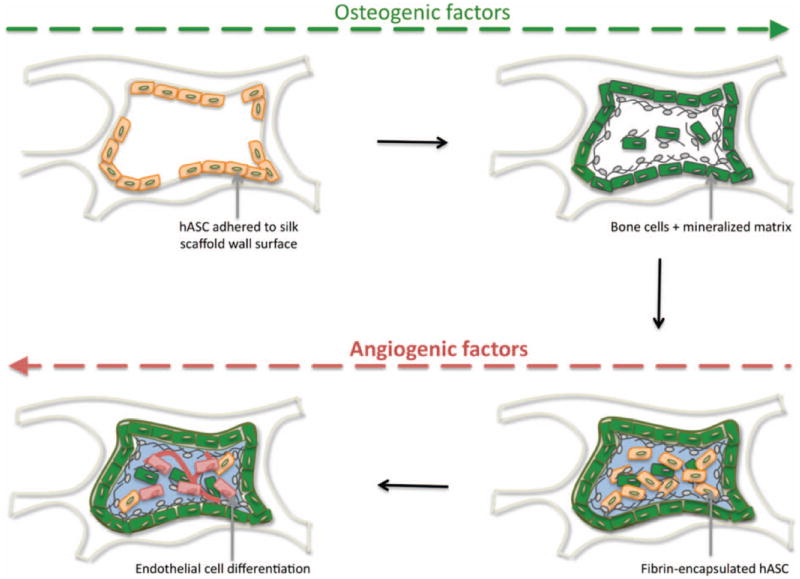
hASC (beige spindle-shape) adhered to silk scaffold wall, sense the hard surface and osteogenic factors provided, stimulate osteogenic differentiation (green cells) and deposition of mineralized matrix (grey). At a second stage, hASC encapsulated in fibrin hydrogel (light blue) are triggered by smooth vasculogenic surface growth factors provided, to undergo endothelial differentiation (red cells).
We postulate that hASC adhered to the silk scaffold wall, sense its stiff surface, which, in combination with osteogenic cues, trigger cells to undergo osteogenic differentiation and secrete mineralized matrix. At a second stage, hASC-encapsulated in fibrin hydrogel sense the surrounding smooth environment and inherent vasculogenic properties, which in combination with vasculogenic growth factors such as VEGF and bFGF present in endothelial growth media, trigger undifferentiated hASC to undergo differentiation into endothelial cells. These endothelial cells may subsequently produce osteogenic GFs, responsible to maintain the osteogenic phenotype of differentiated bone cells. The pro-osteogenic function of endothelial cells, as well as their capacity to recruit osteoblast precursors, has been described (Cenni et al. 2009). Endothelial cells secrete BMPs, including BMP-2 (Kaigler et al. 2005), that play an essential role on differentiation and maintenance of osteogenic phenotype of cells. Moreover, several studies have shown that BMP stimulate VEGF expression in osteoblasts (Deckers et al. 2002), and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells (Bouletreau et al. 2002). In addition, PDGF (Platelet Derived Growth Factor) has been recently reported to play a significant role on the significance in the vasculature-pericyte-MSC-osteoblast dynamics (Caplan,Correa 2011).
The results obtained in this study are promising. However, fully developed networks within the bone compartment were not achieved. In a similar study performed by our group, where HUVECs and hMSC were co-cultured in order to develop a vascularized bone model, we obtained well-developed vasculature, with visible lumen formed after in vitro culture, which further anastomosed with host vasculature after 2 weeks of sub-cutaneous implantation in nude mice. Vasculogenic induction with endothelial growth media was performed for 2 weeks, as in the present study, although HUVECs, a mature endothelial cell, were capable to successfully form tube-like structures and nourish the bone tissue (Correia, Grayson et al. 2011). This reinforces the hypothesis that a distinct approach may be needed to account for the undifferentiated state of the adipose stem cell. Actually, the fact that the opposite spatio-temporal application of cells and differentiation cues resulted in most promising outcomes is already an insight to guide future experiments. Moreover, our results are additive to those obtained by Gardin and co-workers, which remains, to our knowledge, the only study that previously explored the concurrent osteogenic and endothelial in vitro commitment of hASC (Gardin et al. 2011). In this work, hASC loaded to a fibronectin coated hydroxyapatite scaffold were exposed to either osteogenic induction only, vasculogenic induction only, or a mixture of both osteogenic and vasculogenic growth factors for 21 days, in vitro. In their hands, concurrent gene expression of both osteogenic markers osteopontin, osteonectin, osteocalcin, collagen type I and vasculogenic markers CD31, von Willebrand Factor, VEGF was achieved. Our study extends the work by Gardin et al to design an engineered vascularized bone graft. We further explored the development of both tissues by investigating: 1) the sequential application of cells and/or osteogenic and vasculogenic growth factors to the system; and 2) evaluation of outcomes based on tissue formation where, besides gene expression, we also assessed the presence of bone - and endothelial - specific proteins within the engineered graft, as well as mineralization of the tissue construct. Therefore, we may furthermore propose our approach as an in vitro model for studying and optimizing both spatial and temporal effects of cell and growth factor delivery.
4. Conclusions
This study supports the hypothesis that human adipose derived stem cells may be used as a single cell source for the formation of vascularized bone. Three culture approaches were tested in order to differentiate both osteogenic and endothelial lineage: 1) simultaneous construct nourishment with bone and vascular growth factors; 2) induction of vasculogenesis before osteogenesis; and 3) induction of osteogenesis before vasculogenesis, to show that the osteogenic induction was necessary before supplying the construct with fresh cells and growth factors for vascularization. This last combination, in particular the osteo-induction of hASC seeded to silk scaffold for 3 weeks, followed by addition (to the free pore spaces of the construct) of fibrin-encapsulated hASC to which vasculogenic cues were provided for 2 weeks, resulted in the most promising outcomes towards engineered vascularized bone grafts. Nevertheless, extensive research has yet to be performed in order to understand cell crosstalk and underlying mechanisms associated with multilineage cell differentiation of hASC and interactions responsible for the development of a robust functional vascularized bone graft useful for successful therapeutic applications.
Acknowledgments
We gratefully acknowledge funding support of this work by the NIH (DE161525 and EB02520 to GVN), and the FCT PhD grant (SFRH/BD/42316/2007 to CC). The authors thank Darja Marolt and Supansa Yodmuang for their help with the experiments, and David Kaplan for providing silk scaffolds used in this study.
References
- Aust L, Devlin B, Foster SJ, Halvorsen YDC, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6(1):7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- Beck GR, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. P Natl Acad Sci USA. 2000;97(15):8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhumiratana S, Grayson WL, Castaneda A, Rockwood DN, Gil ES, Kaplan DL, Vunjak-Novakovic G. Nucleation and growth of mineralized bone matrix on silk-hydroxyapatite composite scaffolds. Biomaterials. 2011;32(11):2812–2820. doi: 10.1016/j.biomaterials.2010.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouletreau PJ, Warren SM, Spector JA, Peled ZM, Gerrets RP, Greenwald JA, Longaker MT. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg. 2002;109(7):2384–2397. doi: 10.1097/00006534-200206000-00033. [DOI] [PubMed] [Google Scholar]
- Cao Y, Sun Z, Liao LM, Meng Y, Han Q, Zhao RCH. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Bioph Res Co. 2005;332(2):370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- Caplan A, Correa D. PDGF in Bone Formation and Regeneration: New Insights into a Novel Mechanism Involving MSCs. J Orthop Res. 2011;29:1795–1803. doi: 10.1002/jor.21462. [DOI] [PubMed] [Google Scholar]
- Cenni E, Ciapetti G, Granchi D, Fotia C, Perut F, Giunti A, Baldini N. Endothelial cells incubated with platelet-rich plasma express PDGF-B and ICAM-1 and induce bone marrow stromal cell migration. J Orthop Res. 2009;27(11):1493–1498. doi: 10.1002/jor.20896. [DOI] [PubMed] [Google Scholar]
- Correia C, Bhumiratana S, Yan LP, Oliveira AL, Gimble JM, Rockwood D, Kaplan DL, Sousa RA, Reis RL, Vunjak-Novakovic G. Development of silk-based scaffolds for tissue engineering of bone from human adipose derived stem cells. Acta Biomat. 2011 doi: 10.1016/j.actbio.2012.03.019. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia C, Grayson WL, Park M, Hutton D, Zhou B, Guo XE, Niklason L, Sousa RA, Reis RL, Vunjak-Novakovic G. In vitro model of vascularized bone: synergizing vascular development and osteogenesis. PLoS ONE. 2011;6(12):e28352. doi: 10.1371/journal.pone.0028352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers MM, van Bezooijen RL, van der Horst G, Hoogendam J, van Der Bent C, Papapoulos SE, Lowik CW. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143(4):1545–1553. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- Fischer LJ, McIlhenny S, Tulenko T, Tulenko T, Golesorkhi N, Zhang P, Larson R, Lombardi J, Shapiro I, DiMuzio PJ. Endothelial Differentiation of Adipose-Derived Stem Cells: Effects of Endothelial Cell Growth Supplement and Shear Force. J Surg Res. 2009;152(1):157–166. doi: 10.1016/j.jss.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich M, Grayson WL, Marolt D, Gimble JM, Kregar-Velikonja N, Vunjak-Novakovic G. Bone Grafts Engineered from Human Adipose-Derived Stem Cells in Perfusion Bioreactor Culture. Tissue Eng Pt A. 2010;16(1):179–189. doi: 10.1089/ten.tea.2009.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardin C, Bressan E, Ferroni L, Nalesso E, Vindigni V, Stellini E, Pinton P, Sivolella S, Zavan B. In vitro concurrent endothelial and osteogenic commitment of adipose-derived stem cells and their genomical analyses through CGH array: novel strategies to increase the successful engraftment of tissue engineered bone grafts. Stem Cells and Devel. 2011 doi: 10.1089/scd.2011.0147. in press. [DOI] [PubMed] [Google Scholar]
- Ghazalpour A, Bennett B, Petyuk VA, Orozco L, Hagopian R, Mungrue IN, Farber CR, Sinsheimer J, Kang HM, Furlotte N, Park CC, Wen PZ, Brewer H, Weitz K, Camp DG, 2nd, Pan C, Yordanova R, Neuhaus I, Tilford C, Siemers N, Gargalovic P, Eskin E, Kirchgessner T, Smith DJ, Smith RD, Lusis AJ. Comparative analysis of proteome and transcriptome variation in mouse. PLoS Genet. 2011;7(6):e1001393. doi: 10.1371/journal.pgen.1001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5(5):362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- Grayson WL, Marolt D, Bhumiratana S, Frohlich M, Guo XE, Vunjak-Novakovic G. Optimizing the medium perfusion rate in bone tissue engineering bioreactors. Biotechnol Bioeng. 2010 doi: 10.1002/bit.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL, Paschalis EP, Wilkison WO, Gimble JM. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7(6):729–741. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- Heydarkhan-Hagvall S, Schenke-Layland K, Yang JQ, Heydarkhan S, Xu YH, Zuk PA, MacLellan WR, Beygui RE. Human adipose stem cells: A potential cell source for cardiovascular tissue engineering. Cells Tissues Organs. 2008;187(4):263–274. doi: 10.1159/000113407. [DOI] [PubMed] [Google Scholar]
- Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. Faseb Journal. 2005;19(1):665–7. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26(15):2775–2785. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Koob S, Torio-Padron N, Stark GB, Hannig C, Stankovic Z, Finkenzeller G. Bone formation and neovascularization mediated by mesenchymal stem cells and endothelial cells in critical-sized calvarial defects. Tissue Eng Pt A. 2011;17(3-4):311–321. doi: 10.1089/ten.TEA.2010.0338. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Malafaya PB, Pedro AJ, Peterbauer A, Gabriel C, Redl H, Reis RL. Chitosan particles agglomerated scaffolds for cartilage and osteochondral tissue engineering approaches with adipose tissue derived stem cells. J Mater Sci Mater Med. 2005;16(12):1077–1085. doi: 10.1007/s10856-005-4709-4. [DOI] [PubMed] [Google Scholar]
- McIntosh K, Zvonic S, Garrett S, Mitchell JB, Floyd ZE, Hammill L, Kloster A, Halvorsen YD, Ting JP, Storms RW, Goh B, Kilroy G, Wu XY, Gimble JM. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells. 2006;24(5):1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110(3):349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, McIntosh K, Zvonic S, Garretta S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu XY, Gimble JM. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24(2):376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- Natesan S, Zhang G, Baer DG, Walters TJ, Christy RJ, Suggs LJ. A Bilayer Construct Controls Adipose-Derived Stem Cell Differentiation into Endothelial Cells and Pericytes Without Growth Factor Stimulation. Tissue Eng Pt A. 2011;17(7-8):941–953. doi: 10.1089/ten.tea.2010.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells - Physiological and therapeutic perspectives. Circulation. 2004;109(5):656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- Rehman J, Traktuev D, Li JL, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- Scherberich A, Muller AM, Schafer DJ, Banfi A, Martin I. Adipose tissue-derived progenitors for engineering osteogenic and vasculogenic grafts. J Cell Physiol. 2010;225(2):348–353. doi: 10.1002/jcp.22313. [DOI] [PubMed] [Google Scholar]
- Suga H, Shigeura T, Matsumoto D, Inoue K, Kato H, Aoi N, Murase S, Sato K, Gonda K, Koshima I, Yoshimura K. Rapid expansion of human adipose-derived stromal cells preserving multipotency. Cytotherapy. 2007;9(8):738–745. doi: 10.1080/14653240701679873. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Montagne K, Nishihara A, Watabe T, Miyazono K. BMPs promote proliferation and migration of endothelial cells via stimulation of VEGF-A/VEGFR2 and angiopoietin-1/Tie2 signalling. J Biochem. 2008;143(2):199–206. doi: 10.1093/jb/mvm215. [DOI] [PubMed] [Google Scholar]
- Tian L, George SC. Biomaterials to prevascularize engineered tissues. J Cardiovasc Transl Res. 2011;4(5):685–698. doi: 10.1007/s12265-011-9301-3. [DOI] [PubMed] [Google Scholar]
- Tsigkou O, Pomerantseva I, Spencer JA, Redondo PA, Hart AR, O’Doherty E, Lin YF, Friedrich CC, Daheron L, Lin CP, Sundback CA, Vacanti JP, Neville C. Engineered vascularized bone grafts. P Natl Acad Sci USA. 2010;107(8):3311–3316. doi: 10.1073/pnas.0905445107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RE, Ghanaati S, Orth C, Sartoris A, Barbeck M, Halstenberg S, Motta A, Migliaresi C, Kirkpatrick CJ. The rapid anastomosis between prevascularized networks on silk fibroin scaffolds generated in vitro with cocultures of human microvascular endothelial and osteoblast cells and the host vasculature. Biomaterials. 2010;31(27):6959–6967. doi: 10.1016/j.biomaterials.2010.05.057. [DOI] [PubMed] [Google Scholar]
- Usami K, Mizuno H, Okada K, Narita Y, Aoki M, Kondo T, Mizuno D, Mase J, Nishiguchi H, Kagami H, Ueda M. Composite implantation of mesenchymal stem cells with endothelial progenitor cells enhances tissue-engineered bone formation. J Biomed Mater Res A. 2009;90(3):730–741. doi: 10.1002/jbm.a.32142. [DOI] [PubMed] [Google Scholar]
- Valdimarsdottir G, Goumans MJ, Rosendahl A, Brugman M, Itoh S, Lebrin F, Sideras P, ten Dijke P. Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation. 2002;106(17):2263–2270. doi: 10.1161/01.cir.0000033830.36431.46. [DOI] [PubMed] [Google Scholar]
- Yu G, Wu X, Dietrich MA, Polk P, Scott LK, Ptitsyn AA, Gimble JM. Yield and characterization of subcutaneous human adipose-derived stem cells by flow cytometric and adipogenic mRNA analyzes. Cytotherapy. 2010;12(4):538–546. doi: 10.3109/14653241003649528. [DOI] [PMC free article] [PubMed] [Google Scholar]


