Abstract
Objectives
To evaluate the contributions of patient and treatment factors to overall expenditures and regional variation for initial treatment of localized prostate cancer (CaP) in the Medicare program.
Research Design
Using the Surveillance, Epidemiology, and End Results–Medicare database, we identified 47,517 beneficiaries with localized CaP during 2005–2009 and matched non-cancer controls. We employed hierarchical generalized linear models to estimate risk-standardized cancer-related expenditures for each hospital referral region. To identify key contributors to the variation, we sequentially added patient characteristics, treatment intensity (the percentage of patients receiving curative treatments), ancillary procedures (biopsy, hormone therapy, and imaging), and specific treatment modalities into the model. We categorized the expenditures according to the type of services to identify their relative impact on the expenditure variations.
Results
The mean expenditure on CaP-related care per CaP beneficiary was $15,900, including $1,800 on surgery, $11,200 on radiotherapy, and $1,900 on ancillary procedures. The expenditure difference between quintiles 5 and 1 was $6,200. Patient characteristics explained 8.4% of this difference. Treatment intensity and treatment modalities accounted for an additional 21.2% and 31.2% of the variation, respectively. Between the highest and lowest expenditure quintiles, the difference in radiotherapy expenditure was $5,000, whereas that in surgery or ancillary procedures was less than $200.
Conclusions
There is substantial geographic variation in CaP expenditures, and the specific modality of radiotherapy is the most important contributor to this variation. Efforts to address the CaP care costs, such as bundled payment development, require targeting both treatment intensity and use of costly modalities.
Keywords: expenditures, regional variation, prostate cancer, radiotherapy, treatment modalities, bundled payment
Introduction
Prostate cancer (CaP) imposes a substantial burden on patients, their caregivers, and the healthcare system. National costs for CaP care in 2010 were estimated at $12 billion in the United States.1 As newer and more expensive technologies for surgery and radiotherapy have been readily adopted into clinical practice,2–4 costs are likely to increase. In response to the concerns about rising costs of cancer care, including CaP care, there has been substantial interest in using a bundled payment for a specific episode of cancer care.5,6 The Center for Medicare & Medicaid Services recently announced the Bundled Payments for Care Improvement initiative;7 where a “bundled” payment covers all services delivered across providers during a single episode of care.8 This payment is designed to improve care coordination and reduce the use of inefficient services. However, setting up an appropriate bundled payment for all of the initial care in a CaP episode is challenging.9
Since a large variation may indicate inefficiency in the high-spending areas, bundled payments have the potential to reduce costs and improve quality in these areas.10,11 Understanding which factors are responsible for the highest expenditure variation can help policy makers prioritize efforts to create payment bundles for CaP care. Conceptually, several factors can lead to variation in CaP expenditures: first, patient and tumor characteristics affect treatment options and care patterns, and thus expenditures. Second, expenditures can differ substantially among treatment options for localized CaP, including curative therapy (surgery or radiotherapy) and conservative management (active surveillance or watchful waiting).12 Third, the propensity of adoption of costly modalities can exert a profound impact on treatment expenditures. To establish a bundled payment, it is important to know about how these different factors – patient clinical characteristics, treatment options, and the use of specific modality – contribute to overall CaP care expenditures, or variation across regions.
Furthermore, an important component of bundled payments is to hold multiple providers jointly accountable for the payment of a bundle of services. Bundled payments are perfectly suitable for CaP care, which involves collaborative efforts across multi-disciplinary specialties. Thus, assessing which service (e.g., surgery, radiation, biopsy, hormone therapy, and imaging) contributes more to expenditure variation across different providers can assist in setting up bundled payment schema. While prior literature has shown large variations in practice patterns for CaP care,13–20 few studies examined variations in expenditures. When studies examined this issue, they usually focused on services from one single treatment option.21,22 Hence, they provided limited information within the bundled payment framework. To date, little is known to what extent different services across specialties influence regional variation of overall CaP care expenditures.
To address these knowledge gaps, we estimated Medicare expenditures for the initial care of beneficiaries with newly diagnosed CaP across multiple providers. We then assessed the geographic variation of CaP-related expenditures across hospital referral regions (HRRs), and determined the contributions of treatment modality to CaP expenditures and variation. Finally, we categorized the expenditures according to the type of services to identify their relative impact on the expenditure variations.
Methods
Model Overview
We first calculated CaP-related expenditures by computing the difference between the mean expenditures for cancer patients and for patients without cancer who are otherwise compatible, also known as the “net costs” approach.23 We ranked HRRs according to CaP-related expenditures, and grouped them into quintiles. We then used sequential models to determine the relative contribution of different factors to the overall expenditure variation in CaP care. We estimated service-specific CaP-related expenditures, including services of surgery, radiotherapy, and ancillary procedures (biopsy, hormone therapy, and imaging).
We constructed the ranking by “net costs” rather than by total expenditures from cancer patients for two reasons: Prior literature has shown that a substantial proportion of older Americans are estimated to have at least two chronic physical or behavioral health conditions.24 Using Medicare expenditure among CaP patients without subtracting expenditure among controls incorrectly assumes no Medicare expenditure for other comorbidities. Our approach could also address the issue of variation of expenditures for different diseases within an HRR,25 because non-cancer expenditures were teased out at each HRR level.
Data Source
We used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database. The SEER program collects information on patients’ sociodemographic and tumor characteristics. Use of health services and the corresponding expenditures were derived from Medicare claims. The Yale Human Investigation Committee determined that this study did not directly involve human subjects.
Study Population
Using the SEER–Medicare database, our “case” group consisted of men who were newly diagnosed with localized CaP during 2005–2009 who had the following: known month of diagnosis, diagnosis not reported from autopsy or death certificate, continuous Medicare Parts A and B fee-for-service coverage from two years prior to diagnosis through one year after diagnosis, and known zip code of residence to be linked to HRRs as defined by the Dartmouth Atlas of Healthcare.26,27 We excluded patients who had a history of a prior or concurrent malignancy. We only included men 67 years of age or older at diagnosis in order to have two years of claims history before diagnosis to assess comorbidity. To reduce variability caused by low HRR volumes, we excluded data from HRRs with fewer than 25 patients during the study period.
Each man identified as a CaP “case” was 1:1 frequency matched to a man without cancer who served as a “control,” using non-cancer patients from the 5% random sample of Medicare beneficiaries residing in the same HRR. We created an index date for each “control” using the first day of a randomly selected month and year during 2005–2009 in which the control was alive and met the same enrollment criteria used for cases except for CaP diagnosis. “Cases” and “controls” were matched by age at the time of diagnosis for cases and the index date for controls (in quartiles), comorbidity (yes/no), race (white/black/other), year of diagnosis/index date, and pre-cancer Medicare expenditure (from 18 months through 6 months prior to diagnosis/index date).
Covariates
Patient characteristics included age at diagnosis, race, year of diagnosis, urban versus rural residence, median household income, and comorbidities. Age at diagnosis of CaP was based on the Patient Entitlement and Diagnosis Summary File (PEDSF) variable which incorporated the site and corresponding diagnostic dates. We used selected Elixhauser comorbidity conditions, adapting an approach that requires the International Classification of Diseases, 9th revision (ICD-9) diagnosis code to appear on an inpatient claim or ≥2 outpatient claims greater than 30 days apart during the time period 24 months to 3 months prior to diagnosis or index date.28 Tumor characteristics included stage, prostate specific antigen level, and Gleason score, as reported by SEER. Treatment type and modality were identified from claims based on ICD-9 diagnosis and procedure codes and Healthcare Common Procedure Coding System codes (see online supplementary material for a list of treatment type and identified modality). Treatment intensity was defined as the percentage of patients receiving curative treatment, either surgery of radiotherapy.
Estimation of Medicare Spending
We defined initial phase of care as the period from two months prior through 12 months after CaP diagnosis/index date, consistent with prior literature.29 Medicare claims files including inpatient, outpatient, physician, home health, durable medical equipment, and hospice services during the initial phase of care were used to estimate CaP initial treatment expenditures. We adjusted for inflation30 and geographic price differences.31,32 All expenditures were expressed in 2009 U.S. dollars.
Statistical Analysis
We estimated the CaP-related expenditure for each HRR by calculating the difference in total Medicare expenditure for cases and for controls, using hierarchical generalized linear models (HGLMs) with a log link function and gamma distribution. Because there was heterogeneity in the effect of age between the “cases” and “controls” groups, we used separate risk adjustment models for these two groups. We Winsorized total expenditures by assigning the 97.5th percentile value to data above the 97.5th percentile to eliminate the influence of extreme values.33–35 We calculated the expected mean CaP-related expenditure per patient for HRRi as the difference of the mean expected expenditures of the cases in HRRi and the mean expected expenditures of the control in HRRi. Then, for HRRi, we estimated its adjusted per patient CaP-related expenditure as:
where Ōcase,i and Ōcontrol,i are the mean observed expenditure over all cases and controls in HRRi; Ēcase,i and Ēcontrol,i are the mean expected expenditure over all cases and controls in HRRi; and Ōcase, and Ōcontrol are the mean observed expenditures over all cases and controls, respectively, in the entire sample. This measure reflects expenditures per CaP Medicare beneficiary across HRRs after adjusting for patient and/or treatment factors.
We used sequential models to determine the relative contribution of different factors to the overall expenditure variation in CaP care (detailed in the Appendix). We estimated an unconditional model with just an intercept (Model 0). We then used a multivariate model to adjust for patient demographics and tumor characteristics (Model 1). Lastly, we sequentially added four sets of dichotomous variables that reflected the treatment received: receipt of any curative treatment (Model 2); receipt of surgery and/or radiotherapy (two dichotomous variables representing each treatment separately, Model 3); receipt of ancillary procedures (Model 4); and receipt of specific surgery or radiotherapy modalities (open surgery, laparoscopic surgery, external beam radiation therapy, intensity-modulated radiation therapy [IMRT], brachytherapy, proton beam radiotherapy, and stereotactic radiosurgery, Model 5).
Using a similar approach but only limited to data from the “cases,” we estimated Medicare expenditure on the major components of CaP care (i.e., surgery, radiotherapy, and ancillary procedures). Because not all CaP patients received each of these treatments/services, many cases had zero values on one or more component expenditure. Therefore, we adapted the HGLMs by integrating them with two-part models while allowing for correlated HRR random effects and accounting for the “semi-continuous” nature of these expenditure data.36 We used these models to calculate for each CaP patient his “expected” Medicare expenditure based on his predicted probability of having non-zero expenditure and his predicted expenditure conditional on having non-zero expenditure (while setting the HRR random effect at zero). The adjusted per patient Medicare expenditure on each component of CaP care for HRRi was calculated as:
where Ōcase,i,l, Ēcase,i,,l, and Ōcase,l were defined as above except that they now refer to the l-th component of cancer care.
Estimated Medicare expenditures were then summarized across HRRs. We assigned the HRRs to five quintiles with each quintile accounting for one-fifth of the CaP patients represented in the SEER–Medicare database. The degrees of regional variation in total expenditure, as well as in the different components of care, were evaluated by comparing the corresponding mean estimates across the HRR quintiles. Descriptive statistics were used to summarize the characteristics of patients stratified by HRR quintiles, using means for continuous variables and proportions for categorical variables. We calculated the mean expenditures on a specific service per service-received patient. Applying a point system developed by van Walraven et al,37 we demonstrated similar distributions of comorbidities between the case and control groups across quintiles (see online supplementary figures). All data analysis was performed using SAS 9.2 (SAS Inc., Cary, NC).
Results
There were 47,517 patients (mean age 74 years) with newly diagnosed localized CaP during the study period in 95 HRRs (Table 1). The unadjusted mean expenditure on CaP-related care per beneficiary was $15,700, ranging from $12,800 in quintile 1 to $18,900 in quintile 5 (difference = $6,100; 47.7% of the expenditure in quintile 1). Distributions of patients’ demographics and tumor characteristics varied significantly across the quintiles. Patients in the highest expenditure areas were more likely to reside in metropolitan or higher income areas and to have less advanced disease (Table 1). After adjusting for patient demographics and tumor characteristics, the mean expenditure on CaP-related care per beneficiary was $15,900: specifically, $1,800 on surgery, $11,200 on radiotherapy, $1,200 on biopsy, $500 on hormone therapy, and $300 on imaging (Table 2).
Table 1.
Mean Prostate Cancer Treatment Spending and Patients’ Characteristics, According to Quintiles of Medicare Spending*
| Total | Quintile of Prostate Cancer Treatment
Spending per Patient |
||||||
|---|---|---|---|---|---|---|---|
| 1 n=9,736 |
2 n=9,376 |
3 n=9,384 |
4 n=8,352 |
5 n=10,669 |
P Value, 1 vs. 5 | ||
| Mean initial expenditures among cases | $24,500 | $22,300 | $22,800 | $23,800 | $25,700 | $27,500 | <.001 |
|
| |||||||
| Mean initial expenditures among controls | $8,800 | $9,500 | $8,800 | $8,400 | $8,700 | $8,600 | <.001 |
|
| |||||||
| Mean cancer-related expenditures | $15,700 | $12,800 | $14,000 | $15,400 | $17,000 | $18,900 | <.001 |
|
| |||||||
| Patient’s demographics | |||||||
|
| |||||||
| Age group (%) | <.001 | ||||||
| 67–69 | 22.4 | 22.4 | 23.4 | 21.9 | 21.5 | 22.7 | |
| 70–74 | 27.8 | 27.5 | 27.9 | 27.6 | 27.4 | 28.6 | |
| 74–77 | 22.9 | 22.4 | 22.3 | 22.3 | 24.2 | 23.5 | |
| 78+ | 26.9 | 27.7 | 26.4 | 28.3 | 26.9 | 25.2 | |
|
| |||||||
| Race (%) | 0.01 | ||||||
| White | 83.3 | 83.0 | 83.4 | 85.5 | 81.3 | 83.2 | |
| Black | 9.7 | 13.0 | 6.8 | 8.8 | 6.9 | 12.2 | |
| Other | 7.0 | 3.9 | 9.8 | 5.8 | 11.8 | 4.7 | |
|
| |||||||
| Comorbidity (%) | 0.79 | ||||||
| 0 | 50.2 | 50.4 | 51.6 | 51.4 | 46.9 | 50.5 | |
| 1–2 | 36.8 | 36.3 | 36.1 | 36.3 | 39.0 | 36.6 | |
| 3+ | 13.0 | 13.2 | 12.3 | 12.3 | 14.2 | 12.9 | |
|
| |||||||
| Urban/Rural (%) | <.001 | ||||||
| Metro | 52.8 | 43.8 | 58.8 | 40.4 | 50.6 | 68.5 | |
| Urban | 30.4 | 38.1 | 18.1 | 38.9 | 40.2 | 19.0 | |
| Less urban | 5.9 | 5.9 | 6.7 | 8.6 | 5.9 | 2.9 | |
| Rural | 8.7 | 11.0 | 12.0 | 10.2 | 2.8 | 7.1 | |
| Unknown | 2.1 | 1.2 | 4.4 | 1.9 | 0.5 | 2.4 | |
|
| |||||||
| Median household income at ZIP code level (%) | <.001 | ||||||
| Less than $33,000 | 18.9 | 22.6 | 25.6 | 23.5 | 10.1 | 12.3 | |
| $33,000–40,000 | 15.9 | 17.9 | 16.0 | 18.3 | 12.8 | 14.5 | |
| $40,000–50,000 | 21.0 | 22.8 | 21.0 | 23.7 | 21.2 | 16.8 | |
| $50,000–63,000 | 19.4 | 17.8 | 18.1 | 14.6 | 26.2 | 20.9 | |
| More than $63,000 | 20.6 | 13.4 | 15.3 | 15.5 | 26.6 | 31.6 | |
| Unknown | 4.2 | 5.5 | 4.1 | 4.3 | 3.1 | 4.0 | |
|
| |||||||
| Tumor characteristics | |||||||
|
| |||||||
| Prostate specific antigen (%) | <.001 | ||||||
| 0.1–10.0 | 61.0 | 59.2 | 59.5 | 60.3 | 63.0 | 62.9 | |
| 10.1–20.0 | 14.8 | 13.5 | 15.3 | 17.0 | 14.5 | 14.1 | |
| >20.0 | 8.6 | 8.3 | 9.6 | 9.5 | 7.0 | 8.4 | |
| Unknown | 15.6 | 19.1 | 15.6 | 13.2 | 15.4 | 14.7 | |
|
| |||||||
| Gleason score (%) | 0.09 | ||||||
| 1–6 | 43.8 | 43.8 | 43.4 | 42.9 | 43.3 | 45.1 | |
| 7 | 38.3 | 38.9 | 39.4 | 38.3 | 37.3 | 37.3 | |
| >7 | 16.6 | 15.6 | 16.0 | 17.7 | 17.7 | 16.0 | |
| Unknown | 1.4 | 1.6 | 1.2 | 1.1 | 1.6 | 1.5 | |
|
| |||||||
| Stage (%) | <.001 | ||||||
| T1 | 57.1 | 49.2 | 56.4 | 55.0 | 58.8 | 65.2 | |
| T2 | 40.2 | 47.7 | 40.9 | 42.4 | 38.1 | 32.2 | |
| T3–T4 | 2.4 | 2.8 | 2.1 | 2.4 | 2.5 | 2.1 | |
| Unknown | 0.4 | 0.3 | 0.5 | 0.3 | 0.6 | 0.5 | |
Quintiles were based on HRR level total prostate cancer – related spending in the null model (Model 0).
Table 2.
Prostate Cancer Treatment Spending by Type of Services According to Medicare Spending Quintiles*
| All HRRs | Quintile† | Difference between quintiles 1 and 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean | Median | 95% CI | Lowest | Highest | |||||
|
| |||||||||
| (1) | (2) | (3) | (4) | (5) | |||||
|
| |||||||||
| Total | $15,400 | $16,800 | $15,400–$15,500 | $13,300 | $14,100 | $15,700 | $17,400 | $19,000 | $5,700 |
|
| |||||||||
| Surgery | $1,800 | $1,200 | $1,600–$1,600 | $1,800 | $2,400 | $1,800 | $1,600 | $1,400 | (−$500) |
| Radiotherapy | $10,900 | $11,100 | $10,800–$10,900 | $9,100 | $9,300 | $10,800 | $12,600 | $14,100 | $5,000 |
| Biopsy | $1,100 | $1,100 | $1,100–$1,100 | $1,200 | $1,000 | $1,100 | $1,300 | $1,300 | $100 |
| Hormone therapy | $500 | $400 | $500–$500 | $500 | $500 | $500 | $500 | $500 | $40 |
| Imaging | $200 | $200 | $200–$200 | $200 | $200 | $200 | $300 | $300 | $100 |
Minor discrepancies may exist due to rounding.
Treatment spending by type of services was adjusted for age, comorbidity, race, year of diagnosis, urban/rural residency, median household income at ZIP code, and prostate cancer–related factors, including prostate-specific antigen, Gleason score, and stage.
Quintiles were based on HRR level total prostate cancer–related spending in the null model (Model 0).
HRR: Hospital referral region; CI: Confidence interval
Explaining Geographic Differences
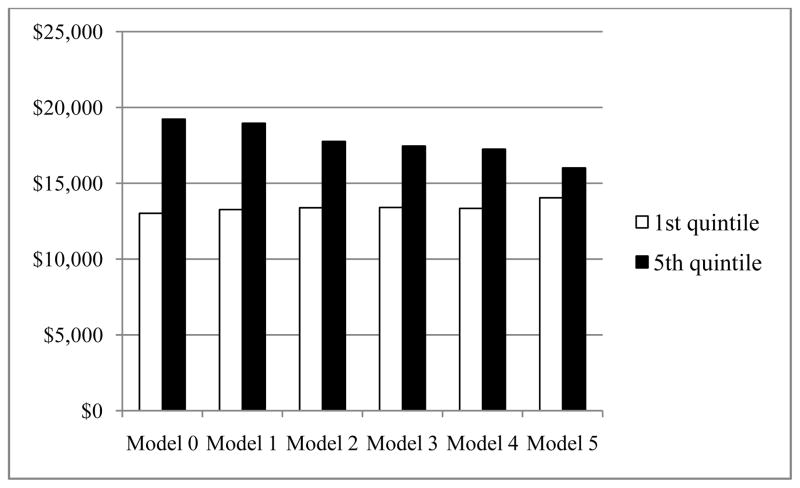
Sequentially adjusting for patient characteristics, treatment intensity, ancillary procedures, and treatment modalities reduced the differences in CaP-related expenditures between the highest and lowest quintiles (Figure 1). The difference between quintiles 5 and 1 was $6,200 in Model 0. After adjusting for demographics and tumor characteristics, the difference between quintiles 5 and 1 was still as high as $5,700 (Model 1), indicating that patients’ characteristics only explained 8.4% of the difference. Adding an indicator for treatment intensity (Model 2) reduced the size of the variation to $4,400, which indicated that treatment intensity could explain an additional 21.2% of the original difference. When we specified surgery and radiotherapy, the difference between quintiles 5 and 1 was decreased to $4,100 (Model 3), explaining an additional 5.2% of the original difference. Including an expanded set of ancillary procedures explained another 2.4% of the original difference (Model 4). The estimation of Model 5, which further specified surgery and radiotherapy modalities, showed that the magnitude of the unexplained variation between quintiles 5 and 1 was substantially reduced to $2,000, indicating that the specific treatment modality explained an additional 31.2% of the original difference. Still, 31.6% of the geographic variation could not be explained by the factors we evaluated.
Figure 1. Regional Variations in Mean Total Prostate Treatment Spending After Adjustment Using a Series of Sequential Models.
Model 0: Null model.
Model 1: Adjusted for age, comorbidity, race, year of diagnosis, urban/rural residency, median household income at ZIP code, prostate-specific antigen level, Gleason score, and stage.
Model 2: Model 1 + curative treatment.
Model 3: Model 1 + any surgery + any radiotherapy.
Model 4: Model 3 + biopsy, hormone therapy, and imaging.
Model 5: Model 4 + specific modalities (open surgery and robotic surgery for surgery; external beam radiation therapy, intensity-modulated radiation therapy, brachytherapy, proton, stereotactic radiosurgery, and other radiotherapy for radiotherapy).
Regional Variation in Utilization Patterns and Corresponding Expenditures
The spending on the individual type of services varied across CaP expenditure quintiles and contributed to the variation of total expenditures differently (Table 2). Radiotherapy accounted for the largest portion of the expenditure variation across HRRs. The difference of spending on radiotherapy per CaP beneficiary between quintiles 5 and 1 was $5,000. In contrast, spending variation in other services was a smaller driver of regional difference in total CaP expenditures. The difference of spending per CaP beneficiary between quintiles 5 and 1 on biopsy, imaging, or hormone therapy only contributed to less than $200 of the total expenditure variation. Furthermore, HRRs with lower total expenditures had higher spending per CaP beneficiary on surgery than HRRs with higher total expenditures.
Treatment intensity varied across quintiles of CaP expenditures, ranging from 56.3% of patients receiving curative treatment in the lowest spending quintile to 63.7% in the highest spending quintile (Table 3). Compared with patients living in quintile 1 HRRs, patients living in quintile 5 HRRs were more likely to receive radiotherapy (57.8% vs. 46.5%), hormone therapy (37.6% vs. 32.9%), and imaging (61.5% vs. 48.9%), and were less likely to receive surgery (13.4% vs. 17.7%). Notably, the largest difference between quintiles 1 and 5 was the percentage of patients receiving IMRT (24.7% vs. 41.9%; p-values<.001 for all comparisons).
Table 3.
Utilization of Prostate Cancer Treatment Modalities According to Medicare Spending Quintiles*
| Mean | Quintile |
Difference between quintiles 1 and 5 | |||||
|---|---|---|---|---|---|---|---|
| Lowest | Highest | ||||||
|
| |||||||
| (1) | (2) | (3) | (4) | (5) | |||
|
| |||||||
| Any curative therapy (i.e., treatment intensity) | 59.8% | 56.3% | 58.3% | 56.9% | 63.7% | 63.7% | 7.4% |
|
| |||||||
| Surgery† | 16.9% | 17.7% | 22.2% | 16.7% | 14.9% | 13.4% | (−4.3%) |
| Robotic surgery† | 6.7% | 6.6% | 8.8% | 6.7% | 7.1% | 4.8% | (−1.8%) |
|
| |||||||
| Radiotherapy† | 50.5% | 46.5% | 43.5% | 47.9% | 56.5% | 57.8% | 11.3% |
| External beam radiotherapy† | 28.1% | 25.1% | 23.8% | 25.9% | 30.7% | 34.6% | 9.5% |
| Intensity-modulated radiation therapy† | 33.0% | 24.7% | 28.1% | 29.9% | 40.1% | 41.9% | 17.2% |
| Brachytherapy† | 8.8% | 11.4% | 5.7% | 6.1% | 8.0% | 12.0% | 0.6% |
| Proton beam radiotherapy† | 0.7% | 0.4% | 0.8% | 1.5% | 0.7% | 0.3% | (−0.1%) |
| Stereotactic radiosurgery† | 0.4% | 0.1% | 0.1% | 0.6% | 0.5% | 0.6% | 0.5% |
|
| |||||||
| Biopsy | 90.7% | 91.6% | 89.2% | 89.4% | 92.0% | 91.2% | (−0.4%) |
|
| |||||||
| Hormone therapy | 36.9% | 32.9% | 35.9% | 38.8% | 39.4% | 37.6% | 4.7% |
|
| |||||||
| Imaging | 55.5% | 48.9% | 51.7% | 52.0% | 63.5% | 61.5% | 12.6% |
Quintiles were based on HRR level total prostate cancer–related spending in the null model (Model 0).
The proportions summed may be larger than the cumulative proportions because patients may receive multiple treatments or different kinds of treatment modalities.
HRR: Hospital referral region
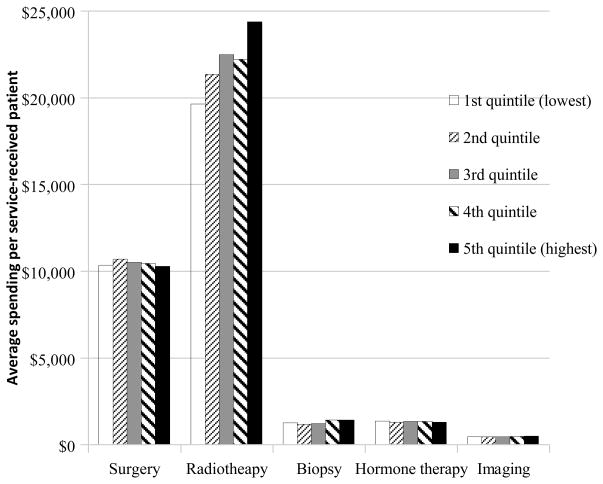
The mean service-specific expenditures per service-received patient across five quintiles demonstrated that radiotherapy expenditures were the key factor driving the regional variation (Figure 2). The mean expenditure on radiotherapy varied from $19,600 per radiated patient in the quintile 1 areas to $24,400 per radiated patient in the quintile 5 areas, a difference of $4,800. In contrast, the average expenditures of surgery or ancillary procedures among patients who received that service did not differ substantially among quintiles.
Figure 2.
Mean Service-Specific Expenditures per Service-Received Patient by the Type of Service, According to the Quintile of Total Prostate Cancer–Related Expenditures
Discussion
We identified substantial geographic variation in initial treatment expenditures among Medicare beneficiaries with localized CaP. Patient and tumor characteristics provided little explanatory power, while treatment intensity and the type of treatment modalities received were the main factors driving the observed variation.
Our findings build upon prior work in important ways. We found that radiotherapy expenditures not only were the largest component of CaP-related spending overall, but also accounted for the largest portion of regional variation. High-spending areas not only provided more radiotherapy services but also used more expensive radiotherapy modalities. In contrast, spending for surgery and ancillary procedures represented a small portion of the variation. Our results suggest that the use of radiotherapy and the cost per-radiated patient are the most important factors driving geographic variation in CaP costs.
Second, we have defined how specific components of CaP care contribute to cancer-related expenditures. Although recent cost-containment initiatives such as the Choosing Wisely Campaign have focused on reducing imaging costs,38 our findings suggest that a decrease in imaging may not control CaP spending substantially. Similarly, there has been increasing concern about biopsy and pathology expenditures,39 with some reports finding that unit costs are estimated as high as $1,100 for prostate biopsy and $700 for pathology,40 yet we found that biopsy and pathology costs contributed little to overall spending for CaP care. In contrast, we found that radiotherapy expenditures accounted for 70% of overall spending, and such expenditures varied substantially, largely due to the variation in the percentage of patients receiving radiotherapy and the treatment modality. While expensive radiotherapy modalities may provide advantages over conventional treatments, such as reducing the likelihood of treatment-related toxicity and tumor recurrence,41 very low-risk CaP patients may not benefit from these modalities since the probability of progression is low. Indeed, conservative management can provide an equitable outcome for these patients. Recent research has shown that the use of expensive treatment modalities has increased among CaP patients who were unlikely to achieve a survival benefit.42 Thus, monitoring the use of advanced technologies for men with low-risk CaP is warranted and is closely aligned to the American Society for Radiation Oncology’s (ASTRO’s) top-five list that suggests that physicians should avoid giving curative therapy without discussing active surveillance first.43
Third, our study underscores the need for research examining expenditure variation for a specific disease. Examining geographic variation in expenditures across populations and including a variety of diseases, researchers found that medical spending was largely explained by disease burden.44 Yet when we focus on a specific disease, we find that comorbidities and tumor characteristics contribute little to this variation. Recent research has also shown that spending for different conditions within an HRR was not strongly correlated, suggesting variation in physician aggressiveness within an HRR.25 Our research demonstrates that, even for a single condition, high total CaP spending areas did not necessarily have high use across all types of services, providing further insight regarding bundling payments for an episode of care.
The services provided by urologists and radiation oncologists counteract their impact on regional variation, as low-spending areas had a relatively high usage rate of surgery but low usage rate of radiotherapy, and vice versa in the high-spending areas. We acknowledge that integrated practices between urologists and radiation oncologists might have different effects on expenditure variations, as patients were more likely to receive IMRT when urologists acquired radiotherapy equipment.45 Therefore, areas of particularly low surgical volume and high radiotherapy volume may represent the practice pattern of integrated urology and radiation oncology practices. As current health reform moves toward targeting clinical decision-making units rather than entire geographic areas, our results indicate that reducing reimbursement for all providers in high-spending areas may unfairly penalize urologists who may have approached CaP treatment with equipoise. Similarly, increasing reimbursement for all providers in low-spending areas may unjustly reward urologists who may have been treating more patients who may have been candidates for active surveillance. Bundled payment without considering different strategies across providers would miss the opportunity to select high-value therapy, and bundled payment across relevant specialties should consider the spectrum of variation that each specialty stands.
Our study has several limitations. Our analyses are based on the SEER–Medicare population, which does not necessarily represent all Medicare beneficiaries. However, we expect that the variation may be even larger in the entire United States.46 We acknowledge that the health status information may not be comprehensive as we only used claims to capture comorbidity. Nevertheless, our results, identifying the key contributors to expenditure variation, are unlikely to change substantially since the SEER program does provide detailed cancer characteristics. Our case-control approach attempted to reduce unmeasured bias regarding expenses for treating diseases other than prostate cancer. We acknowledge that our analysis did not address some unmeasured factors. We might have underestimated the magnitude of the variation in spending on each service across different areas as the quintile is ranked by total expenditure, not by the expenditure of each service. Notably, we still found substantial variation in radiotherapy expenditures. Finally, our study did not assess the association between an area’s spending on indicators of its quality of care, the long-term spending for CaP care, and clinical outcomes, as well as not addressing some important factors such as hospital and physician characteristics on overall variations. Future research is warranted.
In summary, our findings of expenditure variations provide insights for policy makers to develop a Medicare value-based payment system. First, for localized CaP care, patient demographics and tumor characteristics were not a major driver of the expenditure variation. Both the receipt of any curative treatment and the expenditures of services are influential drivers of the overall expenditures. Second, radiotherapy expenditures accounted for 70% of overall spending, and accounted for majority of geographic variation. To develop a bundles payment for CaP care, policy makers should target radiotherapy payment first. Guidelines and/or incentives for appropriate use of expensive modalities are necessary to reduce regional variation in CaP treatment expenditures. Third, expenditures on surgery and radiotherapy in particular are substantial. Since active surveillance is one of the treatment options for low-risk CaP patients, motivating these patients to forgo active treatment implies potential savings without sacrificing clinical effectiveness. Future research should examine the long-term expenditures for conservative management, which could advance our knowledge on the magnitude of cost saving, and may potentially assist in developing a financial incentive to encourage active surveillance for appropriate population.
Supplementary Material
Acknowledgments
Funding: This study was supported by the National Cancer Institute (R01 CA149045 and P30 CA016359).
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER–Medicare database. The interpretation and reporting of the SEER–Medicare data are the sole responsibility of the authors.
Appendix. Sequential models to determine the relative contribution of different factors
For the j-th patient clustered within HRRi, where i=1, 2… 95 and j=1, 2… ni, we calculated the “expected” Medicare expenditure for each beneficiary j as the predicted expenditure conditional on the HRR random effect being equal to zero. We calculated the expected mean CaP-related expenditure per patient for HRRi as the difference of the mean expected expenditures of the cases in HRRi and the mean expected expenditures of the control in HRRi. We assigned the 95 HRRs to five quintiles (k; k=1 to 5) based on their mean CaP-related expenditure per patient in the null model. The adjusted per patient CaP-related expenditure in HRRi was estimated as , where Ōcase,i and Ōcontrol,i are the mean observed expenditure over all cases and controls in HRRi; Ēcase,i and Ēcontrol,i are the mean expected expenditure over all cases and controls in HRRi; and Ōcase, and Ōcontrol are the mean observed expenditures over all cases and controls, respectively, in the entire sample. We then calculated the mean expenditure in quintile k where the estimated CaP-related expenditure of HRRi was weighted by ni (the number of patients in HRRi) in the quintile k. These processes were calculated iteratively foe each model. We specify our sequential models as following:
|
HRR: Hospital referral region; RT: Radiotherapy
References
- 1.National Cancer Institute. [Assessed Feb 7, 2014.];The cost of cancer. http://www.cancer.gov/aboutnci/servingpeople/cancer-statistics/costofcancer.
- 2.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. JCO. 2011;29(12):1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elliott SP, Jarosek SL, Virnig BA. Data Points Publication Series. Rockville (MD): 2011. Changes across time and geography in the use of prostate radiation technologies for newly diagnosed older cancer patients: 2006–2008: Data Points #16. [PubMed] [Google Scholar]
- 4.Yu JB, Soulos PR, Herrin J, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J Natl Cancer Inst. 2013;105(1):25–32. doi: 10.1093/jnci/djs463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantlupe J. [Accessed September 30, 2013.];Bundled payments come to cancer care. HealthLeaders Media [website] 2013 Mar 11; Available at: http://www.healthleadersmedia.com/page-2/COM-289965/Bundled-Payments-Come-to-Cancer-Care.
- 6.Butcher L. ONLINE FIRST: Bundled payments come to oncology. [Accessed September 30, 2013.];Oncology Times. 2011 Nov 1; [online] Available at: http://journals.lww.com/oncology-times/blog/onlinefirst/pages/post.aspx?PostID=318.
- 7.Centers for Medicare and Medicaid Services. [Accessed September 30, 2013.];Bundled Payments for Care Improvement (BPCI) Initiative. 2013 Available at: http://innovation.cms.gov/initiatives/bundled-payments/index.html.
- 8.American Medical Association. [Accessed September 30, 2013.];Evaluating and negotiating emerging payment options [AMA web site] 2012 Available at: http://www.ama-assn.org/resources/doc/psa/payment-options.pdf#page=87.
- 9.Altman SH. The lessons of Medicare’s prospective payment system show that the bundled payment program faces challenges. Health Aff (Millwood) 2012;31(9):1923–1930. doi: 10.1377/hlthaff.2012.0323. [DOI] [PubMed] [Google Scholar]
- 10.Epstein AM. Geographic variation in Medicare spending. N Engl J Med. 2010;363(1):85–86. doi: 10.1056/NEJMe1005212. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland JM, Fisher ES, Skinner JS. Getting past denial--the high cost of health care in the United States. N Engl J Med. 2009;361(13):1227–1230. doi: 10.1056/NEJMp0907172. [DOI] [PubMed] [Google Scholar]
- 12.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11):1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krupski TL, Kwan L, Afifi AA, et al. Geographic and socioeconomic variation in the treatment of prostate cancer. JCO. 2005;23(31):7881–7888. doi: 10.1200/JCO.2005.08.755. [DOI] [PubMed] [Google Scholar]
- 14.Spencer BA, Fung CH, Wang M, et al. Geographic variation across veterans affairs medical centers in the treatment of early stage prostate cancer. J Urol. 2004;172(6 Pt 1):2362–2365. doi: 10.1097/01.ju.0000144064.54670.7b. [DOI] [PubMed] [Google Scholar]
- 15.Harlan L, Brawley O, Pommerenke F, et al. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. JCO. 1995;13(1):93–100. doi: 10.1200/JCO.1995.13.1.93. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton AS, Wu XC, Lipscomb J, et al. Regional, provider, and economic factors associated with the choice of active surveillance in the treatment of men with localized prostate cancer. J Natl Cancer Inst Monogr. 2012;2012(45):213–220. doi: 10.1093/jncimonographs/lgs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nambudiri VE, Landrum MB, Lamont EB, et al. Understanding variation in primary prostate cancer treatment within the Veterans Health Administration. Urology. 2012;79(3):537–545. doi: 10.1016/j.urology.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Wilt TJ, Cowper DC, Gammack JK, et al. An evaluation of radical prostatectomy at Veterans Affairs Medical Centers: time trends and geographic variation in utilization and outcomes. Med Care. 1999;37(10):1046–1056. doi: 10.1097/00005650-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Lai S, Lai H, Krongrad A, et al. Radical prostatectomy: geographic and demographic variation. Urology. 2000;56(1):108–115. doi: 10.1016/s0090-4295(00)00557-4. [DOI] [PubMed] [Google Scholar]
- 20.Lai S, Lai H, Lamm S, et al. Radiation therapy in non-surgically-treated nonmetastatic prostate cancer: geographic and demographic variation. Urology. 2001;57(3):510–517. doi: 10.1016/s0090-4295(00)01034-7. [DOI] [PubMed] [Google Scholar]
- 21.Makarov DV, Loeb S, Landman AB, et al. Regional variation in total cost per radical prostatectomy in the healthcare cost and utilization project nationwide inpatient sample database. J Urol. 2010;183(4):1504–1509. doi: 10.1016/j.juro.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Williams SB, Gu X, Lipsitz SR, et al. Utilization and expense of adjuvant cancer therapies following radical prostatectomy. Cancer. 2011;117(21):4846–4854. doi: 10.1002/cncr.26012. [DOI] [PubMed] [Google Scholar]
- 23.Barlow WE. Overview of methods to estimate the medical costs of cancer. Medical care. 2009;47(7 Suppl 1):S33–36. doi: 10.1097/MLR.0b013e3181a2d847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Rep. 2004;119(3):263–270. doi: 10.1016/j.phr.2004.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newhouse JP, Garber AM. Geographic variation in Medicare services. N Engl J Med. 2013;368(16):1465–1468. doi: 10.1056/NEJMp1302981. [DOI] [PubMed] [Google Scholar]
- 26.Dartmouth Medical School. Center for the Evaluative Clinical Sciences. . The Dartmouth Atlas of Health Care. Chicago: American Hospital Publishing; 1996. [PubMed] [Google Scholar]
- 27. [Accessed June 9, 2013.];The Dartmouth Atlas of Health Care: crosswalks, ZIP code crosswalks 1995–2010 [downloads] 2011 Available at: http://www.dartmouthatlas.org/tools/downloads.aspx.
- 28.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB, Brown ML. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100(12):888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Market basket data [database online] Baltimore, MD: Centers for Medicare & Medicaid Services; 2013. [Accessed June 9, 2013.]. Available at: http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareProgramRatesStats/MarketBasketData.html. [Google Scholar]
- 31.Centers for Medicare and Medicaid Services. PFS Relative Value Files. 2013. [Google Scholar]
- 32.Centers for Medicare and Medicaid Services. Wage Index Files. 2013. [Google Scholar]
- 33.Dixon WJ. Trimming and winsorization: A review. Statistical Papers. 1974;15(2–3):157–170. [Google Scholar]
- 34.Adams JL, Mehrotra A, Thomas JW, et al. Physician cost profiling: reliability and risk of misclassification. N Engl J Med. 2010;362(11):1014–1021. doi: 10.1056/NEJMsa0906323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jha AK, Orav EJ, Dobson A, et al. Measuring efficiency: the association of hospital costs and quality of care. Health Aff (Millwood) 2009;28(3):897–906. doi: 10.1377/hlthaff.28.3.897. [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Strawderman RL, Cowen ME, et al. A flexible two-part random effects model for correlated medical costs. J Health Econ. 2010;29(1):110–123. doi: 10.1016/j.jhealeco.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Medical care. 2009 Jun;47(6):626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 38.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. JCO. 2012;30(14):1715–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- 39.Ma X, Wang R, Long JB, et al. The cost implications of prostate cancer screening in the Medicare population. Cancer. 2014;120(1):96–102. doi: 10.1002/cncr.28373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keegan KA, Dall’Era MA, Durbin-Johnson B, et al. Active surveillance for prostate cancer compared with immediate treatment: an economic analysis. Cancer. 2012;118(14):3512–3518. doi: 10.1002/cncr.26688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hummel S, Simpson EL, Hemingway P, et al. Intensity-modulated radiotherapy for the treatment of prostate cancer: a systematic review and economic evaluation. Health Technol Assess. 2010;14(47):1–108. iii–iv. doi: 10.3310/hta14470. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA. 2013;309(24):2587–2595. doi: 10.1001/jama.2013.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American Society for Radiation Oncology. [Accessed September 30, 2013.];ASTRO releases list of five radiation oncology treatments to question as part of national choosing wisely campaign [news release] 2013 Sep 23; Available at: https://www.astro.org/uploadedFiles/Main_Site/News_and_Media/News_Releases/2013/Choosing_Wisely_PR_FINAL_091713.pdf.
- 44.Reschovsky JD, Hadley J, Romano PS. Geographic variation in fee-for-service Medicare beneficiaries’ medical costs is largely explained by disease burden. Med Care Res Rev. 2013;70(5):542–63. doi: 10.1177/1077558713487771. [DOI] [PubMed] [Google Scholar]
- 45.Bekelman JE, Suneja G, Guzzo T, et al. Effect of practice integration between urologists and radiation oncologists on prostate cancer treatment patterns. J Urol. 2013;190(1):97–101. doi: 10.1016/j.juro.2013.01.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobson M, Earle CC, Newhouse JP. Geographic variation in physicians’ responses to a reimbursement change. N Engl J Med. 2011;365(22):2049–2052. doi: 10.1056/NEJMp1110117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.