Abstract
A novel heparin- and cellulose-based biocomposite is fabricated by exploiting the enhanced dissolution of polysaccharides in room temperature ionic liquids (RTILs). This represents the first reported example of using a new class of solvents, RTILs, to fabricate blood-compatible biomaterials. Using this approach, it is possible to fabricate the biomaterials in any form, such as films or membranes, fibers (nanometer- or micron-sized), spheres (nanometer- or micron-sized), or any shape using templates. In this work, we have evaluated a membrane film of this composite. Surface morphological studies on this biocomposite film showed the uniformly distributed presence of heparin throughout the cellulose matrix. Activated partial thromboplastin time and thromboelastography demonstrate that this composite is superior to other existing heparinized biomaterials in preventing clot formation in human blood plasma and in human whole blood. Membranes made of these composites allow the passage of urea while retaining albumin, representing a promising blood-compatible biomaterial for renal dialysis, with a possibility of eliminating the systemic administration of heparin to the patients undergoing renal dialysis.
Keywords: biomaterials, heparin, renal dialysis, ionic liquids, cellulose
INTRODUCTION
The first generation of biomaterials (such as gold) was used as inert structural materials, but recent biomaterials are active materials that interact appropriately with tissues and organs.1,2 These bioactive materials often exhibit biocompatibility, blood compatibility, and biodegradability, and are currently being investigated as kidney dialysis membranes, artificial organs, drug delivery matrices, and tissue engineering scaffolds. The membranes used for kidney dialysis should have excellent biocompatibility and blood compatibility to render its function, without activating the complement and coagulation cascade and triggering the blood clotting.3 Heparin (or low molecular weight heparins) is administered, either by intravenous or subcutaneous route, to maintain blood flow in patients on extracorporeal therapy,4 such as blood oxygenation or kidney dialysis, or in the treatment of clots formed in disease states, such as deep vein thrombosis.5 Often significant side effects result, including life-threatening medical implications, such as hemorrhage, requiring the application of heparin reversal agents or methods, which have their own set of side effects.6,7
Advances have been made in reducing or eliminating the need for systemic heparinization in extracorporeal therapy by preparing biomaterials with improved blood compatibility.8 Heparin immobilized to a surface, enhances the blood compatibility of that surface, reducing platelet adhesion, loss of blood cells, and increasing plasma recalcification time and activated partial thromboplastin time (APTT). Immobilized heparin, unlike soluble heparin, also inhibits initial contact activation enzymes through an antithrombin-mediated pathway, and thus has enhanced anticoagulant properties.9 Improving the biocompatibility of various polymers has primarily focused on the surface immobilization of heparin.10–12
Cellulose, a carbohydrate polymer, has very good thermal, mechanical, biostable, and biocompatible properties.13,14 Chemically modified celluloses find a wide range of medical applications, particularly in the preparation of dialysis membranes and implantable sponges. The chemical modification of cellulose is complicated by its high degree of crystallinity, resulting in its insolubility in water and most conventional organic solvents (other than N-methylmorpholine-N-oxide, CdO/ethylenediamine, LiCl/N,N′-dimethylacetamide, and near supercritical water). Room temperature ionic liquids (RTILs) are organic liquid salts that are generating increased interest as “green” solvents, because of their low vapor pressure and recycling possibility.15,16 RTILs that are capable of dissolving many nonpolar and polar compounds, including carbohydrates, have been designed and synthesized.17,18 Recently, Swatloski and coworkers employed microwave irradiation to dissolve up to 25% (w/w) of unmodified cellulose in the RTIL 1-butyl-3-methylimidazolium chloride [bmIm][Cl].19 We recently reported the dissolution of up to 10 mg/mL heparin (originally soluble only in dimethyl sulfoxide, dimethyl formamide, and formamide, apart from water) in the RTILs 1-ethyl-3-methylimidazolium benzoate ([emIm][ba]) and 1-butyl-3-methylimidazolium benzoate ([bmIm][ba]).20 Here, we demonstrate the fabrication of a novel heparin and cellulose-based biocomposite by exploiting the enhanced dissolution of polysaccharides in RTILs. Even though the biomaterial can be fabricated in many forms and shapes, composite membranes were chosen as a model form in this study. This novel composite exhibits superior clotting kinetics, as measured by APTT and thromboelastography (TEG). Membranes made of these composites allow the passage of urea while retaining albumin, representing a promising blood-compatible biomaterial membrane for renal dialysis, without requiring systemic administration of anticoagulants.
MATERIALS AND METHODS
Materials
Heparin (sodium salt, extracted from porcine intestinal mucosa, USP activity 169 U/mg) was bought from Celsus Laboratories. Cellulose, [bmIm][Cl], [emIm][Cl], and Dowex® cationic resin were bought from Sigma Aldrich. Benzoic acid and imidazole were bought from Fisher Scientific. [EmIm][ba] was prepared by following the existing protocol.20
Biocomposite Material Fabrication
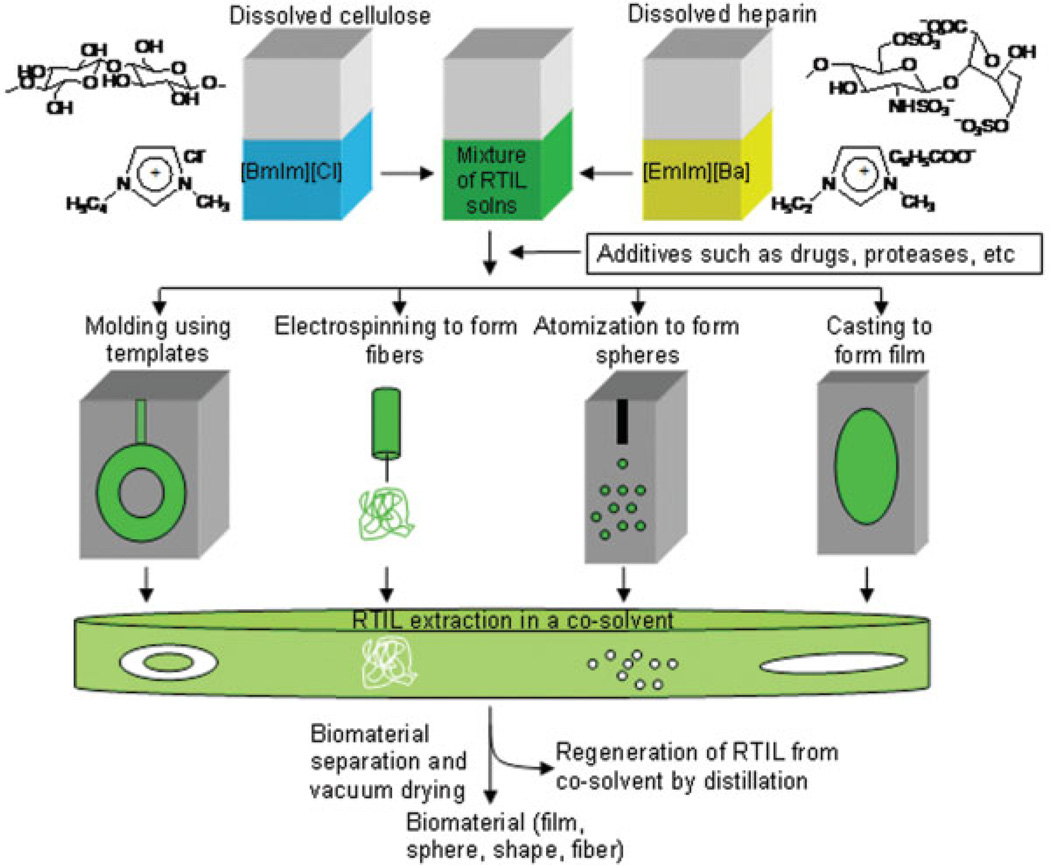
The imidazolium salt of heparin was prepared from the sodium salt using ion exchange chromatography (Dowex cationic (H+) resin) followed by neutralization with imidazole. Approximately 7 mg of imidazolium heparin was added to 400 mg (500 µL) of [emIm][ba]. The contents were then mixed by vortexing and heated to 35°C for about 20 min, to afford a clear solution of heparin in [emIm][ba]. Cellulose was dissolved in [bmIm][Cl] (1.0 g) by preheating the RTIL to 70°C, and then adding 100 mg of cellulose. The contents were then mixed by vortexing and microwaved for 4–5 s, to afford a 10% (w/w) cellulose in [bmIm][Cl]. The solution of heparin and cellulose in RTIL were combined and mixed by vortexing for 5–10 s (other materials such as drugs or enzymes can be added at this step) to afford a solution of both polysaccharides. The resulting solution of heparin and cellulose can be fabricated into biomaterials in various shapes and forms, including films or membranes (by membrane casting), micro- or nanospheres (atomization), micro- or nanofibers (electrospinning), or any other shapes molded by using templates (Figure 1). Once solidified, this biocomposite was immersed in ethanol, which could dissolve both the RTILs, leaving a composite of cellulose matrix with immobilized heparin, The biocomposite film was washed extensively with ethanol, to completely extract all RTIL (as confirmed by the absence of residual RTIL in the ethanol wash), and the biocomposite film was then dried in vacuo.
Figure 1.
Schematic representation for the preparation of heparin–cellulose composite biomaterials. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Scanning Electron and Atomic Force Microscopic Techniques
The cellulose and the heparin–cellulose composite films were analyzed with an electron beam at an acceleration voltage of 5 kV under field emission scanning electron microscopy (FESEM), using a JEOL JSM-6332 equipped with secondary electron detectors. Prior to FESEM analysis, the film was subjected to gold sputtering, to make the film conductive. The surface morphological differences between the cellulose film and heparin–cellulose composite film were characterized using tapping mode–atomic force microscopy (TM-AFM) on a Veeco D3100 scanning probe microscope. The films were placed on an atomically flat silicon wafer surface and dried by applying weights in vacuo. Both the topography and the phase were recorded.
Activated Partial Thromboplastin Time
In a typical experiment, the film (0.5 × 0.5 cm2) was affixed to a cup of fibrometer (BBL Fibrometer, Beckton Dickinson Microbiology Systems, Cockeysville, MD) followed by the addition of an automated APTT reagent (100 µL) and warmed for a minute at 37°C. Platelet-poor-human plasma (100 µL) was added, followed by 5 min incubation at 37°C; CaCl2 (100 µL of 0.025M) was then added to recalcify the citrated plasma. The plasma solution was observed for clotting by using the fibrometer. APTT was measured in triplicate.
Thromboelastography
The clotting kinetics of the human whole blood was also assessed in the presence of biocomposites by using TEG. TEG has been used as a widely useful technique in hospitals to study the abnormalities in the coagulation pathway of the patients. TEG works by measuring the physical viscoelastic characteristics of blood. Typically, a biocomposite film (0.5 × 0.5 cm2) was placed in a TEG cup, followed by the addition of 350 µL of human whole blood and incubated for 5 min. Ten microliters of 0.01M CaCl2 was added to recalcify the citrated blood. A coaxially suspended stationary piston was then placed on the cup, with a clearance of 1 mm. This pin is suspended by a torsion wire that transduces the torque. The cup is oscillated at an angle of 4° 45′ in either direction every 4.5 s. During the clot formation, fibrin fibrils link the cup to the pin, which influences the rotation of the pin, and the disturbance is measured and displayed by a computer. The display called thromboelastogram plots the torque experienced by the pin as a function of time. TEG studies the coagulation by measuring various factors, including the latent time for clot initiation (R), the time to initiate a fixed clot firmness of around 20 mm amplitude (k), the kinetics of clot development (angle α), and the maximum amplitude (MA) of the clot. This is another way of measuring the blood compatibility of the heparin–cellulose biocomposite films.
Equilibrium Dialysis of Urea and BSA
The film was fixed into an equilibrium dialysis cell between equal volumes of buffer. An 8 mg sample of either urea or BSA in PBS buffer (8 mL) was loaded onto the high-concentration-donor side (H) and 8 mL of PBS buffer was loaded onto the low-concentration-receiver side (L). Aliquots were simultaneously removed at periodic intervals from each side, and their solute concentration was analyzed by measuring UV absorbance (206 nm for urea and 280 nm for BSA). Concentrations were calculated using a standard curve of each solute.
RESULTS AND DISCUSSION
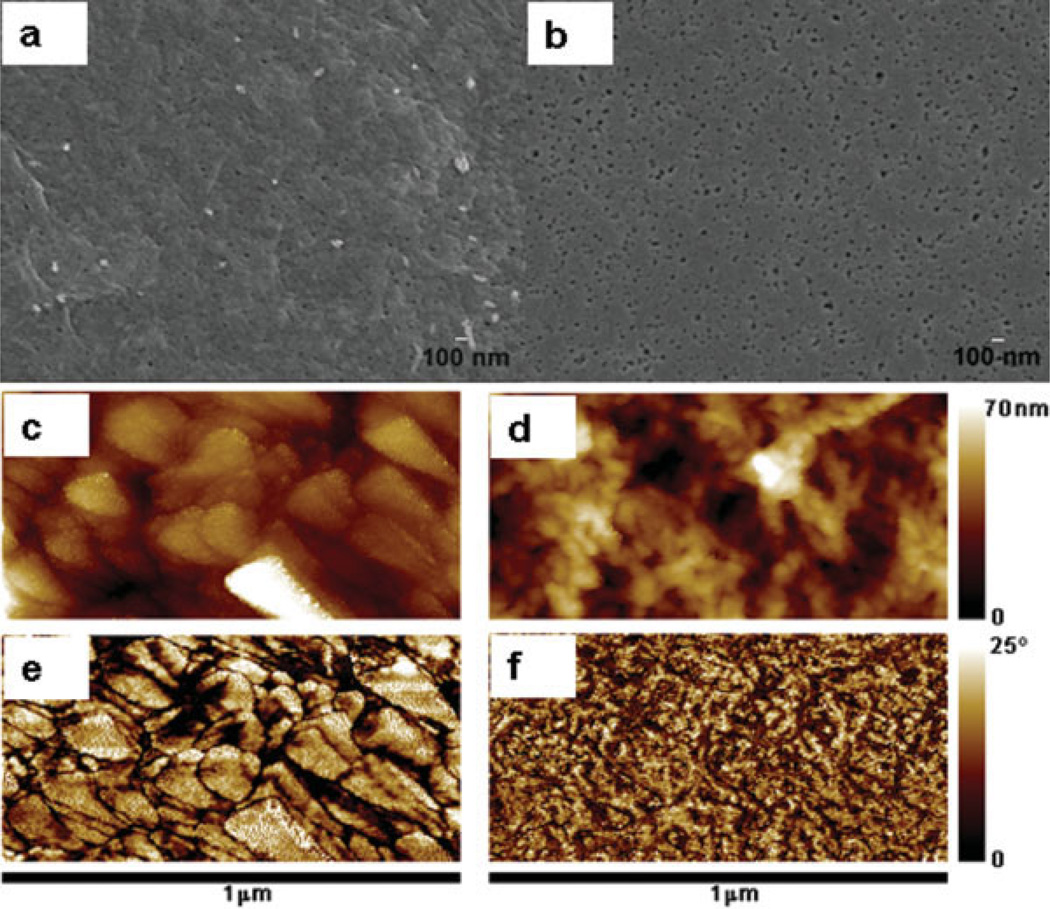
The FESEM micrographs of the cellulose and the heparin–cellulose composite films [Figure 2(a,b)] show uniformly formed nanosized pores throughout the films. This property suggested the utility as a membrane, particularly for dialysis applications. The heparin–cellulose composite film [Figure 2(b)] does not show the flaking, observed in pure cellulose films [Figure 2(a)], and it has a uniform smooth surface with a larger number of nanopores. The nanopores found on the surface of this film have diameters ranging from 20 to 40 nm. The TM-AFM imaging [Figure 2(c–f)] clearly distinguished the cellulose and the heparin–cellulose heparin composite films. The topography [Figure 2(c)] and the phase [Figure 2(e)] images of the cellulose film correlated to each other, both showing a monomodal distribution with a flaky structure in consistence with the FESEM observation. Phase imaging is sensitive to sudden changes in topography, such as at the edge of the flakes. However, the flakes themselves do not reveal any phase contrast, indicating that they are of uniform elasticity, and composed of the same material (cellulose). In the case of heparin–cellulose composite film, the topographic image [Figure 2(d)] did not correlate with the phase image [Figure 2(f)]. The topography image (d) of heparin–cellulose composite film revealed the formation of globular-shaped heparin domains in the cellulose matrix. This globular alignment was found uniformly across the surface. The phase image (f) reveals distinct bimodal contrast that does not correlate to topography. This contrast arises from difference in elasticity between the cellulose and heparin, indicating that there are two distinct phases (cellulose and heparin). This phase image (f) demonstrates that heparin is uniformly distributed in the cellulose matrix.
Figure 2.
Surface morphology of the cellulose and the heparin–cellulose composite films. (a, b) FESEM; (c, d) AFM topography; and (e, f) AFM phase images of the cellulose-only film (a, c, and e) and heparin–cellulose composite film (b, d, and f). FESEM images are presented at ×30,000 magnification. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
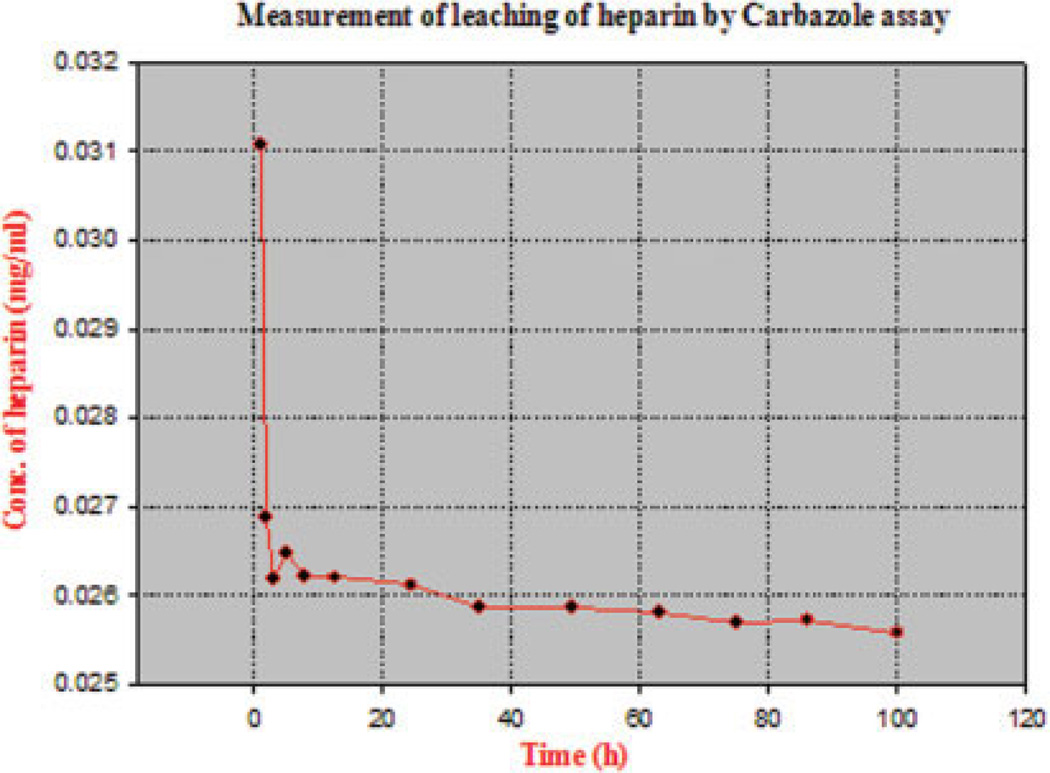
The degree of entrapment of the water-soluble heparin by the water-insoluble cellulose was studied by incubating the composite film (105 mg) in water (10 mL). Samples of water were withdrawn at periodic time intervals, and the heparin concentration was determined by carbazole assay against a standard curve.21 Residual heparin in the composite film could also be estimated by dissolving the composite film and subjecting this solution to the carbazole assay. The maximum leaching of heparin (Figure 3) was observed in the first 20 min of the experiment, consistent with a commonly observed “burst effect.”22,23 Little leaching was observed after this initial burst effect even after prolonged (100 h) washing, with less than 500 µg being lost from the 7 mg of heparin in the composite film. The results of carbazole assay used to determine the amount of heparin present before and after leaching of a heparin–cellulose composite suggest that 92% of the heparin within the heparin–cellulose composite film was not leachable. Further, elemental analysis (C, H, N, and S) (Galbraith Laboratories, Knoxville, TN, USA) of cellulose film showed the absence of nitrogen or sulphur, while composite films showed the expected values based on the synthesis described in Figure 1.
Figure 3.
The concentration of heparin measured, releasing out of film, plotted as a function of time. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Alcian blue (a cationic dye that binds to the negative groups) staining clearly demonstrated the presence of the dye-accessible heparin even after leaching (data not shown), demonstrating that the heparin present within the composite was sufficiently accessible for chemical reaction.
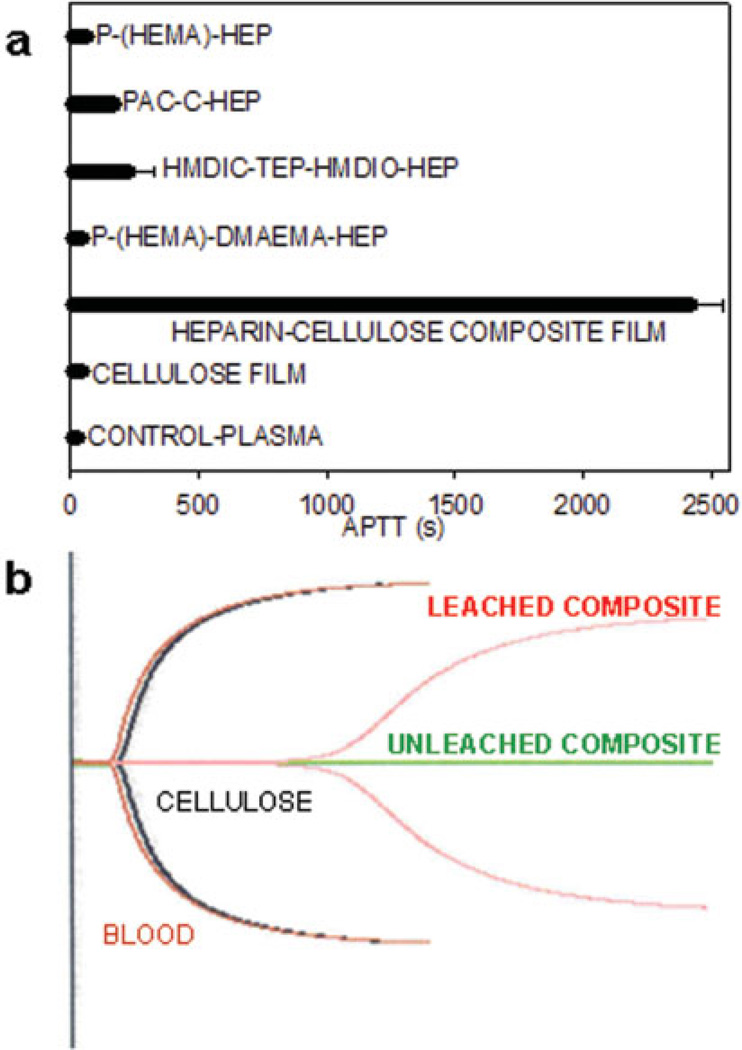
APTT is routinely used for evaluating the blood compatibility of various heparinized polymer surfaces.24,25 APTT was used to measure the in vitro plasma anticoagulant activity of heparin present in both leached and unleached composite films against a cellulose control [Figure 4(a)]. Plasma on the unleached heparin–cellulose composite film did not clot, as the film releases heparin into the plasma, providing no measurable APTT. Plasma on cellulose behaved similar to control APTT of 32.3 ± 2 s. Plasma on unleached composite showed no clot in over 1 h, while the leached composite showed an APTT value of 2424.3 ± 120.5 s (n = 3)). This value compared favorably to other heparinized polymers, including poly(2-hydroxyethylmethacrylate)-dimethylaminoethylmethacrylate (P-(HEMA)-DMAEMA-HEP),24 hexamethylene diisocyanate–tetraethylenepentamine–hexamethylene diisocyanate (HMDIO-TEP-HMDIC-HEP),26 polyacyrlonitrile–chitosan (PAN-C-HEP),12 and poly(2-hydroxyethylmethacrylate) (P-(HEMA)-HEP).27
Figure 4.
Clotting properties of heparin–cellulose composite membrane. (a) APTT values of the cellulose and heparin–cellulose composite membranes compared with existing heparinized biomaterials. (b) TEGs of blood alone (control in red), cellulose film (black), leached heparinized biofilm (pink) and intact heparinized biofilm (green); The characteristics of the pink TEG (elongated clotting time, reduced clot formation, formation of weaker clot, and slower rate of clot formation) clearly demonstrates heparin is present in its bioactive form. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
TEG measures anticoagulant activity in human whole blood.28 A comparison of TEGs in the absence and presence of cellulose film reveals nearly identical curves [Figure 4(b)]. The TEG of the leached heparin–cellulose composite film shows an extended latent time of clotting initiation. The shape of this TEG is also different from the controls, demonstrating the presence of active anticoagulant heparin on the composite surface. The clotting parameters measured by TEG are given in Table I. The time taken to form 20 mm clot (K) was prolonged, and the rate of clot formation (α) and the maximum clot strength (MA) were reduced when treated with leached heparin–cellulose composite film. Again, as with APTT, no clot was formed during the 2 h evaluation of the unleached composite, and this was evaluated as due to the release of weakly bound heparin. The clotting kinetic parameters of 1 U/mL solution heparin are also provided from the literature for comparison. Thus, the heparin–cellulose composite film has excellent blood-compatible properties, as observed by both plasma-based APTT 2424.3 ± 120.5 s and whole blood-based TEG studies.
TABLE I.
Clotting Kinetics in the Presence of Cellulose and Heparin–Cellulose Composite Film Determined in Human Whole Blood Using TEG
| Entity | R (min) | K (min) | α (degrees) | MA (mm) |
|---|---|---|---|---|
| Human whole blood (control) | 3.9 | 1.7 | 65.6 | 55.9 |
| Plain cellulose film (control) | 4.6 | 1.7 | 65.9 | 55.9 |
| Heparin–cellulose composite film-leached | 23.0 | 7.8 | 20.4 | 44.8 |
| Heparin–cellulose composite film-unleached | 121.7a | 0 | 0 | 0 |
| Free heparin (1 U/mL)29 | 41.5 | 20 | 16.5 | 58.5 |
Latent time for clot initiation (R), time to initiate a fixed clot firmness of 20 mm amplitude (k), kinetics of clot development (α angle), and the maximum amplitude (MA) of the clot were recorded.
No clot observed.
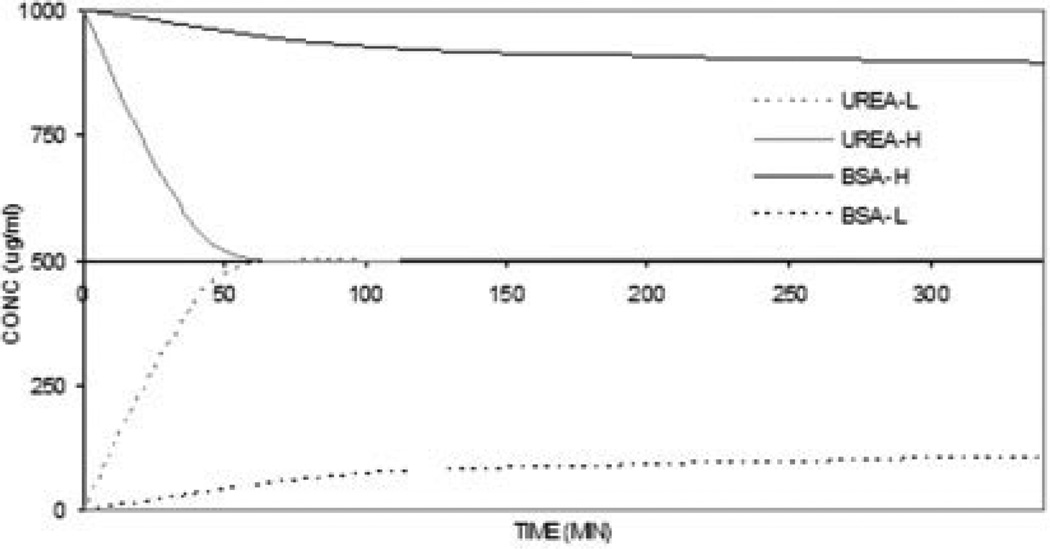
The utility of the leached heparin–cellulose composite film for dialysis was evaluated next. The film was fixed into an equilibrium dialysis cell between equal volumes of buffer. The low MW (60.1) urea and the high MW (67,000) albumin involved in kidney dialysis were chosen for this equilibrium dialysis study across the composite film (Figure 5) from high concentration to low concentration side. An 8 mg sample of either urea or BSA in PBS buffer (8 mL) was added to the first compartment of the equilibrium dialysis cell, and the second compartment was filled with PBS buffer. Aliquots were taken at various time points from the second compartment, and the solute concentration was analyzed by measuring UV absorbance (206 nm for urea and 280 nm for BSA). Urea freely dialyzed across the membrane, reaching equilibrium in less than 60 min. The diffusivity of urea across this membrane was found to be 3.6 × 10−10 m2/s. Only slight movement of BSA was seen across the membrane, reaching <10% of its equilibrium value even after 45 h (data shown only for 5.6 h), suggesting a promising application for these heparin–cellulose composite films as kidney dialysis membranes.
Figure 5.
Dialysis properties of heparin–cellulose composite membrane. Equilibrium dialysis of urea and BSA across the heparin–cellulose composite film from high concentration side (H) to the low concentration side (L).
Protamine sulfate, commonly used as a heparin reversal agent, can cause severe complications.30 The current approach avoids systemic heparinization, thus, eliminating the need for protamine neutralization. Also during hemodialysis, on repeated exposure to heparin, patients can develop antiheparin-platelet factor 4 antibodies.6 These antibodies are a major risk factors involved in many thrombotic complications, including heparin-induced thrombocytopenia (HIT). The current approach should significantly reduce the concentration of circulating heparin. Hence, the chances for the generation of antiheparin-platelet factor 4 antibodies and HIT may be significantly reduced.
In conclusion, a novel technique has been used to fabricate a heparin–cellulose-based biocomposite using RTILs. While this composite can be prepared in a variety of forms, preliminary studies evaluated a membrane film of this composite. This film was characterized using FESEM, AFM, dye binding, carbazole, and elemental analysis. These analyses showed a very uniform composite in which heparin was stably immobilized but still chemically and biologically accessible. The biocompatibility of the film was clearly demonstrated by APTT and TEG measurements and implied superior performance to other heparinized biomaterials reported in the literature. Finally, these membranes can be used in dialysis, suggesting a potential, new and valuable material to make blood-compatible, hollow fiber, nanoporous membranes for kidney dialysis that might eliminate the requirement for systemic heparinization.
Acknowledgments
We thank Prof. Georges Belfort, Prof. Chang Ryu, Dr. Ashavani Kumar, Dr. Anand Kumar, and Dr. Gunaranjan Viswanathan for their informative discussions.
Contract grant sponsor: NIH; contract grant numbers: HL 52622, GM 38060, and HL 62244
REFERENCES
- 1.Amass W, Amass A, Tighe B. A review of biodegrable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradable studies. Polym Int. 1998;47:89–144. [Google Scholar]
- 2.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 3.Modi GK, Pereira BJG, Jaber BL. Hemodialysis in acute renal failure : Does the membrane matter? Semin Dial. 2001;14:318–321. doi: 10.1046/j.1525-139x.2001.00078.x. [DOI] [PubMed] [Google Scholar]
- 4.Brieterman-White R. A review of heparin use in hemodialysis and peritoneal dialysis. ANNA J. 1995;22:491–493. [PubMed] [Google Scholar]
- 5.Rydberg EJ, Westfall MJ, Nicholas RA. Low molecular weight heparin in preventing and treating DVT. Am Fam Physician. 1999;59:1607–1612. [PubMed] [Google Scholar]
- 6.O’Shea SI, Ortel TL, Kovalik EC. Alternative methods of anticoagulation for dialysis-dependent patients with heparin-induced thrombocytopenia. Semin Dial. 2003;16:61–67. doi: 10.1046/j.1525-139x.2003.03014.x. [DOI] [PubMed] [Google Scholar]
- 7.Langer R, Linhardt RJ, Hoffberg S, Larsen AK, Cooney CL, Tapper D, Klein M. An enzymatic system for removing heparin in extracorporeal therapy. Science. 1982;217:261–263. doi: 10.1126/science.7089564. [DOI] [PubMed] [Google Scholar]
- 8.Chanard J, Lavaud S, Randoux C, Rieu P. New insights in dialysis membrane biocompatibility: Relevance of adsorption properties and heparin binding. Nephrol Dial Transplant. 2003;18:252–257. doi: 10.1093/ndt/18.2.252. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez J, Elgue G, Riesenfeld J, Olsson P. Studies of adsorption, activation and inhibition of factor XII on immobilized heparin. Thromb Res. 1998;89:41–50. doi: 10.1016/s0049-3848(97)00310-1. [DOI] [PubMed] [Google Scholar]
- 10.Keuren JFW, Wielders JH, Driessen A, Verhoeven M, Hendricks M, Lindhout T. Covalently-bound heparin makes collagen thromboresistant. Arterioscler Thromb Vasc Biol. 2004:613–617. doi: 10.1161/01.ATV.0000116026.18945.66. [DOI] [PubMed] [Google Scholar]
- 11.Kang I, Kwon OH, Lee YM, Sung YK. Preparation and surface characterization of functional group-grafted and heparin-immobilized polyurethanes by plasma glow discharge. Biomaterials. 1996;17:841–847. doi: 10.1016/0142-9612(96)81422-0. [DOI] [PubMed] [Google Scholar]
- 12.Yang MC, Lin WC. Surface modification and blood compatibility of polyacrylonitrile membrane with immobilized chitosan–heparin conjugate. J Polym Res. 2002;9:201–206. [Google Scholar]
- 13.Risbud MV, Bhonde RR. Suitability of cellulose molecular dialysis membrane for bioarificial pancreas: In vitro biocompatibility studies. J Biomed Mater Res. 2001;54:436–444. doi: 10.1002/1097-4636(20010305)54:3<436::aid-jbm180>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto T, Takahashi S, Ito H, Inagaki H, Noishiki Y. Tissue compatibility of cellulose and its derivatives. J Biomed Mater Res. 1989;23:125–133. doi: 10.1002/jbm.820230110. [DOI] [PubMed] [Google Scholar]
- 15.Sheldon R. Catalytic reactions in ionic liquids. Chem Commun (Camb) 2001;7:2399–2407. doi: 10.1039/b107270f. [DOI] [PubMed] [Google Scholar]
- 16.Seddon KR. Ionic liquids: Designer solvents for green synthesis. Tce. 2002;730:33–35. [Google Scholar]
- 17.Murugesan S, Karst N, Islam T, Wiencek JM, Linhardt RJ. Dialkyl imidazolium benzoates—Room temperature ionic liquids useful in peracetylation and perbenzoylation of simple and sulfated saccharides. Synlett. 2003;9:1283–1286. [Google Scholar]
- 18.Murugesan S, Linhardt RJ. Ionic liquids in carbohydrate chemistry—Current trends and future directions. Curr Org Synth. 2005;2:437–451. [Google Scholar]
- 19.Swatloski RP, Spear SK, Holbrey JD, Rogers RD. Dissolution of cellulose with ionic liquids. J Am Chem Soc. 2002;8:4974–4975. doi: 10.1021/ja025790m. [DOI] [PubMed] [Google Scholar]
- 20.Murugesan S, Wiencek JM, Ren RX, Linhardt RJ. Benzoate-based room temperature ionic liquids—Thermal properties and glycosaminoglycan dissolution. Carbohydr Polym. 2006;63:268–271. [Google Scholar]
- 21.Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73:121–136. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 23.Narasimhan B, Langer R. Zero-order release of micro and macromolecules from polymeric devices: The role of burst effect. J Control Release. 1997;47:13–20. [Google Scholar]
- 24.Denizli A. Heparin immobilized poly(2-hydroxyethyl methacrylate)-based microspheres. J Appl Polym Sci. 1999;74:655–662. [Google Scholar]
- 25.Lin WC, Liu TY, Yang MC. Hemocompatibility of polyacrylonitrile dialysis membrane immobilized with chitosan and heparin conjugate. Biomaterials. 2004;25:1947–1957. doi: 10.1016/j.biomaterials.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Marconi W, Benvenuti F, Piozzi A. Covalent bonding of heparin to a vinyl copolymer for biomedical applications. Biomaterials. 1997;18:885–890. doi: 10.1016/s0142-9612(97)00015-x. [DOI] [PubMed] [Google Scholar]
- 27.Duncan AC, Boughner D, Campbell G, Wan WK. Preparation and characterization of a poly(2-hydroxyethyl methacrylate) biomedical gel. Eur Polym J. 2001;37:1821–1826. [Google Scholar]
- 28.Vance NG. The detection of changes in heparin activity in the rabbit: A comparison of anti-Xa activity, thromboelastography, activated partial thromboplastin time, and activated coagulation time. Anesth Analg. 2002;95:1503–1506. doi: 10.1097/00000539-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Monte S, Lyons G. In vitro evidence of gender-related heparin resistance. Int J Obstet Anesth. 2004;13:91–94. doi: 10.1016/j.ijoa.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Shenoy S, Harris RB, Sobel M. Development of heparin antagonists with focused biological activity. Curr Pharm Des. 1999;5:965–986. [PubMed] [Google Scholar]