Abstract
Objectives: Stress is a well-known predictor of smoking relapse, and cortisol is a primary biomarker of stress. The current pilot study examined changes in levels of cortisol in hair within the context of two time-intensity matched behavioral smoking cessation treatments: mindfulness training for smokers and a cognitive-behavioral comparison group.
Participants: Eighteen participants were recruited from a larger randomized controlled trial of smoking cessation.
Outcome Measures: Hair samples (3 cm) were obtained 1 month after quit attempt, allowing for a retrospective analysis of hair cortisol at preintervention and post–quit attempt time periods. Self-reported negative affect was also assessed before and after treatment.
Intervention: Both groups received a 7-week intensive intervention using mindfulness or cognitive-behavioral strategies.
Results: Cortisol significantly decreased from baseline to 1 month after quit attempt in the entire sample (d=−0.35; p=.005). In subsequent repeated-measures analysis of variance models, time by group and time by quit status interaction effects were not significant. However, post hoc paired t tests yielded significant pre–post effects among those randomly assigned to the mindfulness condition (d=−0.48; p=.018) and in those abstinent at post-test (d=−0.41; p=.004). Decreased hair cortisol correlated with reduced negative affect (r=.60; p=.011).
Conclusions: These preliminary findings suggest that smoking cessation intervention is associated with decreased hair cortisol levels and that reduced hair cortisol may be specifically associated with mindfulness training and smoking abstinence. Results support the use of hair cortisol as a novel objective biomarker in future research.
Introduction
Quitting smoking is notoriously difficult, and high relapse rates in smokers have encouraged investigation of physiologic and psychological processes associated with smoking and smoking cessation. Cortisol is a glucocorticoid hormone that plays a key regulatory role in the immune, metabolic, and central nervous systems.1 The hypothalamic-pituitary-adrenocortical (HPA) axis, which regulates cortisol secretion, is highly responsive to nicotine.2 Smokers display higher salivary cortisol levels relative to nonsmokers,3–5 with cortisol decreasing when smokers quit.4,6,7 Increased cortisol in smokers is generally believed to be attributable to nicotine exposure.4
While the effects of smoking on cortisol have been consistent and robust, findings on cortisol as a predictor of smoking behavior have been less consistent and less intuitive. For example, lower levels of absolute salivary cortisol and decreases in mean salivary cortisol have been associated with an increased risk for smoking relapse, increased self-reported ratings of smoking urges, and increased withdrawal related distress.6–8 Therefore, researchers have investigated additional variables that could further explain variability in smoking cessation outcomes.
Negative affect is a potent risk factor for smoking relapse9 and may form a link between cortisol and smoking. A substantial body of research supports an affective model of nicotine dependence, with nicotine use maintained through negative reinforcement with the expectation that substance use will alleviate unpleasant feelings.9
Acute and chronic negative affect have been linked to HPA axis activity.10,11 Chronic stress exposure has been linked in meta-analysis12 to a flatter diurnal cortisol slope (Cohen d=0.39) and a higher daily cortisol output volume (d=0.31). Higher cortisol levels have also been associated with higher ratings of subjective distress13 and rise and fall in tandem with changes in negative affect.14
Taken together, these findings linking negative affect, increased risk of smoking relapse, and increased cortisol output under stress suggest that stress reduction interventions designed to cultivate emotion regulation skills and target social-emotional processes could conceivably aid smoking cessation, and may be marked by lower levels of cortisol.
Historically, cortisol has been assessed through plasma, saliva, or urine, all of which are reliable methods for obtaining real-time estimates of cortisol in the body.12 While these methods provide valuable insight into short-term fluctuations in cortisol secretion, their experimental or clinical use presents substantial challenges. Serum and saliva cortisol are highly responsive to diurnal physiologic processes and respond quickly to environmental stressors, yet these methods are susceptible to technical problems with sample collection and processing, often leading to substantial missing data. Urinary cortisol provides information on HPA axis activity over a somewhat longer period of time, but sample collection is time intensive and provides only limited information on mean cortisol output over weeks or months.
Hair cortisol measurement is a novel assessment method gaining popularity and validity in the past decade.15,16 To date, hair cortisol correlates highly with other methods of cortisol measurement in both humans and animals.17,18 Levels have also been shown to change in expected directions in response to well-characterized stressors such as peer-raising in macaques19 and the third trimester of human pregnancy.20 Hair cortisol is also responsive to substance abuse, with higher hair cortisol levels found in alcohol-dependent participants during an active drinking phase compared with abstinent alcohol-dependent participants or controls.21
Hair cortisol holds substantial promise for measuring HPA axis activity because sample collection is simple (see Materials and Methods below). In addition, it is possible to assess changes over the course of several months in a single sample,15 thereby reducing vulnerability to missing data. Hair cortisol may be particularly useful for assessing phenomena involving long-term affective changes, such as those that might occur in the context of alternative and complementary medicine interventions.
Hair Cortisol in Mindfulness Training for Smokers
The current pilot study sought to examine changes in hair cortisol in a subsample of smokers participating in a larger randomized trial of smoking cessation comparing mindfulness training for smokers22 (MTS) to a cognitive-behavioral therapy (CBT) active control group. Mindfulness-based interventions have demonstrated efficacy for reducing stress in both clinical and nonclinical populations.23 Support exists for applications of mindfulness to a range of medical and psychological conditions, including substance use disorders.24,25 Evidence for the impact of mindfulness-based interventions on cortisol, however, remains mixed.26 For example, Marcus et al.27 reported significant decreases in the cortisol awakening response in a sample of 21 individuals receiving Mindfulness-Based Stress Reduction (MBSR) within residential substance abuse treatment, while others28 failed to detect pre–post differences for MBSR participants in a self-selected community sample. Difficulties in salivary cortisol measurement (e.g., diurnal fluctuation) may present a key methodologic barrier to accurate assessment.26 Hair cortisol assessment may provide an alternative method—one less susceptible to the aforementioned methodologic challenges with cortisol measurement—for indexing HPA axis-relevant physiologic effects.
The current pilot study examined changes in hair cortisol in a sample of smokers participating in a randomized trial comparing MTS22,29 to a time-intensity matched CBT control group. Mindfulness-based interventions have demonstrated efficacy for reducing stress23 and specifically for the treatment of substance abuse.24,25 Evidence for the use of cortisol as a useful biomarker in mindfulness-based interventions, however, remains inconclusive.26
The pilot study was designed to answer the following questions:
Q1. Is participation in a smoking cessation intervention associated with a decrease in hair cortisol levels?
Q2. Do intervention effects on hair cortisol differ by treatment type (i.e., mindfulness vs. CBT)?
Q3. Is smoking abstinence following an intervention associated with decreased hair cortisol levels?
Q4. Are changes in hair cortisol associated with changes in negative affect?
Materials and Methods
Study design
Participants were randomly assigned to receive one of two time-intensity matched 7-week behavioral smoking cessation interventions: MTS22 or Freedom from Smoking Enhanced (FFS-E, based on Freedom from Smoking).30 The MTS group received mindfulness instruction and the FFS-E group received relaxation and CBT strategies targeted to smoking cessation. Both groups had a total of eight meetings, made quit attempts approximately 1 month after the first meeting, and received 2 weeks' worth of nicotine patches. The institutional review board approved all study procedures and participants provided informed consent.
Participants
The larger randomized trial included 175 adult smokers; 135 participants elected to be randomly assigned to a high-intensity intervention (MTS or FFS-E), and 86 attended a 1-month postquit study visit. To qualify for hair donation for the cortisol substudy, individuals must have attended five or more of the eight MTS or FFS-E classes and the 1-month postquit study visit. On the basis of guidelines from prior studies,17,31 participants were excluded from hair donation if they reported using dye or bleach on their hair in the past year, were younger than age 25 or older than age 65 years, or were currently using pharmaceutical glucocorticoids. The hair cortisol substudy sample included 18 individuals drawn from an eligible pool of 36 who were offered $30 to donate 3 cm of hair. When compared at baseline to the eligible participants who did not donate hair, the substudy sample was more likely to be female (p=.015), smoke fewer cigarettes per day (p=.029), and report lower nicotine dependence on the Fagerström Test for Nicotine Dependence (p=.025). No other differences were noted between the substudy sample and the eligible sample.
Measures
Baseline demographic questionnaire
At baseline, all participants completed a brief questionnaire that assessed demographic characteristics and smoking history.
Smoking status. Biochemically confirmed 7-day point prevalence abstinence was assessed. A cutoff of 7.0 parts per million exhaled carbon monoxide32 was used to confirm abstinence.
Nicotine dependence. The Fagerström Test for Nicotine Dependence33 was administered at baseline to assess severity of smoking addiction (α=.60 in the current trial).
Negative affect. The Depression Anxiety Stress Scale34 asks participants to rate negative affect and somatic symptoms over the past week. The total score was used in analyses (α=.95 in the current trial), with higher scores indicating greater negative affect.
Hair cortisol. A single 3-cm hair sample was obtained from each participant at the 1-month postquit study visit. On the basis of previous work,35 the 1-cm segment most proximal to the scalp was assayed to indicate postquit cortisol output. The second most proximal 1-cm segment, representing the month before the quit day, was not analyzed. The third most proximal 1-cm segment was assayed to indicate cortisol output the month before the intervention.
Hair samples were analyzed by following standard procedures.17 Cortisol concentrations were adjusted to mass to provide a hair cortisol concentration in picograms of cortisol per milligram of hair. Intra- and interassay coefficients of variation were 6.1% and 10.6%, respectively.
Data analysis
Repeated-measures analysis of variance (RMANOVA; SPSS software, version 18.0; SPSS, Inc., Chicago, Illinois), independent and paired t-tests, and Pearson correlations were used. Effect sizes were computed as Cohen36 d using the post–pre mean difference divided by the pooled standard deviation.
Results
Demographic characteristics of the study sample included the following: white, 88.9%; female, 55.6%; education beyond high school, 77.8%; and average age, 42.2±11.4 years. Smoking history variables were as follows: years smoked, 22.3±9.7; cigarettes per day, 14.5±5.3; past quit attempts, 6.0±6.7; average Fagerström Test for Nicotine Dependence score, 3.6±1.6. The MTS and FFS-E groups did not differ at baseline for any tested variable (p>.10). Biochemically confirmed 7-day point prevalence smoking abstinence at 1 month after quitting did not differ by group: MTS, 80.0%; FFS-E, 62.5% (Pearson's chi-square [1]=.68; p=.410).
A significant main effect for time was found in an RMANOVA model with hair cortisol as the outcome and no other predictors (F[1,17]=10.43; p=.005; post–pre d=−0.35). The main effect for time remained significant in a model that included group and a time-by-group interaction as predictors of change in hair cortisol (F[1,16]=9.70; p=.007), although the time-by-group interaction was not significant (F[1,16]=1.40; p=.254). The main effect for time also remained significant in a model predicting cortisol with quit status and a time-by-quit status interaction as predictors (F[1,16]=6.05; p=.026), although the time-by-quit status interaction was not significant (F[1,16]=.92; p=.353).
Paired samples t-tests were used post hoc to examine within-group change in hair cortisol (Table 1). Cortisol significantly decreased in the MTS group (t[9]=2.89; p=.018; d=−0.48) but not the FFS-E group (t[7]=1.56; p=.162; d=−0.22). A significant drop in cortisol was found in the quit group (t[12]=3.51; p=.004; d=−0.41) but not the relapsed group (t[4]=0.74; p=.502; d=−0.20).
Table 1.
Pre–Post Changes in Hair Cortisol Reported for Full Sample and Subsamples
| Group | Participants (n) | Baseline cortisol level (pg/mg) | Postquit cortisol level (pg/mg) | t-statistic | p-Value | d-Value |
|---|---|---|---|---|---|---|
| Full sample | 18 | 220.07±87.75 | 190.56±80.88 | 3.23 | .005* | −0.35 |
| Quit | 13 | 207.26±92.10 | 172.30±77.14 | 3.51 | .004* | −0.41 |
| Relapsed | 5 | 253.38±73.20 | 238.02±77.82 | 0.74 | .502 | −0.20 |
| MTS | 10 | 197.68±89.83 | 158.60±73.38 | 2.89 | .018** | −0.48 |
| FFS-E | 8 | 248.06±81.93 | 230.51±75.38 | 1.56 | .162 | −0.22 |
Cortisol values are expressed as mean±standard deviation.
*p<.01; **p<.05.
d, Cohen d (1988) computed by using post–pre mean difference divided by pooled standard deviation; MTS, mindfulness training for smokers; FFS-E, Freedom from Smoking Enhanced.
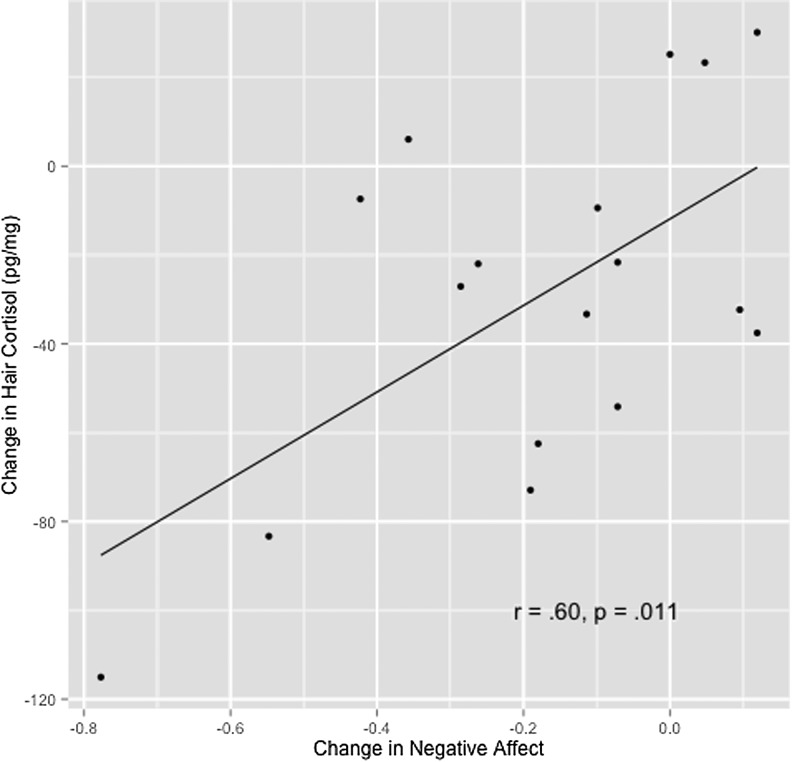
One outlier (3 standard deviations below the mean) was found for change in negative affect and was removed before analysis. A significant correlation was found between change in negative affect and change in cortisol (r=.60; p=.011) (Fig. 1), with larger drops in cortisol associated with greater decreases in negative affect.
FIG. 1.
Change in cortisol and negative affect. Change scores computed as post–pre. Blood pressure, mean arterial blood pressure; Cort, hair cortisol; neg affect, Depression Anxiety Stress Scales total score.34
Discussion
This pilot study examined change in hair cortisol within the context of two high-intensity smoking cessation interventions. The primary finding was that participation in an intensive smoking cessation intervention was associated with reduction in hair cortisol (Q1). In addition, preliminary evidence suggested that participation in mindfulness training and smoking cessation itself may each be independently associated with reduction in hair cortisol (Q2, Q3).
The effect size for the overall drop in cortisol (d=−0.35) following participation in a smoking cessation intervention was small by Cohen's36 standards. For intervention participants who were abstinent at the 1-month postquit study visit, the magnitude of cortisol decrease was roughly double (d=−.41) compared with that in participants who had relapsed (d=−.20). This finding supports research showing that long-term nicotine exposure is associated with increased cortisol output.3–5 A similar difference in magnitude was observed comparing changes in the MTS (d=−0.48) and FFS-E group (d=−0.22). The large drop in cortisol seen in the mindfulness condition is consistent with preliminary evidence that mindfulness training (e.g., MBSR) may reduce cortisol.26
Notably, the nonsignificant RMANOVA interaction terms suggested no difference in cortisol decrease between groups (i.e., quit status or treatment assignment). The small overall sample provided low statistical power to detect interaction effects, which may explain why significant differences were observed only with use of paired t tests.
The significant positive correlations in the expected direction between changes in hair cortisol and changes in self-reported negative affect support the notion that hair cortisol may be a useful biomarker for psychological stress (Q4).
In conclusion, this pilot study found that participation in an intensive behavioral treatment for smoking cessation is associated with decreased hair cortisol. This is meaningful given that hair sampling provides a measure of long-term cortisol secretion, not easily ascertained through other means (i.e., blood or saliva). The additional findings are intriguing but require replication in a larger sample. Overall, this study supports hair cortisol as a straightforward, objective biomarker for use in future studies, particularly interventions designed to affect health through changes in stress.
Acknowledgments
Primary funding was provided through National Institute on Drug Abuse grant K23DA022471. SBG was supported in part by a National Institute of Child Health and Human Development core grant to the Waisman Center (P30 HD03352). JMG was supported by National Center for Complementary and Alternative Medicine grant R00AT004945. Funding sources had no involvement in study design, collection, analysis, interpretation of data, writing of manuscript, or decision to submit the manuscript for publication.
We would like to thank Wendy Theobald, Kristin Stankevitz, Andy Sandgren, Allison Couillard, Joseph Chase, James Neitzel, Steve Gilbert, Chelsea Davenport, Jim Powell, Janice Sheppard, Beth Wortzel, Jesse Kaye, and Kathryn Hefner for their work in carrying out this study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000;21:55–89 [DOI] [PubMed] [Google Scholar]
- 2.Rohleder N, Kirschbaum C. The hypothalamic–pituitary–adrenal (HPA) axis in habitual smokers. Int J Psychophysiol 2006;59:236–243 [DOI] [PubMed] [Google Scholar]
- 3.Kirschbaum C, Wust S, Strasburger CJ. ‘Normal’ cigarette smoking increases free cortisol in habitual smokers. Life Sci 1992;50:435–442 [DOI] [PubMed] [Google Scholar]
- 4.Steptoe A, Ussher M. Smoking, cortisol and nicotine. Int J Psychophysiol 2006;59:228–235 [DOI] [PubMed] [Google Scholar]
- 5.Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab 2007;92:819–824 [DOI] [PubMed] [Google Scholar]
- 6.Frederick SL, Reus VI, Ginsberg D, Hall SM, Munoz RF, Ellman G. Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success in smokers. Biol Psychiatry 1998;43:525–530 [DOI] [PubMed] [Google Scholar]
- 7.Ussher M, West R, Evans P, et al. Reduction in cortisol after smoking cessation among users of nicotine patches. Psychosom Med 2006;68:299–306 [DOI] [PubMed] [Google Scholar]
- 8.al'Absi M, Hatsukami D, Davis G, Wittmers L. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depen 2004;73:267–278 [DOI] [PubMed] [Google Scholar]
- 9.Baker T, Piper M, McCarthy D, Majeskie M, Fiore M. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev 2004;111:33. [DOI] [PubMed] [Google Scholar]
- 10.Biondi M, Picardi A. Psychological stress and neuroendocrine function in humans: the last two decades of research. Psychother Psychosom 1999;68:114–150 [DOI] [PubMed] [Google Scholar]
- 11.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 2004;130:355. [DOI] [PubMed] [Google Scholar]
- 12.Miller G, Chen E, Zhou E. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 2007;133:25–45 [DOI] [PubMed] [Google Scholar]
- 13.Morgan CA, Rasmusson AM, Wang S, Hoyt G, Hauger RL, Hazlett G. Neuropeptide-Y, cortisol, and subjective distress in humans exposed to acute stress: replication and extension of previous report. Biol Psychiatry 2002;52:136–142 [DOI] [PubMed] [Google Scholar]
- 14.Buchanan TW, al'Absi M, Lovallo WR. Cortisol fluctuates with increases and decreases in negative affect. Psychoneuroendocrinology 1999;24:227–241 [DOI] [PubMed] [Google Scholar]
- 15.Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 2012;37:589–601 [DOI] [PubMed] [Google Scholar]
- 16.Stalder T, Kirschbaum C. Analysis of cortisol in hair: state of the art and future directions. Brain Behav Immun 2012;26:1019–1029 [DOI] [PubMed] [Google Scholar]
- 17.Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum S. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med 2007;30:E183–E191 [DOI] [PubMed] [Google Scholar]
- 18.Manenschijn L, Koper JW, Lamberts SW, van Rossum EF. Evaluation of a method to measure long term cortisol levels. Steroids 2011;76:1032–1036 [DOI] [PubMed] [Google Scholar]
- 19.Feng X, Wang L, Yang S, et al. Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proc Natl Acad Sci U S A 2011;108:14312–14317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production—increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 2009;34:32–37 [DOI] [PubMed] [Google Scholar]
- 21.Stalder T, Kirschbaum C, Heinze K, et al. Use of hair cortisol analysis to detect hypercortisolism during active drinking phase in alcohol-dependent individuals. Biol Psychol 2010;85;357–360 [DOI] [PubMed] [Google Scholar]
- 22.Davis JM, Fleming M, Bonus K, Baker T. A pilot study on mindfulness based stress reduction for smokers. BMC Complement Altern Med 2007;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoury B, Lecomte T, Fortin G, et al. Mindfulness-based therapy: a comprehensive meta-analysis. Clin Psychol Rev 2013;33:763–771 [DOI] [PubMed] [Google Scholar]
- 24.Zgierska A, Rabago D, Chawla N, Kushner K, Koehler R, Marlatt A. Mindfulness meditation for substance use disorders: a systematic review. Subst Abuse 2009;30:266–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanna S, Greeson JM. A narrative review of yoga and mindfulness as complimentary therapies for addiction. Complement Ther Med 2013;21:244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matousek R, Dobkin P, Pruessner J. Cortisol as a marker for improvement in mindfulness-based stress reduction. Complement Ther Clin Pract 2010;16:13–19 [DOI] [PubMed] [Google Scholar]
- 27.Marcus MT, Fine PM, Moeller FG, Khan MM, Pitts K, Swank PR, Liehr P. Change in stress levels following mindfulness-based stress reduction in a therapeutic community. Addict Disord Their Treat 2003;2:63–68 [Google Scholar]
- 28.Galantino M, Baime M, Maguire M, Szapary P, Farrar J. Association of psychological and physiological measures of stress in health-care professionals during an 8-week mindfulness meditation program: mindfulness in practice. Stress Health 2005;21:255–261 [Google Scholar]
- 29.Davis JM, Goldberg SB, Anderson MC, Manley AR, Smith SS, Baker TB. Randomized trial on Mindfulness Training for Smokers targeted to a disadvantaged population. Subst Use Misuse 2014;49:571–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lando H, McGovern P, Barrios F, Etringer B. Comparative evaluation of American Cancer Society and American Lung Association smoking cessation clinics. Am J Public Health 1990;80:554–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereg D, Gow R, Mosseri M, et al. Hair cortisol and the risk for acute myocardial infarction in adult men. Stress 2011;14:73–81 [DOI] [PubMed] [Google Scholar]
- 32.Middleton E, Morice A. Breath carbon monoxide as an indication of smoking habit. Chest 2000;117:758–763 [DOI] [PubMed] [Google Scholar]
- 33.Heatherton T, Kozlowski L, Frecker R, Fagerstrom K. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991;86:1119–1127 [DOI] [PubMed] [Google Scholar]
- 34.Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2nd ed. Sydney, Australia: Psychology Foundation, 1995 [Google Scholar]
- 35.Wennig R. Potential problems with the interpretation of hair analysis results. Forensic Sci Int 2000;107:5–12 [DOI] [PubMed] [Google Scholar]
- 36.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Erlbaum, NJ: Routledge, 1997 [Google Scholar]