Abstract
BACKGROUND:
Paraquat (PQ) is an effective herbicide and is widely used in agricultural production, but PQ poisoning is frequently seen in humans with the lung as the target organ. Clinically pulmonary pathological changes are often used to predict the severity and prognosis of the patients. In this study, we observed the expression of heat shock protein 70 (HSP70) in rat lung after PQ poisoning and to investigate the therapeutic effects of ulinastatin.
METHODS:
Seventy-two adult healthy SD rats were randomly divided into a control group (group A, n=24), a poisoning group (group B, n=24), and an ulinastatin group (group C, n=24). The rat models of acute PQ poisoning were established by intra-gastric administration of 80 mg/kg PQ to rats of groups B and C, and the rats of group C were intra-peritoneally injected with 100 000 IU/kg ulinastatin 30 minutes after poisoning. The expression of HSP70 in lung tissue was observed, and W/D and histopathological changes in the lung tissue were compared 12, 24, 48 and 72 hours after poisoning. The expression of HSP70 in the lung tissue was assayed by using RT-PCR. All quantitative data were processed with one-way analysis of variance to compare multiple sample means.
RESULTS:
Compared to group A, the expression of HSP70 in the lung of rats in groups B and C increased significantly at all intervals (P<0.05). The pathological changes in lung tissue of rats with PQ poisoning included congestion, leukocytes infiltration and local hemorrhage, whereas those of group C were significantly lessened.
CONCLUSION:
Ulinastatin may ameliorate acute lung injury to some extent after PQ poisoning in rats by enhancing the expression of HSP70.
KEY WORDS: Paraquat, Poisoning, Ulilnastatin, Heat shock protein, Acute lung injury
INTRODUCTION
Paraquat (PQ), an effective herbicide, is widely used in agricultural production, but PQ poisoning occurs frequently in humans. The function of organs such as the lung, liver, kidney and heart is impaired after poisoning, and the lung is the main target organ. At the early stage of poisoning, most patients die of acute lung injury, and at the late stage, some patients may develop pulmonary fibrosis with a mortality of over 80%.[1-5] Clinically, pulmonary pathological changes are often used to predict the severity and prognosis of the patients, and it is the main task to alleviate the pulmonary damage. Ulinastatin, a kind of broad spectrum proteinase inhibitors, is effective to inhibit the activity of hydrolases, release inflammatory mediators, and produce oxygen radicals and hyperoxide.[6,7] In this study, we produced the rat models of PQ poisoning to observe the changes of pulmonary heat shock protein 70 (HSP70) and the effect of ulinastatin.
METHODS
Main experimental reagents
20% PQ solution was produced by Shanghai Xianzhengda Company. Ulinastatin was purchased from Tianpu Biochemistry Medical Limited Company, and Coomassie brilliant blue kit from Nanjing Jiancheng Bioengineering Institute. HSP70 sheep polyclonal antibody was the product of Santa Cruz Company, USA, and cochlearia hydrogen peroxidase labelling rabbit-anti-sheep IgG was the product of Beijing Tiangen Biochemistry Technology Limited Company.
Grouping of animals and model establishment
A total of 72 clean Sprague-Dawley rats, body weight 220±40 g, were provided by the Department of Animal Science, Medical College of Nanchang University. The rats were randomly divided into a control group (group A), a poisoning group (group B) and an ulinastatin group (group C). Models of acute PQ poisoning were established according to Tong et al. The rats in groups B and C were intragastrically administered with PQ (80 mg/kg), while the rats in group A were intragastrically administered with the same volume of stroke-physiological saline. The rats in group C were peritoneally injected with 1000 000 U/kg per day ulinastatin at 30 minutes after poisoning, and the rats in groups A and B were peritoneally injected with the same volume of stroke-physiological saline. At 12, 24, 48, 72 hours after poisoning, the rats were anesthetized peritoneally with 3% pentobarbital (35 mg/kg) and executed through blood letting from the abdominal aorta.
Tissue specimens
After the rats were killed, the superior part of the left lung was taken for the measurement of the ratio of wet to dry weight. The central part of the left lung was washed completely, put in nitrogen canister and stored in a freezer at -80 °C for measurement of the expression of HSP70 by Western blotting. The inferior part of the left lung was fixed in 10% formaldehyde and used for pulmonary histopathological observation.
Measurement of arterial partial pressure of oxygen
The 0.5 ml blood was collected from the abdominal aorta 12, 24, 48, 72 hours after poisoning. The arterial partial pressure of oxygen was measured by a blood gas analyzer produced by Roche Company.
Measurement of the expression of pulmonary HSP70 protein
Lung tissues of rats in each group were put into cell lysate and homogenated on ice at corresponding time points. Bradford method was adopted to quantitate protein, and tissue sample was added into 2×SDS loading buffer, and then processed with SDS-PAGE gel separation, and the protein strap was electrotransferred onto the PVDF membrane. TTBS was used to block for 1 hour, then HSP70, HSP70 one antibody and IgG-HRP two antibody were used to brood in succession, finally enhanced chemiluminescence method was applied to detect positive signal. Images were gathered and analyzed semiquantitatively, the level of HSP70 protein was indicated with the ratio of HSP70/β-actin. The process was repeated three times in each group.
Statistical analysis
Experimental data were analyzed with SPSS 12.0 software. Quantitative data were demonstrated in the form of mean±SD, and group comparison was made with one-way analysis of variance and two samples t test. The difference was considered statistically significant when P<0.05.
RESULTS
General conditions of rats
Compared with group A, rats in group B had the manifestations of poisoning at 24-72 hours. The main manifestations included poor spirit, slow reaction, cyanosis in oral lips and four limbs at varying degrees, increased respiratory frequency, less activity, and easy capture. Blood-like substance outflowed from the nasal cavity in some rats. In group C, the above manifestations alleviated, activity increased, dyspnea reduced, but compared with group A, there were significant differences.
Histopathological observation
In group A, the lung tissue was pink and retracted well under naked eyes. Under a light microscope, the alveolus structure was distinct, the alveolar wall was thick, inflammatory cell infiltration was not found in the alveolar space (Figure 1).
Figure 1.
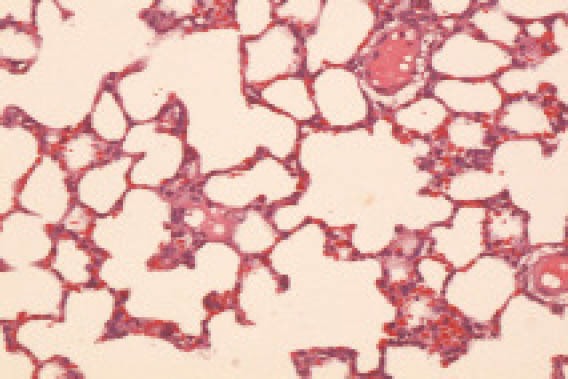
The lung tissue in the group A (HE, original magnification ×100)
In group B, the lung tissue didn’t retract well under naked eyes. The size of the lung increased significantly, the color of the lung was not asymmetrical, a flake of ecchymosis and hemorrhagic spots were observed. Under a light microscope, edematous fluid in the alveolar space and a small quantity of cellulose were observed at 12 hours after poisoning; at 3 days after poisoning, the most obvious clinical manifestations included interstitial pulmonary edema, and alveolar edema. The alveolar space was filled with dissociative neutrophils, macrophages, and homogeneous edema fluid associated with diffuse pulmonary hemorrhage. The alveolar space collapsed, and hyaline membrane formed partially. For a long time, no changes took place in the clinical manifestations but there was a absorption trend (Figure 2).
Figure 2.
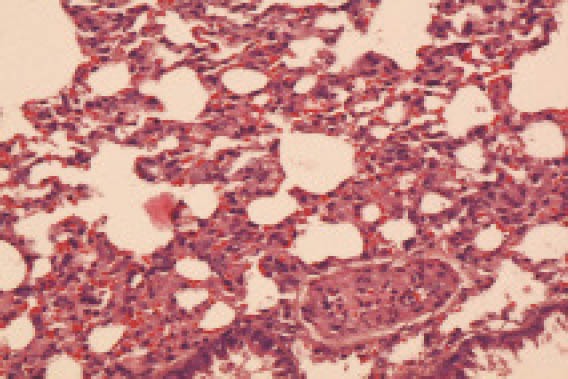
The lung tissue in the group B 24 hours after poisoning (HE, original magnification ×100)
In group C at 12 hours after poisoning, focus or foliated inflammatory cell infiltration was observed, the degree was lower than that in group B. The clinical manifestations were obvious at 3 days after poisoning in group C, but less obvious in group B (Figure 3).
Figure 3.
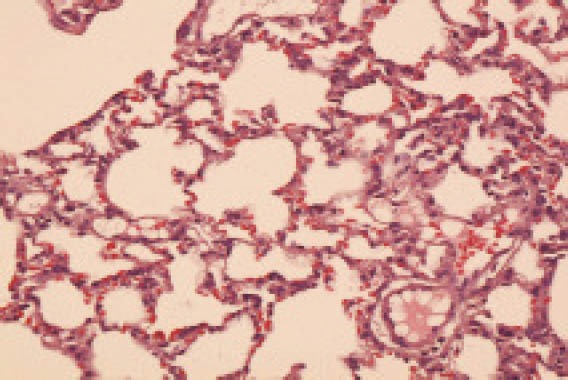
The lung tissue in the group C 24 hours after poisoning (HE, original magnification ×100)
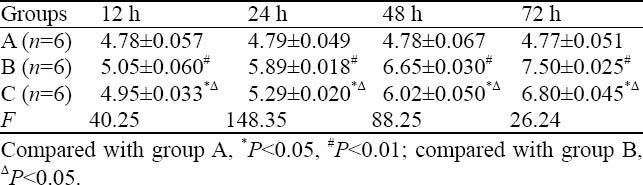
Pulmonary W/D ratio
Compared to group A, the W/D ratio of groups B and C increased significantly at corresponding time points, especially group B, and there was statistical significance among the three groups (P<0.05) (Table 1).
Table 1.
Comparison of lung W/D in different time point in rats (mean ±SD)
Arterial partial pressure of oxygen
Compared to group A, the arterial partial pressure of oxygen in groups B and C in corresponding time points decreased significantly, especially in group B. There was statistical significance among the three groups (P<0.05) (Table 2).
Table 2.
Blood gas analysis changes in each group (mean ±SD, mmHg)

Expression of pulmonary HSP70 and effect of ulinastatin
The expression of pulmonary HSP70 in group A at corresponding time points was low, and there was no statistical significance among subgroups (P>0.05). Compared to group A, the expression of pulmonary HSP70 in group B increased gradually with time, and peaked at 24 hours. Compared to group B, the expression of pulmonary HSP70 in group C increased, and peaked at 24 hours (Table 3, Figure 4).
Table 3.
Comparison of the expression of HSP70 in different groups (mean ±SD)
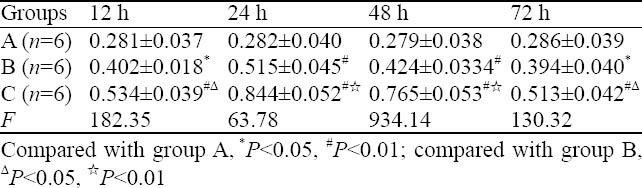
Figure 4.
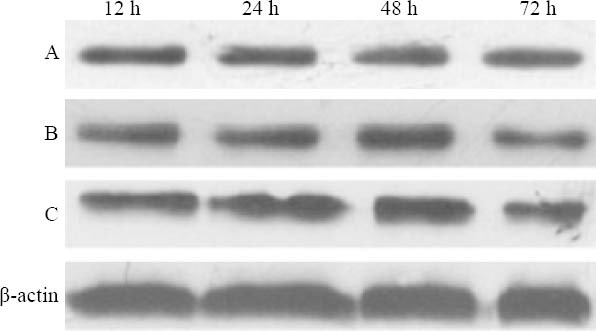
Expression of HSP70 in different groups
DISCUSSION
The lung is the major target organ of PQ poisoning. Acute lung injury, multiple organ dysfunctions, and pulmonary interstitial fibrosis are the main causes of death for PQ poisoning.[8,9] PQ can cause histocytic pathophysiological damage by producing oxygen radicals, releasing inflammatory mediators, and inducing lipid peroxidation, especially pulmonary oxidative damage.[10,11] Some studies show that antioxidant treatment can significantly reduce the degree of lung injury for the PQ poisoning.[12-15] In this study, the rats had the following clinical manifestations after poisoning, including poor spirit, slow reaction, cyanosis in oral lips and limbs at different degrees, increased respiratory frequency, inactivity, easy capture, and blood-like substance in the nasal cavity. Diffuse hemorrhage, alveolar space collapse, hyaline membrane formation, increased pulmonary water content, inflammotary cells aggregation in alveoli were observed. These indicated the occurrence of lung injury.
Heat shock protein is a kind of protein, which is conservative in organic evolution. Many physiological, pathological and stress factors can induce the production of heat shock protein, and this is important in protecting the body from excessive stress damage. The HSP70 family is closely associated with pulmonary biology, and has protective effects on lung injury induced by anti-inflammation, anti-oxidation, anti-apotosis or molecular chaperone roles.[16-19] Weiss et al[20] administered a vector containing the porcine HSP-70 cDNA driven by a CMV promoter (AdHSP) into the lungs of rats subjected to 2CLP or sham operation. The administration of AdHSP after either sham operation or 2CLP increased HSP-70 protein expression in lung tissue, as determined by immunohistochemistry and Western blot hybridization. The administration of AdHSP significantly attenuated interstitial and alveolar edema and protein exudation and dramatically decreased neutrophil accumulation, relative to the control of adenovirus. Hiratsuka et al[21] transduced HSP70 DNA into the lungs of rats using adenovirus vector, and found that donor adenovirus-mediated gene transfer of HSP70 decreases subsequent ischemia-reperfusion injury in rat lung isografts.
Other studies found that glutaminate and rhubarb can both increase the expression of HSP70 after lung injury and exert protective effects.[22-24] Ulinastatin is a broad spectrum proteinase inhibitor, and can protect the lung from injury through antioxidation, inhibiting the release of inflammatory mediators, increasing stability of pulmonary cell membrane and mitochondria, endoplasmic reticulum, lysosome membrane, and improving cell energy metabolism.[25-29] Another study discovered that ulinastatin can inhibit the release of TNF-α, IL-1β, IL-2, SOD, MDA, MMP-9 and neutrophil elastase in serum of rats after acute PQ poisoning, exerting protective effect againt lung injury. Chen et al[30] found that ulinastatin can protect the kidney in the early phase of trauma by upregulating the renal expression of HSP70. In this study, we found that compared with the control group, the pulmonary expression of HSP70 in poisoning group increased significantly at 12 hours, and peaked at 24 hours. The results indicated that PQ poisoning may enhance the expression of HSP70 while inducing acute lung injury, but serious pulmonary damage indicated the production of HSP70 is unable to induce injury after poisoning. In the ulinastatin group, the pulmonary expression of HSP70 in the corresponding time points was obviously higher than that in the poisoning group. At the same time, pulmonary pathological changes minimized and pulmonary water content decreased, indicating that ulinastatin may alleviate PQ-induced lung injury by enhancing the pulmonary expression of HSP70. Hence our study provided theoretical evidence for the application of ulinastatin in treatment of PQ poisoning.
Footnotes
Funding: None.
Ethical approval: Not needed.
Conflicts of interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributors: Zhang ZJ wrote the main body of the article. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Bertolote JM, Fleischmann A, Eddleston M, Gunnell D. Deaths from pesticide poisoning: a global response. Br J Paychiatry. 2006;189:201–203. doi: 10.1192/bjp.bp.105.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang KY, Lee EY, Hong SY. Paraquat intoxication in Korea. Arch Environ Health. 2002;57:162–166. doi: 10.1080/00039890209602931. [DOI] [PubMed] [Google Scholar]
- 3.Sittipunt C. Paraquat poisoning. Respir Care. 2005;50:383–385. [PubMed] [Google Scholar]
- 4.Dinis-Oliveira RJ, Sarmento A, Reis P, Amaro A, Remião F, Bastos ML, et al. Acute paraquat poisoning:report of a survival case following intake of a potential lethal dose. Pediatr Emerg Care. 2006;22:537–540. doi: 10.1097/01.pec.0000223179.07633.8a. [DOI] [PubMed] [Google Scholar]
- 5.Podprasart V, Satayavivad J, Riengrojpitak S, Wilairat P, Wananukul W, Chavalittumrong P, et al. No direct hepatotoxic potential following a multiple-low dose paraquat exposure in rat as related to its bioaccumulation. Toxicol Lett. 2007;170:193–202. doi: 10.1016/j.toxlet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Heikkila JJ. Heat shock protein gene expression and function in amphibian model systems. Comp Biochem Physiol A Mol Integr Physiol. 2010;158:19–33. doi: 10.1016/j.cbpa.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Yang Q, Liu X, Liu M, Zhang L, Guan Y. Ulinastatin-mediated protection against zymosan-induced multiple organ dysfunction in rats. Biologicals. 2010;38:552–556. doi: 10.1016/j.biologicals.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Satomi Y, Sakaguchi K, Kasahara Y, Akahori F. Novel and extensive aspects of paraquat-induced pulmonary fibrogenesis: comparative and time-course microarray analyses in fibrogenic and non-fibrogenic rats. J Toxicol Sci. 2007;32:529–553. doi: 10.2131/jts.32.529. [DOI] [PubMed] [Google Scholar]
- 9.Bertsias GK, Katonis P, Tzanakakis G, Tsatsakis AM. Review of clinical and toxicological features of acute pesticide poisonings in Crete (Greece) during the period 1991-2001. Med Sci Monit. 2004;10:622–627. [PubMed] [Google Scholar]
- 10.Shuvaev VV, Tliba S, Nakada M, Albelda SM, Muzykantov VR. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J Pharmacol Exp Ther. 2007;323:450–457. doi: 10.1124/jpet.107.127126. [DOI] [PubMed] [Google Scholar]
- 11.Tomita M, Okuyama T, Hidaka K, Ishikawa T, Adachi J, Nohno T. Early differential gene expression of rat lung after exposure to paraquat. Free Radic Res. 2004;38:821–829. doi: 10.1080/10715760410001713826. [DOI] [PubMed] [Google Scholar]
- 12.Dinis-Oliveira RJ, De Jesús Valle MJ, Bastos ML, Carvalho F, Sánchez Navarro A. Kinetics of paraquat in the isolated rat lung: influence of sodium depletion. Xenobiotica. 2006;369:724–737. doi: 10.1080/00498250600790331. [DOI] [PubMed] [Google Scholar]
- 13.Huang M, Zhang P, Chang XL, Wu Q, Zhou ZJ. Change of oxidative stress and nuclear factor-κB in acute paraquat poisoned rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2009;27:457–462. [PubMed] [Google Scholar]
- 14.Jian XD, Sui H, Chu ZH, Zhang ZW, Kan BT, Zhang L, et al. Changes of serum cytokine caused by acute paraquat poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2007;25:230–232. [PubMed] [Google Scholar]
- 15.He XY, Zhao GJ, Lu ZQ, Hong GL, He F, Liang H, et al. Oxidative stress of acute paraquat poisoned rats and sodium dimercaptopropane sulfonate intervention. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2009;27:476–479. [PubMed] [Google Scholar]
- 16.Wheeler DS, Wong HR. Heat shock response and acute lung injury. Free Radic Biol Med. 2007;42:1–14. doi: 10.1016/j.freeradbiomed.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuboki S, Schuster R, Blanchard J, Pritts TA, Wong HR, Lentsch AB. Role of heat shock protein 70 in hepatic ischemia-reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:1141–1149. doi: 10.1152/ajpgi.00491.2006. [DOI] [PubMed] [Google Scholar]
- 18.Song JY, Li L, Ahn JB, Park JG, Jo JS, Park DH, et al. Acute liver toxicity by carbon tetrachloride in HSP70 knock out mice. Exp Toxicol Pathol. 2007;59:29–34. doi: 10.1016/j.etp.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Imao M, Nagaki M, Moriwaki H. Dual effects of heat stress on tumor necrosis factor-alpha-induced hepatocyte apoptosis in mice. Lab Invest. 2006;86:959–967. doi: 10.1038/labinvest.3700451. [DOI] [PubMed] [Google Scholar]
- 20.Weiss YG, Maloyan A, Tazelaar J, Raj N, Deutschman CS. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J Clin Invest. 2002;110:801–806. doi: 10.1172/JCI15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiratsuka M, Mora BN, Yano M, Mohanakumar T, Patterson GA. Gene transfer of heat shock protein 70 protects lung grafts from ischemia-reperfusion injury. Ann Thorac Surg. 1999;67:1421–1427. doi: 10.1016/s0003-4975(99)00164-2. [DOI] [PubMed] [Google Scholar]
- 22.Singleton KD, Wischmeyer PE. Glutamine's protection against sepsis and lung injury is dependent on heat shock protein 70 expression. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1839–1845. doi: 10.1152/ajpregu.00755.2006. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi Y, Sawa Y, Fukuyama N, Nakazawa H, Matsuda H. Preoperative glutamine administration induces heat-shock protein 70 expression and attenuates cardiopulmonary bypass-induced inflammatory response by regulating nitric oxide synthase activity. Circulation. 2002;106:2601–2607. doi: 10.1161/01.cir.0000035651.72240.07. [DOI] [PubMed] [Google Scholar]
- 24.Eggleton P, Harries LW, Alberigo G, Wordsworth P, Viner N, Haigh R, et al. Changes in apoptotic gene expression in lymphocytes from rheumatoid arthritis and systemic lupus erythematosus patients compared with healthy lymphocytes. J Clin Immunol. 2010 doi: 10.1007/s10875-010-9429-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Ito K, Mizutani A, Kira S, Mori M, Iwasaka H, Noguchi T. Effect of Ulinastatin, a human urinary trypsin inhibitor, on the oleic acid-induced acute lung injury in rats via the inhibition of activated leukocytes. Injury. 2005;36:387–394. doi: 10.1016/j.injury.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Pan F, Chen AM, Guo FJ, Zhu CL. Effect of tacrolimus on apoptosis and expression of heat shock protein 70 after acute spinal cord injury in rats. Zhonghua Wai Ke Za Zhi. 2006;44:1708–1712. [PubMed] [Google Scholar]
- 27.Bao P, Gao W, Li S, Zhang L, Qu S, Wu C, et al. Effect of pretreatment with high-dose ulinastatin in preventing radiation-induced pulmonary injury in rats. Eur J Pharmacol. 2009;603:114–119. doi: 10.1016/j.ejphar.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Wang RK, Zhao SP, Yang XP, Cai HW. Effect of ulinastatin on lung injury after dilute hydrochloric acid aspiration. Zhong Nan Da Xue Bao Yi Xue Ban. 2004;29:305–380. [PubMed] [Google Scholar]
- 29.Zhou LW, Wang YL, Yan XT, He XH. Urinary trypsin inhibitor treatment ameliorates acute lung and liver injury resulting from sepsis in a rat model. Saudi Med J. 2008;29:368–373. [PubMed] [Google Scholar]
- 30.Mao WD, Sun SH. Ulinastatin's early effects on kidney's expression of HSP70 and IL-6 after mechanical injury of kidney. Sichuan Da Xue Xue Bao Yi Xue Ban. 2008;39:591–594. [PubMed] [Google Scholar]


