Abstract
BACKGROUND:
The tumor necrosis factor recepter associated factor (TRAF) 6 is an important intracellular adapter protein that plays a pivotal role in activating multiple inflammatory and immune related processes induced by cytokines. TRAF6 represents a strong candidate susceptibility factor for sepsis. We investigated whether polymorphisms at the TRAF6 gene are associated with the susceptibility to and severity of sepsis.
METHODS:
A hospital-based case-control study was conducted with 255 patients with sepsis and 260 controls who were recruited from Zhengzhou, China. Haplotype tagging single nucleotide polymorphisms (htSNPs) were selected from the HapMap database and genotyped using the SNPstream genotyping platform. The associations with the susceptibility and disease severity of sepsis were estimated by logistic regression, and adjusted for age, sex, smoking, drinking, chronic diseases status, APACHEII score and critical illness status.
RESULTS:
A total of 13 TRAF6 SNPs were tagged by 7 htSNPs. Five htSNPs (rs5030490, rs5030411, rs5030416, rs5030445 and rs3740961) were genotyped in the case control study. Genotype frequencies of the htSNPs were conformed to the Hardy-Weinberg equilibrium in both patients and controls. No significant association was found between the 5 htSNPs and the susceptibility to and severity of sepsis. Compared with the main haplotype -11120A/-10688T/-9423A/805G/12967G, no certain haplotype was associated with the significantly susceptibility to or severity of sepsis.
CONCLUSION:
TRAF6 gene polymorphisms might not play a major role in mediating the susceptibility to and severity of sepsis in the Chinese population. A larger population-based case-control study is warranted.
KEY WORDS: Sepsis, Tumor necrosis factor recepter associated factor 6, Haplotype tagging singlenucleotide polymorphisms, Linkage disequilibrium, Genetic association
INTRODUCTION
Sepsis is defined as suspected or proven infection plus a systemic inflammatory response syndrome (e.g., fever, tachycardia, tachypnea, and leukocytosis). Severe sepsis is defined as sepsis with organ dysfunction (hypotension, hypoxemia, oliguria, metabolic acidosis, thrombocytopenia, or obtundation).[1] Septic shock is defined as severe sepsis with hypotension, despite adequate fluid resuscitation. Septic shock and multi-organ dysfunction are the most common causes of death in patients with sepsis.[2] Despite significant advances in the development of therapeutic strategies and the understanding of pathophysiological mechanisms of sepsis, the mortality rates of severe sepsis and septic shock remain high.[3,4] The mortality rates associated with severe sepsis and septic shock are 25% to 30%[5] and 40% to 70%,[6] respectively. In China, approximately three million people are diagnosed with sepsis each year, resulting in at least 600 000 deaths.[4] The hospital mortality of severe sepsis is 48.7%, and the mean hospital cost is $11 390 per patient and $502 per patient per day.[7] This response is expressed as a compendium of a variety of different clinical signs and symptoms such as fever, increased blood leukocyte counts, unexplained thrombocytopenia, mental confusion, transient hypotension, and organ stress and dysfunction. The individual response is determined by many factors, including the virulence of the organism, the size of the inoculum, genetic variations, and the patient’s coexisting conditions.[8-10] The genetic variations may identify patients at high risk for the development of sepsis and organ dysfunction during severe infections. The identification of susceptibility genes contributing to the occurrence and development may help to clarify pathophysiologic mechanisms relevant to this disorder.[11-14]
The tumor necrosis factor recepter associated factor (TRAF) 6 is an important intracellular adapter protein involved in the signaling cascade of the Toll/IL-1 receptor (TIR) family,[15-17] which is a key protein that plays a critical role in transducing extracellular cytokine signals from the corresponding cell surface receptors and activating intracellular signaling cascades. The activation of the TIR pathways ultimately leads to translocation of NF-κB into the nucleus resulting in innate host defense mechanisms such as the release of inflammatory cytokines[18] that contribute to sepsis induced organ dysfunction.
We suspect that TRAF6 is a candidate susceptibility gene for sepsis in biology. In this study we determined whether TRAF6 gene polymorphisms have any association with the risk of sepsis in the Han Chinese population. The present case-control genetic association study was designed by using a HapMap haplotype-tagging SNPs (htSNPs) approach.
METHODS
Patients and controls
The case-control population consisted of 255 patients with sepsis and 260 controls. All subjects and controls or their family members signed informed consent. This study was approved by the Medical Ethics Committee of the Institute of Radiation Medicine, Military Academy of Medical Sciences. All subjects and controls were Han residents in Zhengzhou city of Henan Province and the surrounding regions, and they were not biologically related. All patients with sepsis were consecutively recruited from July 2006 to March 2008 at the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China). Sepsis, severe sepsis, septic shock and multiple organ distress syndrome (MODS) was diagnosed according to the International Sepsis Definition Conference.[19] Briefly, sepsis is a clinical syndrome defined by the presence of documented or suspected infection and a systemic inflammatory response. The definition of severe sepsis refers to sepsis complicated by organ dysfunction. Septic shock is diagnosed in cases of sepsis with an arterial systolic pressure of <90 mmHg for at least one hour after fluid resuscitation or required vasopressor therapy to maintain a systolic blood pressure of >90 mmHg. MODS is defined as dysfunction or failure of multiple organ or system happened simultaneously or sequentially. Patients were excluded from the study if they were infected with human immunodeficiency virus, had any neoplasmic disease, had autoimmune disorders, or received immunosuppressive drug therapy. For all enrolled patients, baseline demographic factors, medical history, tobacco use, site of infection, chronic comorbidities and biochemical parameters including hematocrit, white blood cell count, coagulation profile, BUN, serum creatinine, serum albumin and liver function index were recorded via structured questionnaire. The severity of illness was also evaluated through recording the worst reading during the first 24 hours after intensive care unit admission, using the Acute Physiology and Chronic Health Evaluation (APACHE) II score system.[20]
A total of 260 subjects without sepsis, who were not biologically related to any of the patients, were recruited as controls. The controls were randomly selected among 597 volunteers from a community cancer screening program for early detection of cancer in the same regions during the same period. The selection criteria for the controls included no history of sepsis or other serious illness, and frequency matching to the cases on gender, age and ethnicity.
Haplotype-Tagged SNP identification and selection
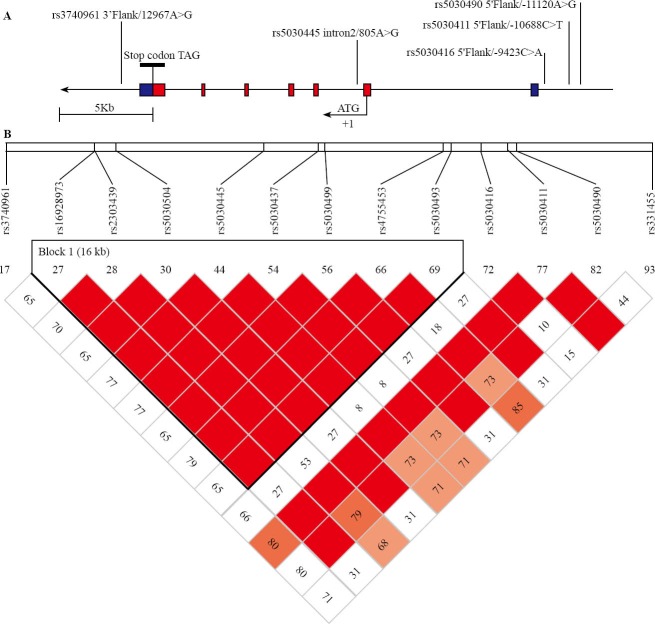
In order to better detect small relative risks, we restricted our attention to common SNPs and haplotypes (frequency > 5%). We selected htSNPs from the HapMap database (http://www.hapmap.org, public releases up to 21a/phaseII Jan 2007, on NCBI B35 assembly, dbSNP b125) using Han Chinese Beijing (HCB) sample with the Haploview 4.01 program, including 3 kilobases upstream and downstream the gene and aiming for a minimum r2 of 0.8. r2 is a measure of correlation between haplotypes defined by all SNPs and haplotypes defined by the selected htSNPs. The 13 common TRAF6 SNPs were tagged by 7 htSNPs: rs5030490 A-11120G, rs5030411 C-10688T, rs5030416 C-9423A, rs5030445 A805G (in absolute linkage disequilibrium with rs5030437, rs2303439 and rs4755453, r2=0.974), rs5030493 (in absolute linkage disequilibrium with rs5030504, rs16928973 and rs5030499, r2=1), rs331455 and rs3740961 A12967G. The gene consisted of only one 16kb LD block (Figure 1). The areas represented by rs5030493 and rs331455 were away from exon, so the other 5 htSNPs were selected as genotyping in this case-control study.
Figure 1.
The structure of TRAF6 polymorphisms related to the human genomic location and pair-wise linkage disequilibrium analysis. A: Genomic location of SNPs was identified in relation to the exon/intron structure of the human TRAF6 gene. The 7 exons marked with boxes, in which blue areas represents untranslated regions. Positions for SNPs were relative to the first nucleotide of open reading frame of the TRAF6 gene. B: SNP positions and data on pair-wise linkage disequilibrium were obtained from the HapMap database (CHB sample; http://www.hapmap. org, public releases up to 21a/phaseII Jan 2007). Pair-wise LD was measured by D’ and r2. The color boxes correspond to the paired r2 between the SNPs.(Red boxes: r2=1, Grey boxes: 1>r22>0 and white boxes: r2=0). Squares without a number indicated D’=1. 7 SNPs in 7 tests captured 13 of 13 (100%) alleles at r21≥0.8. Mean max r2 was 0.994.
Genotyping
Genomic DNA was extracted from 5 ml peripheral blood samples using standard phenol chloroform protocols. DNA samples were diluted to a concentration of 10 ng/ml and were distributed in 96-well plates.
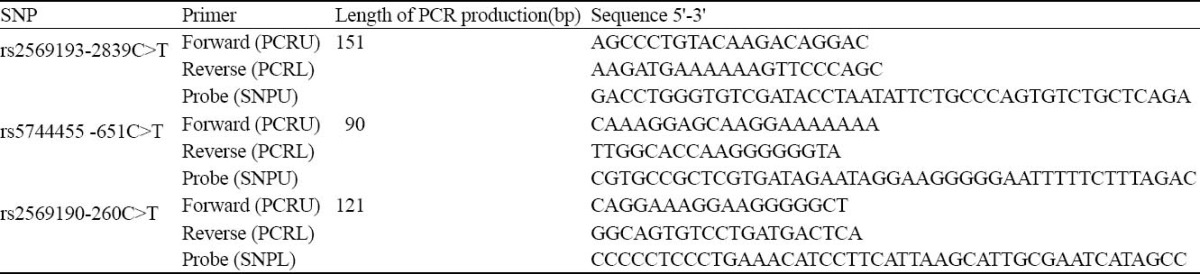
Genotyping was performed with multiplex PCR and SNP analysis based on a GenomeLab SNPstream genotyping platform (Beckman Coulter Inc., Fullerton, CA). The primers for the multiplex PCR and single-base extension reaction (Tabel 1) were designed for the SNP sites by using the web-based software, www. autoprimer.com. The SNPstream genotyping assay was performed according to the methods previously described by Bell et al.[21] Briefly, 10 ng of each DNA sample was used for multiplex PCR amplification. The target regions containing the SNP sites were amplified in a final volume of 5 μl, containing 5 mmol MgCl2, 75 μmol dNTPs, 50 nmol of each primer and 0.1 unit AmpliTaq Gold (Applied Biosystems). The PCR conditions were as follows: 94 °C for 15 minutes to activate AmpliTaq Gold., 34 cycles of denaturation at 94 °C for 30 seconds, annealing at 55 °C for 30 seconds, and primer extension at 72 °C for 1 minute. The PCR products were treated with a cocktail of exonuclease I and shrimp alkaline phosphatase to degrade unincorporated PCR primers and dNTPs. Single base extension primers were extended using single TAMRA- or Bodipy-fluorescein-labeled nucleotide terminator reactions (96 °C 3 minutes, then 45 cycles of 94 °C 20 seconds, 40 °C 11 seconds) and then spatially resolved by hybridization to the complementary oligonucleotides arrayed on the SNPware Tag Array (384-well microplate format). The individual SNPs within the multiplex were identified according to the position of the arrayed oligonucleotides within each well. Genotype data were generated on the basis of the relative fluorescent intensities for each SNP and computer processed for graphical review.
Table 1.
Primers and tagged extension probes used for SNP detection by GenomeLab SNPstream genotyping platform
Failed genotypes were not repeated. Genotyping was performed in a blind manner so that the performers did not know the case/control status of the subjects. For quality control, a 10% masked random sample of cases and controls was tested by DNA sequencing and all genotype results were 100% concordance.
Statistical analysis
Genotype and allele frequencies for each SNP were determined by direct gene counting. The significance of deviation from the Hardy-Weinberg equilibrium was tested using the chi-square test. Descriptive statistics was used for basic characterization of the patients and controls. Differences of continuous variables between the patient and control groups were compared by Student’s t test. Comparisons of categorical data between the groups were done using the chi-square test. Multivariate logistic regression analysis was performed to evaluate whether there was an association between the CD14 polymorphisms and the risk and severity of sepsis. P values, odds ratios (ORs), and 95% confidence intervals (CIs) were calculated and adjusted for age, drinking, smoking, chronic diseases status and APACHEII score. In view of multiple comparisons in the case-control study, the correction factor n(m-1) (in which there are n loci with m alleles each) was applied to correct the significance level. An association was considered significant at a P value of <0.01 (i.e., 0.05 ÷ 5), and all statistical tests were two-sided. These analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Linkage disequilibria and haplotype were analyzed using the Haploview 4.01 program (available at: http://www.broad.mit.edu/haploview/ haploview).
RESULTS
Characteristics of patients
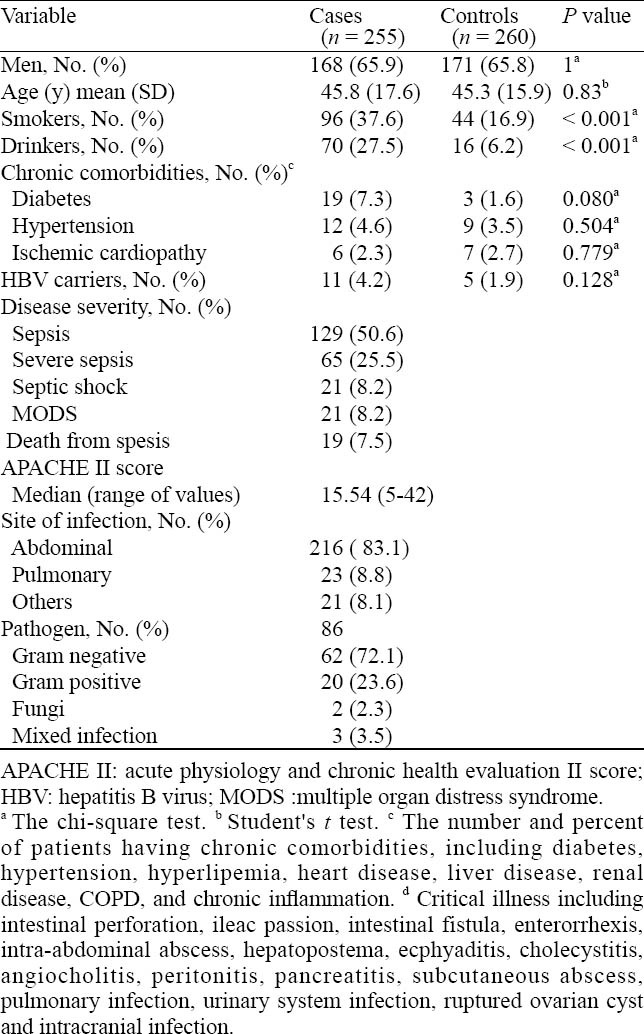
Table 2 shows the selected characteristics of the patients and controls. The controls were comparable with the patients in terms of age, gender and chronic diseases status. However, more smokers and drinkers were in the patients compared with the controls (37.6% vs. 16.9%, χ2=16.7, P<0.001 and 27.5% vs. 6.2%, χ2=12.3, P<0.001, respectively). Of the 255 patients and 260 controls, 58 and 45 had chronic comorbid conditions, including diabetes mellitus, hypertension and ischemic cardiopathy, respectively. According to the International Sepsis Definition Conference, 50.6%, 25.5%, 8.2%, and 8.2% of the patients were classified with sepsis, severe sepsis, septic shock and MODS. 19 patients with septic shock and MODS died. Abdominal infection (83.1%) was the most frequent infection followed by pumanary infection (8.8%) and others (8.1%). A majority of pathogens were Gram negative (72.1%) and positive bacteria (23.6%).
Table 2.
Distributions of selected characteristics by case-control status
Susceptibility and severity analysis of sepsis
The genotyping results of the five polymorphisms are shown in Table 3. The observed genotype frequencies of the three polymorphisms conformed to the Hardy-Weinberg equilibrium in both patients and controls (all P > 0.05). The distribution of TRAF6 polymorphisms allele frequency was similar in the patients and controls (-11120A 78.5%, -10688T 46.1%, -9423A 73.3%, 805G 68.9% and 12967G 21.3% vs80.4%, 47.5%, 72.2%, 73.5% and 25.9% respectively). On the basis of multivaribale logistic regression analysis with adjustment for age, drinking, smoking and chronic diseases status, no significant association was found between the 5 TRAF6 polymorphisms and susceptibility to sepsis.
Table 3.
The distribution of TRAF6 polymorphisms genotype in the controls and patients with different disease severity and adjusted OR for sepsis risk
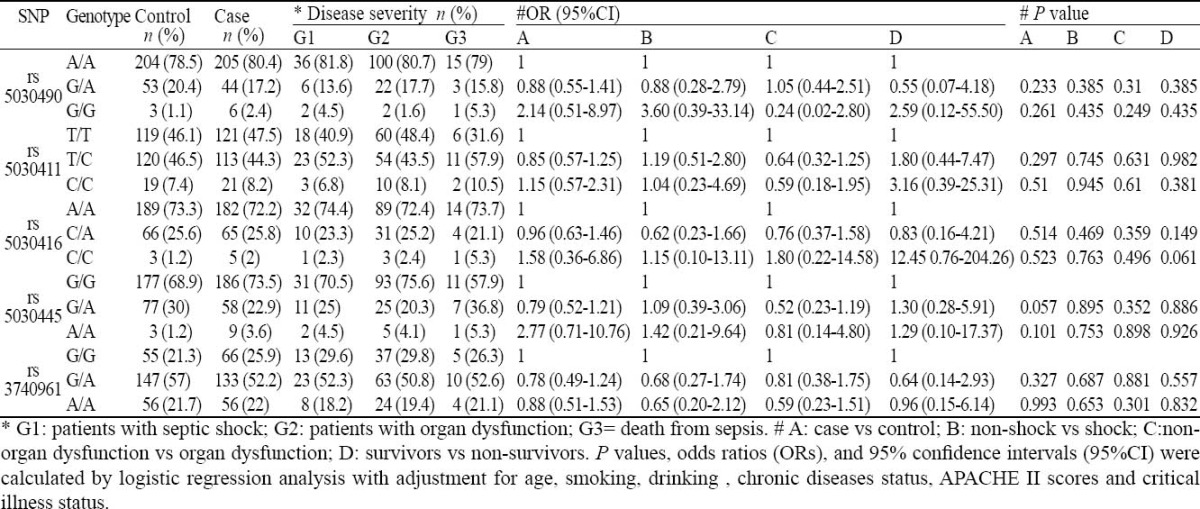
The patients were subdivided into groups according to shock or non-shock, death or survival, the number of dysfunction organs, and the distribution of five htSNPs allelic loci and genotype frequency was analyzed. The severity of sepsis was measured by organ dysfunction, septic shock and non-survivor subgroups. On the basis of multivaribale logistic regression analysis with adjustment for age, drinking, smoking, chronic diseases status, APACHEII scores and critical illness status, slimily, the genotype distributions of the three polymorphisms were not significantly different among the subgroups.
We also assessed whether there were any associations between the haplotypes derived from the 5 TRAF6 polymorphisms and susceptibility to and severity of sepsis. Haplotypes based on the 5 polymorphisms were observed, and among them only 4 haplotypes had allele frequency more than 5%. The estimated haplotype distribution was not significantly different between the patients with sepsis and controls, and among the subgroups with severity. Furthermore, compared with the main haplotype -11120A/-10688T/-9423A/805G/12967G (with the frequency 0.449), haplotype was not associated with significantly increased susceptibility to or severity of sepsis (data not shown).
DISCUSSION
Previous studies have suggested that TRAF6 plays a central role in both innate immunity and the pathogenesis of sepsis. The genetic associations between TRAF6 and sepsis are plausible from a biological perspective. In TRAF6-deficient mice, TLR2, TLR5, TLR7, and TLR9 failed to induce activation of NF-κB and MAPKs or production of inflammatory cytokines and TLR4 ligand-induced cytokine production was remarkably reduced.[22] CpG-ODN pre-treatment in LPS-challenged animals decreased LPS-induced leukocyte–endothelial cell interaction, sinusoidal perfusion failure and attenuated hepatic protein expression of TRAF6 and NF-κB.[23] TRAF6 also plays an important role in LPS-induced apoptosis in sepsis.[24] In the present study, however, we did not find any association between the TRAF6 gene polymorphisms and the susceptibility to or subsequent severity of sepsis and not confirm the initial hypothesis.
There are several possible reasons for the negative results. First, the pathophysiology of sepsis involves highly complex interactions between invading microorganisms, environmental and genetic components. Our findings suggested that the TRAF6 gene polymorphisms might not play a major role in mediating susceptibility and severity of sepsis. Alternatively, lack of statistical performance results also caused the negative correlation. For example, for the rs5030490 A-11120G polymorphism, this study had 80% power of test to detect an OR of >1.805 or <0.482 for carriers of the AA genotype relative to the carriers of the GA and GG genotype. Additional larger population-based case-control studies are warranted to understand the roles of these TRAF6 polymorphisms in the etiology of sepsis. Third, our negative results may be due to an inherent selection bias. As a hospital-based study, our sepsis cases were enrolled from the hospitals and the control subjects were selected from the community population. Thus, inherent selection bias cannot be completely excluded. By matching on age, sex and residential area, however, potential confounding factors might have been minimized and any inadequacy in matching might have been controlled in data analyses with further adjustment and stratification. Finally, because of strong LD among the associated polymorphisms, we were unable to determine if any particular site is driving the associations. Further studies are needed to identify the causative polymorphism and elucidate the exact molecular mechanism underlying this association.
In summary, the TRAF6 gene polymorphisms might not play a major role in mediating susceptibility to sepsis. However, these results do not exclude that a still unknown TRAF6 SNP may influence the course of sepsis. Additional studies are warranted before the importance of TRAF6 polymorphisms in sepsis risk can be fully ascertained. First, data from larger population-based case-control studies in other populations, such as other Chinese, Singaporeans, and Taiwanese, are required to confirm our observation. Second, the polymorphisms in additional components of the innate immune pathway association with sepsis risk should be systematically investigated.
Footnotes
Funding: This study was supported in part by grants from Major State Basic Research Program of China (973 Program) (2005CB522602) and Beijing Science & Technology NOVA Program (2006A54).
Ethical approval: This study was approved by the Ethics Committee of the Institute of Radiation Medicine, Military Academy of Medical Sciences.
Conflicts of interest: None.
Contributors: Fang Y proposed and wrote the paper. All authors read and approved the final version.
REFERENCES
- 1.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 2009;136:e28. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Lidicker L, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. CUB-Rea Network. Current epidemiology of septic shock. Am J Respir Crit Care Med. 2003;168:165–172. doi: 10.1164/rccm.2201087. [DOI] [PubMed] [Google Scholar]
- 5.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 6.Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: the CUB-Rea Network. Am J Respir Crit Care Med. 2003;168:165–172. doi: 10.1164/rccm.2201087. [DOI] [PubMed] [Google Scholar]
- 7.Kumpf O, Schumann RR. Genetic influence on bloodstream infections and sepsis. Int J Antimicrob Agents. 2008;32:S44–50. doi: 10.1016/j.ijantimicag.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, et al. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med. 2007;35:2538–2546. doi: 10.1097/01.CCM.0000284492.30800.00. [DOI] [PubMed] [Google Scholar]
- 9.Gao H, Evans TW, Finney SJ. Bench-to-bedside review: sepsis, severe sepsis and septic shock -does the nature of the infecting organism matter? Crit Care. 2008;12:213. doi: 10.1186/cc6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villar J, Maca-Meyer N, Pérez-Méndez L, Flores C. Bench-tobedside review: Understanding genetic predisposition to sepsis. Crit Care. 2004;8:180–189. doi: 10.1186/cc2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 12.Clark MF, Baudouin SV. A systematic review of the quality of genetic association studies in human sepsis. Intensive Care Med. 2006;32:1706–1712. doi: 10.1007/s00134-006-0327-y. [DOI] [PubMed] [Google Scholar]
- 13.Schuetz P, Christ-Crain M, Muller B. Biomarkers to improve diagnostic and prognostic accuracy in systemic infections. Curr Opin Crit Care. 2007;13:578–585. doi: 10.1097/MCC.0b013e3282c9ac2a. [DOI] [PubMed] [Google Scholar]
- 14.Wurfel MM. Genetic insights into sepsis: what have we learned and how will it help? Curr Pharm Des. 2008;14:1900–1911. doi: 10.2174/138161208784980554. [DOI] [PubMed] [Google Scholar]
- 15.Takashi K, Matthew CW, Choi YW. The role of TRAF6 in signal transduction and the immune response. Microbes and Infection. 2004;6:1333–1338. doi: 10.1016/j.micinf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Twomey C, Qian S, McCarthy JV. TRAF6 promotes ubiquitination and regulated intramembrane proteolysis of IL-1R1. Biochemical and Biophysical Research Communications. 2009;381:418–423. doi: 10.1016/j.bbrc.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 17.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 18.Underhill DM, Ozinsky A. Toll-like receptors: key mediators of microbe detection. Current Opinion in Immunology. 2002;14:103–110. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 19.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 21.Bell PA, Chaturvedi S, Gelfand CA, Huang CY, Kochersperger M, Kopla R, et al. SNPstream UHT: Ultra-high throughput SNP genotyping for pharmacogenomics and drug discovery. Bio Techniques. 2003;34:496. [PubMed] [Google Scholar]
- 22.Gohda J, Matsumura T, Inoue J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domainYcontaining adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J Immunol. 2004;173:2913–2917. doi: 10.4049/jimmunol.173.5.2913. [DOI] [PubMed] [Google Scholar]
- 23.Slotta JE, Scheuer C, Menger MD, Vollmar B. Immunostimulatory CpG-oligodeoxynucleotides (CpG-ODN) induce early hepatic injury, but provide a late window for protection against endotoxin-mediated liver damage. J Hepatol. 2006;44:576–585. doi: 10.1016/j.jhep.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Hull C, McLean G, Wong F, Duriez PJ, Karsan A. Lipopolysaccharide signals an endothelial apoptosis pathway through TNF receptor-associated factor 6-mediated activation of c-Jun NH2-terminal kinase. J Immunol. 2002;169:2611–2618. doi: 10.4049/jimmunol.169.5.2611. [DOI] [PubMed] [Google Scholar]