Abstract
BACKGROUND:
With mechanical ventilation widely used in intensive care unit, the ventilator associated pneumonia (VAP) has become a common and serious complication in critically ill patients. Compared with adults, the incidence of VAP and the mortality are higher in children in pediatric intensive care unit (PICU) because of immune deficiency, severe basic diseases, and increased use of artificial airway or mechanical ventilation. Hence it is of significance to study the epidemiology and changes of antibacterial susceptibility in order to reduce the incidence and mortality of VAP in children.
METHODS:
From January 2008 to June 2010, 2758 children were treated in PICU of Wuhan Children’s Hospital. Among them, 171 received mechanical ventilation over 48 hours in PICU, and 46 developed VAP. The distribution and drug-resistance pattern of the pathogenic bacteria isolated from lower respiratory tract aspirations were analyzed.
RESULTS:
A total of 119 pathogenic microbial strains were isolated. Gram-negative bacilli (G−) were the most (65.55%), followed by fungi (21.01%) and gram-positive cocci (G+, 13.45%). Among them, the most common pathogens were Acinetobacter baummannii, Escherichia coli, Klebsiella pneumoniae, candida albicans and coagulase-negative staphylococci. Antibiotic susceptibility tests indicated that the multiple drug-resistances of G− and G+ to antibiotics were serious. Most of G− was sensitive to ciprofloxacin, amikacin, imipenem, meropenem, cefoperazone-sulbactam and piperacillin-tazobactam. The susceptibility of G+ to vancomycin, teicoplanin and linezolid were 100%. Fungi were almost sensitive to all the antifungal agents. The primary pathogens of VAP were G−, and their multiple drug-resistances were serious.
CONCLUSION:
In clinical practice we should choose the most sensitive drug for VAP according to pathogenic test.
KEY WORDS: Pediatric, Intensive care unit, Ventilator-associated pneumonia, Pathogen, Drug-resistance, Retrospective clinical study
INTRODUCTION
Patients in the intensive care unit (ICU) are at risk for dying not only from their critical illness but also from secondary processes such as nosocomial infection. With mechanical ventilation widely used in ICU, ventilator associated pneumonia (VAP) has become a common and serious complication in critically ill patients. VAP is defined as pneumonia occurring more than 48 hours after patients have been intubated and received mechanical ventilation. In adult ICU, the incidence of VAP can reach 9%-27%, and the mortality of VAP has been reported to range from 20% to 50%.[1-4] The incidence and mortality of VAP are higher in children in pediatric intensive care unit (PICU) than in adults because of immune deficiency, severe basic diseases, and increased use of artificial airway or mechanical ventilation. Hence it would be of significance to study the epidemiology and changes of antibacterial susceptibility in order to reduce the incidence and mortality of VAP in children. We retrospectively studied the pathogenic bacteria distribution and drug resistance in VAP children in the PICU of Wuhan Children’s Hospital between January 2008 and June 2010.
METHODS
General data
From January 2008 to June 2010, 2758 children were treated in the PICU of Wuhan Children’s Hospital. Among them, 171 received mechanical ventilation over 48 hours in the PICU, and 46 developed VAP.
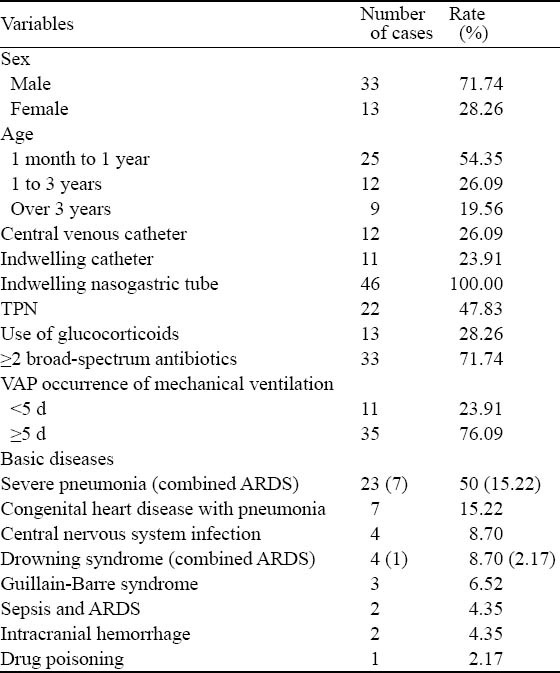
The diagnostic criteria of VAP were as follows: 1) pneumonia occurring more than 48 hours after intubation and mechanical ventilation; 2) two or more of the four criteria (i) fever of >38.3 °C, (ii) leukocytosis of 10 000 cells/mL or<5 000 cells/mL, (iii) purulent tracheobronchial secretion, (iv) and/or new pathogenic bacteria isolated from bronchial secretions; 3) a new and persistent (>48 h) infiltrate on chest radiograph.[5-7] The basic information of 46 children with VAP was listed in Table 1.
Table 1.
General data of children with VAP
Sputum test
Under the condition of sterility, a disposable sterile sputum collector was used to collect secretion samples from the trachea at bifurcation through an endotracheal intubation catheter. Routine microscopic examination was performed before sputum culture. Squamous epithelial cells<10/low-power field and multi-nucleated cells>25/low-power field were regarded as qualified specimens. Then the qualified specimens were inoculated into the culture medium, pathogens with a concentration of >105 cfu/mLwere isolated, and then antimicrobial susceptibility test was carried out.
Instruments and reagents
Bacteria were identified using a VITEK-32 Auto Mcrobic System produced by Marcel Mérieux. Susceptibility paper and susceptibility medium (MH medium) were purchased from the UK Oxiod and Marcel Mérieux, respectively.
Susceptibility test
Routine drug susceptibility test was performed using the disk diffusion method and the VITEK-32 Auto Mcrobic System. The extended-spectrum β-lactamases (ESBLs) strains were detected by double disk test. Quality control strains included Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, Candida albicans ATCC 90028, and Escherichia coli ATCC 35218. The criteria of drug resistance followed the CLSI set in 2008. If more than two consecutive results were the same in one patient, we recorded the results of sputum culture one time; if the results were different, we recorded the each result of sputum culture.
Statistical analysis
All test data were analyzed by the WHONET 5.4 software.
RESULTS
Distribution of pathogenic bacteria
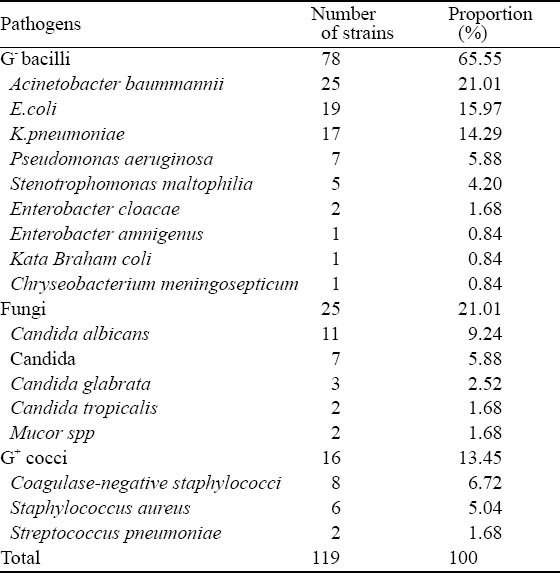
Among the 46 VAP children, 24 (52.17%) had mixed infection. A total of 119 pathogenic microbial strains were isolated, including 78 strains of gram-negative bacilli (G−, 65.55%), 25 strains of fungi (21.01%), and 16 strains of gram-positive cocci (G+, 13.45%). Among pathogens, the most common pathogens were Acinetobacter baummannii, Escherichia coli (E.coli), Klebsiella pneumoniae (K.pneumoniae), Candida albicans and coagulase-negative staphylococci (Table 2).
Table 2.
Distribution of pathogens isolated from patients with VAP
Prevalence of methicillin-resistant staphylococcus and ESBLs
The detection rate of ESBL-producing E.coli strain, ESBL-producing K.pneumoniae strain, methicillin-resistant Staphylococcus aureus (MRSA), and methicillin-resistant coagulase-negative staphylococci (MRCNS) was 57.89 % (11/19), 58.82 % (10/17), 0.75% (6/8), respectively.
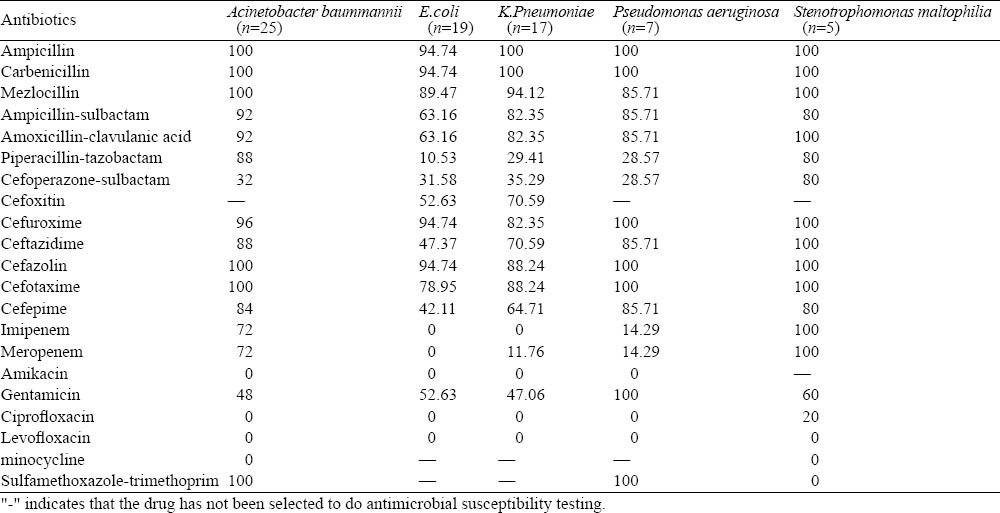
Drug resistance results of the top five G− bacilli
The antimicrobial resistance of G− bacilli showed multiple drug resistance. The most sensitive antibiotics against Acinetobacter baummannii were amikacin, ciprofloxacin, levofloxacin and minocycline (100%), followed by cefoperazone-sulbactam (68%). The resistance rates of penicillins and cephalosporins were more than 70%, and the resistance rates of meropenem and imipenem were up to 72%. E.coli and K.pneumoniae were resistant to most penicillins and cephalosporins, and their antibiotic resistance was lower for imipenem, meropenem, amikacin, ciprofloxacin, levofloxacin, piperacillin-tazobactam and cefoperazone-sulbactam. The antibiotic resistance of Pseudomonas aeruginosa was 0 for amikacin, ciprofloxacin and levofloxacin; 14.29% for imipenem and meropenem; 28.57% for piperacillin-tazobactam and cefoperazone-sulbactam; >70% for other antibiotics. For Stenotrophomonas maltophilia, the antibiotic resistance was 0 for sulfamethoxazole-trimethoprim, minocycline, and levofloxacin; 20% for ciprofloxacin; and > 60% for other antibiotics (Table 3).
Table 3.
Drug-resistance of the top five G− bacilli (%)
Susceptibility results of G+ cocci
G+ cocci were highly sensitive to vancomycin, linazolid and teicoplanin (100%), and highly resistant to penicillin, erythromycin, clindamycin, tetracycline and sulfamethoxazole-trimethoprim (>50%). The resistance rate of oxcillin against coagulase-negative staphylococci was up to 75%. Additionally, G+ cocci had lower resistance to cefuxine, cefazolin and levofloxacin (<30%).
Susceptibility results of fungi
The fungi remained sensitive to antifungal agents. One strain of Candida glabrata was resistant to fluconazole and itraconazole, and one strain of Mucor was resistant to fluconazole.
DISCUSSION
PICU is an area within a hospital specializing in the care of critically ill infants, children, and teenagers. Complex technology and equipment is often in use, particularly mechanical ventilators and patient monitoring systems. Children in PICU are characterized by severe pathophysiological disorders, immune dysfunction and other critical conditions. They are often treated with tracheal intubation, tracheotomy, mechanical ventilation and other rescue measures. At the same time, the impaired respiratory tract barriers allow bacteria to enter the respiratory system, and thus increase bacterial colonization and infection. Most children in PICU are given high-dose broad-spectrum antibiotics in combination, which change the normal bacterial colonization, and thus lead to VAP. In PICU of Wuhan Children’s Hospital from January 2008 to June 2010, 171 children received mechanical ventilation (>48 hours), and 46 children (26.9%) were diagnosed with VAP. In the isolated pathogens and pathogenic bacteria, the detection rate of G− bacilli was 65.55%, followed by 21.01% for fungi and 13.45% for G+ cocci. The most common pathogens were E.coli, K.pneumoniae, Candida albicans and coagulase-negative staphylococci.
In recent years, the infection rate of non-fermentative bacteria such as Acinetobacter baummannii, Stenotrophomonas maltophilia has been increasing year by year. The bacteria have become the main pathogens in nosocomial infections, especially in VAP. In this study the infection rate of non-fermentative bacteria was high, accounting for 50% of G− bacilli (39/78), and Acinetobacter baummannii ranked the first. Acinetobacter baummannii produce antimicrobial resistance by increasing the expression of Ampc enzyme, producing OXA-23 carbapenem enzyme, decreasing the expression of outer membrane pore channel protein, efflux pump system hyperactivity, and loss of PBPs.[6,7] Additionally, Acinetobacter baummannii could induce drug resistance through plasmid integration, while causing multiple drug resistance plasmids.[8] Gundogan et al[9] found that the resistance of cephalosporins and imipenem against Acinetobacter baummannii was very serious. In our study Acinetobacter baummannii was only sensitive to aminoglycoside antibiotics, quinolone antibiotics and cefoperazone-sulbactam, and the resistance rate of carbapenem reached 72%. Imipenem and meropenem are no longer the first choice to treat Acinetobacter baummannii. In the present study, the resistance rate of β-lactam antibiotics such as imipenem and meropenem increased year by year. The resistance rate of imipenem and meropenem to Pseudomonas aeruginosa was 14.39%, which indicated that carbapenems can temporarily serve as the first choice to treat Pseudomonas aeruginosa.[10] The resistance rate of Stenotrophomonas maltophilia to imipenem was 100%. This may be connected with the natural drug-resistance to imipenem.[11] Since compound sulfamethoxazole is highly sensitively to Stenotrophomonas maltophilia, this agent can be used as the first choice for critically ill patients. Because of declined susceptibility in Acinetobacter baummannii, increased resistance in Pseudomonas aeruginosa, and the natural drug resistance in Stenotrophomonas maltophilia, close attention should be paid to the results of bacteria at any time so as to adjust or control the use of carbapenems.
Because β-lactam antibiotics are widely used, strains such as E.coli and K.pneumoniae, can produce extended spectrum β-lactamase (ESBLs). Because ESBLs can hydrolyze cephalosporins and monocyclic β-lactam antibiotics and spread through the formation of plasmids[12] at the same time, cephalosporin can induce the G− bacilli to produce ESBLs. Thus the resistance mediated by ESBLs expands rapidly. In our study the detection rates of E.coli and K.pneumoniae, which produce ESBLs, were 57.89% and 58.82% respectively, which were similar to those reported by Wang et al.[13] Susceptibility test results showed that E. coli and K.pneumoniae were highly sensitive to carbapenems, aminoglycosides, quinolones and some compound formulations containing enzyme inhibitors, but resistant to penicillins and cephalosporins.
The results of sensitivity test also showed that G+ cocci were highly resistant to penicillins. Vancomycin resistant strains were not seen, and vancomycin is still the most effective agent to treat severe Staphylococcus aureus infection. β-lactam antibiotics, aminoglycosides, macrolides, clindamycin and tetracyclines were not recommended to treat critically ill patients.[14] Teicoplanin and linezolide against multi-resistant bacteria were very effective to multidrug resistant bacteria, just as vancomycin.
Our data showed that the proportion of fungal infection increased (21.01%), and took the second position before G+ cocci. This was related to the long use of antibiotics (especially the use of third generation cephalosporins and carbapenem >7d or unreasonable use of corticosteroids), mechanical ventilation and other invasive operations.[15] Therefore corticosteroids and antimicrobial agents must be limited and rationally used to control fungal infection. Candida albicans were the main fungal strains in VAP, but the infection of non-Candida albicans strains increased, and natural drug-resistance fungi-Candida glabrata emerged. In our study, among the three Candida glabrata, one was resistant to fluconazole and itraconazole. In clinical practice, therefore, we should choose the most sensitive agents according to the results of pathogenic test.
Footnotes
Funding: None.
Ethical approval: Not needed.
Conflicts of interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributors: Cai XF proposed and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts. Li WB is the guarantor.
REFERENCES
- 1.Baker AM, Meredith JW, Haponik EF. Pneumonia in intubated trauma patients. Microbiology and outcomes. Am J Respir Crit Care Med. 1996;153:343–349. doi: 10.1164/ajrccm.153.1.8542141. [DOI] [PubMed] [Google Scholar]
- 2.Craig CP, Connelly S. Effect of intensive care unit nosocomial pneumonia on duration of stay and mortality. Am J Infect Control. 1984;12:233–238. doi: 10.1016/0196-6553(84)90114-7. [DOI] [PubMed] [Google Scholar]
- 3.Cunnion KM, Weber DJ, Broadhead WE, Hanson LC, Pieper CF, Rutala WA. Risk factors for nosocomial pneumonia: comparing adult critical-care populations. Am J Respir Crit Care Med. 1996;153:158–162. doi: 10.1164/ajrccm.153.1.8542110. [DOI] [PubMed] [Google Scholar]
- 4.Kirschenbaum L, Azzi E, Sfeir T, Tietjen P, Astiz M. Effect of continuous lateral rotational therapy on the prevalence of ventilator-associated pneumonia in patients requiring long-term ventilatory care. Crit Care Med. 2002;30:1983–1986. doi: 10.1097/00003246-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Chollet-Martin S, Montravers P, Gibert C, Elbim C, Desmonts JM, Fagon JY, Gougerot-Pocidalo MA. High levels of interleukin-8 in the blood and alveolar spaces of patients with pneumonia and adult respiratory distress syndrome. Infect Immun. 1993;61:4553–4559. doi: 10.1128/iai.61.11.4553-4559.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kollef MH. What is ventilator-associated pneumonia and why is it important? Respir Care. 2005;50:714–724. [PubMed] [Google Scholar]
- 7.American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 8.Lee HY, Chen CL, Wang SB, Su LH, Chen SH, Liu SY, et al. Imipenem heteroresistance induced by imipenem in multidrugresistant Acinetobacter baumannii: mechanism and clinical implications. Int J Antimicrob Agents. 2011;37:302–308. doi: 10.1016/j.ijantimicag.2010.12.015. Epub 2011 Feb 25. [DOI] [PubMed] [Google Scholar]
- 9.Gundogan N, Citak S, Yalcin E. Virulence Properties of Extended Spectrum β-Lactamase-Producing Klebsiella Species in Meat Samples. J Food Prot. 2011;74:559–564. doi: 10.4315/0362-028X.JFP-10-315. [DOI] [PubMed] [Google Scholar]
- 10.Hu XH, Xu XM, Mi ZH, Fan YF, Feng WY. Relationship between drug resistance of Pseudomonas aeruginosa isolated from burn wounds and its mobile genetic elements. Zhonghua Shao Shang Za Zhi. 2009;25:103–105. [PubMed] [Google Scholar]
- 11.Gómaz-Garcés JL, Aracil B, Gil Y, Burillo A. Susceptibility of 228 non-fermenting gram-negative rods to tigecycline and six other antimicrobial drugs. J Chemother. 2009;21:267–271. doi: 10.1179/joc.2009.21.3.267. [DOI] [PubMed] [Google Scholar]
- 12.Andriatahina T, Randrianirina F, Hariniana ER, Talarmin A, Raobijaona H, Buisson Y, et al. High prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a pediatric unit in Madagascar. BMC Infect Dis. 2010;10:204. doi: 10.1186/1471-2334-10-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang WP, Shao HF, Wang JN, Zhang XW, Shi LN. Genotype distribution of extended-spectrum beta-lactamases and AmpC beta-lactamases produced in Escherichia coli isolated from men with urinary infection. Zhonghua Nan Ke Xue. 2006;12:1000–1003. [PubMed] [Google Scholar]
- 14.Lu Q, Yang J, Liu Z, Gutierrez C, Aymard G, Rouby JJ, et al. Nebulized Ceftazidime and Amikacin in Ventilator-associated Pneumonia caused by Pseudomonas Aeruginosa. Am J Respir Crit Care Med. 2011 Apr 7; doi: 10.1164/rccm.201011-1894OC. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Morrow BM, Mowzer R, Pitcher R, Argent AC. Investigation into the effect of closed-system suctioning on the frequency of pediatric ventilator-associated pneumonia in a developing country. Pediatr Crit Care Med. 2011 Jan 28; doi: 10.1097/PCC.0b013e31820ac0a2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


