Abstract
BACKGROUND:
Carotid intima media thickness (CIMT) and stiffness are taken as useful surrogate markers of atherosclerosis. In China, the number of elderly patients undergoing hemodialysis has increased year by year, with the increase of dialysis-related cardiovascular events. This study was undertaken to examine carotid stiffness in elderly hemodialysis patients by the ultrasound techniques in order to find out the possible risk factors.
METHODS:
From January 2006 to February 2010, a total of 87 patients (41 males and 46 females) treated with routine hemodialysis at the 97th Hospital of People’s Liberation Army were enrolled in this study. The distensibility coefficient (DC) of the carotid artery was detected by Doppler ultrasonic diagnosis apparatus (Philips HBI5000, frequency 12 MHz) for evaluation of arterial stiffness. Serum albumin, total cholesterol (TC), high density lipoprotein (HDL), low density lipoprotein (LDL), triglyceride (TG), glucose, creatinine, calcium, phosphorus, and intact parathyroid hormone (iPTH) were examined with standard methods. The liner correlation and multiple stepwise regression analysis were used to find correlations between them.
RESULTS:
In this study, the systolic blood pressure was 153.33±25.98 mmHg, DBP 84.22± 10.39 mmHg, TC 4.39±1.05 mmol/L, TG 1.36±0.72 mmol/L, LDL 2.47±0.77 mmol/L, Cr 889.82± 207.38 Mmol/L, Glu 5.36±1.87 mmol/L, Ca I 2.00±2.19±0.21 mmol/L, and DC 13.39±5.32×10−3/kPa. DC was associated with age (r=−0.459, P<0.001), SBP (r=−0.527, P<0.001), and serum calcium (r=−0.273, P=0.011). The multiple stepwise regression analysis showed that SBP, age, increased serum calcium level, and diabetes were independent risk factors for decreasing DC.
CONCLUSION:
Systolic blood pressure, age, increased serum calcium level and diabetes in elderly hemodialysis patients are independent risk factors for increased carotid arterial stiffness.
KEY WORDS: Arterial stiffness, Hemodialysis
INTRODUCTION
Epidemiological and clinical studies have shown that damage to large arteries contributes to the high cardiovascular mortality in patients with end-stage renal disease (ESRD).[1-3] Atherosclerosis is the most frequent cause of arterial damage. It is also the most frequent cause of cardiovascular morbidity in patients with ESRD,[4] with occlusive lesions occurring principally in the medium-sized conduit arteries. Risk factors of atherosclerosis in ESRD patients are coronary risk factors such as hypertension, diabetes, hyperlipidemia, and hyperphosphatemia. Bone associated proteins including osteopontin, matrix Gla protein and osteoprotegerin may be involved in the progression of atherosclerosis. Carotid intima media thickness (CIMT) and stiffness are taken as useful surrogate markers of atherosclerosis. Measurement of CIMT, a method initially described by Pignoli et al,[5] has been increasingly used to assess atherosclerosis, to monitor the progression of the disease and the effects of treatment, and also, as a surrogate end point in clinical trials.
In China, the number of elderly hemodialysis patients has increased year by year, while increasing the dialysis-related cardiovascular events. This study was undertaken to examine the carotid stiffness in elderly hemodialysis patients by ultrasound techniques in order to find out the possible risk factors.
METHODS
Patients
From January 2006 to February 2010, a series of 87 patients (41 males and 46 females) who had been treated with routine hemodialysis at the 97th Hospital of the People’s Liberation Army were enrolled in this study. Their age ranged from 45 to 81 (60.18±9.67) years, and hemodialysis history was 3-204 months (median 47 months). Their primary diseases included chronic glomerulonephritis in 32 patients, diabetic nephropathy in 18, interstitial nephritis in 14, essential hypertension in 10, polycystic kidney in 6, chronic pyelonephritis in 2, obstructive nephropathy in 1, and unknown etiology in 4.
Inclusion criteria for patients were: 1) age>45 years; 2) dialysis>3 months; 3) no cardiovascular event at least for 3 months before the study; 4) no signs of acute infection; 5) no severe malnutrition (serum albumin>30 g/L), and 6) dialysis 3 times per week, four hours per time, KT/V>1.2.
The parameters for the dialysis machine included Fresenius 4008B with an F6 polysulfone membrane dialyzer; a dialyzer membrane area of 1.3 m2; a dialysate flow rate of 500 mL/min; a blood flow rate of 200-300 mL/min; and bicarbonate solutions (138 mmol/L sodium, 2.5 mmol/L potassium, 1.5 mmol/L bicarbonate, 0.5 mmol/L magnesium, and 32 mmol/L bicarbonat).
The patients in this study signed consent forms, and the study was approved by the Ethics Committee of the 97th Hospital of the People’s Liberation Army.
Evaluation of arterial stiffness
A Doppler ultrasonic diagnosis apparatus (Philips HBI5000, frequency 12 MHz) was used to detect distensibility coefficient (DC) of the carotid artery for the evaluation of arterial stiffness.
The formula for DC is as follows: DC=2 [(Dd–Ds)/Dd)]/(SBP–DBP) (Ds: vascular systolic diameter; Dd: vascular diastolic diameter; SBP: systolic blood pressure; DBP: diastolic blood pressure).
The body area for the measurement was set at 1.0-2.0 cm apart form carotid bifurcation. When the artery anterior and posterior intima became clearest, the probe was put at the border of medial and outer membranes to capture the diameter curve of the carotid artery. To avoid the effect of volume overload and hemodynamic change on the test results, ultrasound examination was done at one hour after the dialysis by the same qualified physician.
Laboratory examination
Fasting blood samples were collected on the day of dialysis. Albumin (ALB), calcium (Ca), phosphorus (P), total cholesterol (TC), high density lipoprotein (HDL), low density lipid protein (LDL), triglyceride (TG), glucose and creatinine were measured using a Hitachi 7600 automatic biochemical analyzer. Intact parathyroid hormone (iPTH) was determined using Beckman Access Immunoassay. Laboratory examination and evaluation of arterial stiffness were performed on the same day.
Statistical analysis
The data were presented as mean±standard deviation (SD). Statistical evaluations were performed by linear correlation coefficient and multiple stepwise regression analysis using SPSS software package 11.5 (Chicago, IL). A P value less than 0.05 was considered statistically significant.
RESULTS
General information
In this study, the systolic blood pressure was 153.33 ±25.98 mmHg, DBP 84.22±10.39 mmHg, TC 4.39±1.05 mmol/L, TG 1.36±0.72 mmol/L, LDL 2.47±0.77 mmol/ L, Cr 889.82±207.38 μmol/L, Glu 5.36±1.87 mmol/L, Ca I 2.00±2.19±0.21 mmol/L, and DC 13.39±5.32×10−3/kPa. HDL distribution, iPTH, and dialysis period were skewed, but were transformed into poission distributions by the natural logarithm.
Correlation analysis
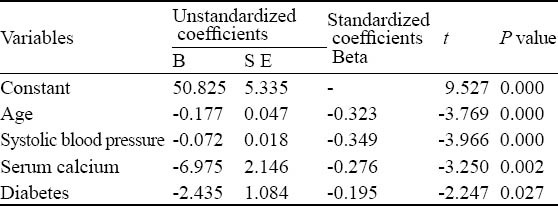
The Pearson’s correlation coefficient analysis showed that DC was associated with age (r=−0.459, P<0.001), SBP (r=−0.527, P<0.001) and serum calcium (r=−0.273, P=0.011). The multiple stepwise regression analysis showed that SBP, age, increased serum calcium, and diabetes were independent risk factors for decreasing DC (Table 1).
Table 1.
The multiple linear regression analysis for DC and its impact factor
DISCUSSION
In ESRD patients, vascular disease develops quickly and early, which is mainly represented as increased arterial extracellular matrix, diffuse thickening of the vascular media, rupture-induced fibrosis in the elastic layer, and the calcification-induced large artery stiffness. Large artery stiffness is an independent predictor for the cardiovascular disease (CVD)-induced death and total mortality of ESRD patients.[6] Ultrasound technology is widely used to precisely track and trace the intima trajectory. By recording the changes of artery diameter during systolic and diastolic phases, we can calculate the expansion of arterial and directly evaluate the vascular stiffness. This method has such advantages as real-time collection, repetitiveness, and non-invasive evaluation. In this study we measured DC in elderly hemodialysis patients and analyzed the possible factors associated with carotid stiffness, and found that systolic blood pressure, age, increased serum calcium and diabetes were independent risk factors for carotid artery DC in middle-aged hemodialysis patients.
ESRD patients are often complicated by hypertension. Studies[7-10] have shown that large artery stiffness in ESRD patients is closely related to hypertension. Our results also indicated that in elderly hemodialysis patients systolic blood pressure was one of the independent risk factors for carotid artery DC. During the arterial stiffness, the pulse wave velocity increased, the arrival of reflected wave at the central artery was brought forward to systolic phase instead of diastolic phase, thus making systolic blood pressure increase, diastolic blood pressure decrease, and pulse pressure increase. In turn the increased blood pressure can lead to impairment of endothelial function, arterial wall dysfunction in systolic and diastolic phases,[11] proliferation and fibrosis of arterial medial smooth muscle cells, and increased collagen synthesis. Therefore large artery atherosclerosis and hypertension interact with each other.
Age is one of the most significant factors of arterial stiffness.[12] In the age-related incidence of arterial stiffness, vascular structural changes play a major role.[13] The mechanism of these changes includes: 1) reduced density of elastin in the arterial wall, fragmentation, collagen synthesis, and increased collagen content in arterial wall, decreased metabolism, hypertrophy of vascular smooth muscle cells, and increased expression of interstitial cell adhesion molecules and growth factors; 2) increased advanced glycation end products (AGEs), cross-linking of increased collagen molecules in the arterial wall, loss of collagen elasticity, and increased wall stiffness. Our study showed that in elderly hemodialysis patients, age was also one of the independent risk factors for carotid arterial stiffness.
In ESRD patients, abnormal calcium deposition and phosphorus metabolism are important causes for vascular calcification in hemodialysis patients.[14] In our study, serum calcium level was one of the independent risk factors for carotid artery DC in the 87 patients, while serum phosphorus and iPTH were not associated with arterial stiffness. As reported, angiosteosis is an active adjustment process which is similar to the formation of bone and cartilage. Its main feature is the bone-like change of vascular smooth muscle cells.[15]
Diabetes is also considered one of the independent risk factors for arterial stiffness in ESRD patients.[8] Takenaka et al[16] after one-year investigation found that large artery stiffness developed more quickly in diabetic hemodialysis patients than in non-diabetic hemodialysis ones. Aoun et al[17] conducted a case-control study in which 122 diabetic patients were compared to 122 non-diabetic patients in terms of sex, age, mean arterial pressure, number and localization of atherosclerotic alterations. They found that the diabetic patients had higher arterial stiffness than the non-diabetic patients with one or more cardiovascular risk factors, manifested by increased aortic PWV and PP. In addition, renal failure, irrespective of its degree and independent of diabetes mellitus, is associated with increased aortic PWV but not PP. Although the morbidity of arterial stiffness significantly increased in the diabetic patients, we didn’t find that fasting blood glucose was an independent risk factor for arterial stiffness. The mechanism may be connected with the formation of AGEs.
In conclusion, systolic blood pressure, age, increased serum calcium, and diabetes in elderly hemodialysis patients are independent risk factors for increased carotid arterial stiffness. Early intervention of reversible risk factors such as blood pressure, serum calcium, blood glucose may reduce the incidences of arterial stiffness and CVD in ESRD patients.
Acknowledgement
We are grateful to Prof. Yi-lun Zhou of Beijing Chaoyang Hospital for his valuable help in this study.
Footnotes
Funding: None.
Ethical approval: The study was approved by the Ethics Committee of the 97th Hospital of People’s Liberation Army.
Conflicts of interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributors: Ren HQ wrote the main body of the article. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Kidney Int. 2003;63:1852–1860. doi: 10.1046/j.1523-1755.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- 2.Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension. 1998;32:570–574. doi: 10.1161/01.hyp.32.3.570. [DOI] [PubMed] [Google Scholar]
- 3.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2349. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 4.Guerin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15:1014–1021. doi: 10.1093/ndt/15.7.1014. [DOI] [PubMed] [Google Scholar]
- 5.Prati P, Vanuzzo D, Casaroli M, Di Chiara A, De Biasi F, Feruglio GA, et al. Prevalence and determinants of carotid atherosclerosis in a general population. Stroke. 1992;23:1705–1711. doi: 10.1161/01.str.23.12.1705. [DOI] [PubMed] [Google Scholar]
- 6.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103:987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
- 7.Beerenhout CM, Konings CJ, Dammers R, Rensma PL, Hoeks AP, Gladziwa U, et al. Determinants of arterial distensibility in patients with renal failure. Nephron Physiol. 2003;95:43–48. doi: 10.1159/000074329. [DOI] [PubMed] [Google Scholar]
- 8.Shoji T, Emoto M, Shinohara K, Kakiya R, Tsujimoto Y, Kishimoto H, et al. Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol. 2001;12:2117–2124. doi: 10.1681/ASN.V12102117. [DOI] [PubMed] [Google Scholar]
- 9.Groothoff JW, Gruppen MP, Offringa M, de Groot E, Stok W, Bos WJ, et al. Increased arterial stiffness in young adults with end-stage renal disease since childhood. J Am Soc Nephrol. 2002;13:2953–2961. doi: 10.1097/01.asn.0000037677.16961.df. [DOI] [PubMed] [Google Scholar]
- 10.Sollinger D, Mohaupt MG, Wilhelm A, Uehlinger D, Frey FJ, Eisenberger U. Arterial stiffness assessed by digital volume pulse correlates with comorbidity in patients with ESRD. Am J Kidney Dis. 2006;48:456–463. doi: 10.1053/j.ajkd.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JN. Arteries, myocardium, blood pressure and cardiovascular risk: towards a revised definition of hypertension. J Hypertens. 1998;16(12 Pt 2):2117–2124. [PubMed] [Google Scholar]
- 12.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 13.Seals DR. Habitual exercise and the age-associated decline in large artery compliance. Exerc Sport Sci Rev. 2003;31:68–72. doi: 10.1097/00003677-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Davies MR, Hruska KA. Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int. 2001;60:472–479. doi: 10.1046/j.1523-1755.2001.060002472.x. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Curinga G, Giachelli CM. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int. 2004;66:2293–2299. doi: 10.1111/j.1523-1755.2004.66015.x. [DOI] [PubMed] [Google Scholar]
- 16.Takenaka T, Kobayashi K, Suzuki H. Pulse wave velocity as an indicator of arteriosclerosis in hemodialysis patients. Atherosclerosis. 2004;176:405–409. doi: 10.1016/j.atherosclerosis.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Aoun S, Blacher J, Safar ME, Mourad JJ. Diabetes mellitus and renal failure: effects on large artery stiffness. J Hum Hypertens. 2001;15:693–700. doi: 10.1038/sj.jhh.1001253. [DOI] [PubMed] [Google Scholar]


