Abstract
BACKGROUND:
Exhaled nitric oxide (eNO) is one of the airway condensate derived markers, reflecting mainly airway inflammation in asthma and other lung diseases. The changes of eNO levels as pathophysiology of neonatal hypoxemic respiratory failure (HRF) in early postnatal life have not been thoroughly studied. The present study was to establish a method for measuring eNO concentrations in neonates with or without HRF.
METHODS:
Twenty-two newborn infants with HRF and 26 non-NRF controls were included within the first 24 hours of postnatal life. Their eNO levels were detected with a rapid-response chemiluminescence analyzer daily during the first week of their postnatal life, and lung mechanics and gas exchange efficiency were monitored at the same time, such as pulse oxygen saturation (SpO2), inspired fraction of oxygen (FiO2) and other parameters.
RESULTS:
During the first two days of postnatal life, eNO values of HRF neonates were significantly higher than those of the control neonates (day 1, 7.9±3.2 vs. 5.8±1.8 parts per billion [ppb], P<0.05; day 2, 8.8±3.2 vs. 6.0±2.4 ppb, P<0.05), but there were no significant differences in the following days. With SpO2/FiO2 increasing, difference of eNO values between the HRF and non-HRF neonates became narrowed, but there was still a two-fold difference of eNO/[SpO2/(FiO2×100)] on days 5-7.
CONCLUSION:
We established a method for measuring eNO and found difference in neonates with or without HRF, which diminished with prolonged postnatal days, reflecting pathophysiological characteristics of HRF.
KEY WORDS: Neonates, Respiratory failure, Respiratory therapy, Nitric oxide, Respiratory physiology, Nitric oxide synthase
INTRODUCTION
Neonatal hypoxemic respiratory failure (HRF) is one of the most common and serious clinical problems, and a major cause of death in newborn infants, especially in preterm newborns.[1] HRF, usually secondary to meconium aspiration, pneumonia with or without sepsis, respiratory distress syndrome (RDS) and primary pulmonary hypertension of the newborn (PPHN), is the most common reason for routine mechanical ventilation. However, long-term ventilator support may lead to acute and chronic lung injury. At present, more and more studies related to lung injury and repair as well as lung development and function compensation have focused on the relationship of physiological functions and bio-pathology or pathophysiology of endogenous factors, such as nitric oxide (NO). NO is an endothelium derived relaxant factor, generated from L-arginine by nitric oxide synthases (NOS), which are predominantly inhabited in vascular endothelial cells, white blood cells and neurons, among other cells of the body. Exhaled nitric oxide (eNO) reflecting the endogenous pulmonary NO production is one of the airway condensate derived markers, reflecting mainly airway inflammation in asthma and other lung diseases.[2] eNO in humans originates mainly in the paranasal sinuses, then goes into the lung during inspiration and out during expiration, which can be detected by means of real-time NO minotor. We examined eNO in neonates with or without HRF in order to find out changes of airway derived endogenous NO levels as pathophysiology of neonatal HRF in early postnatal life.
METHODS
The protocol of study was approved by the Ethics Committee of Children's Hospital of Fudan University and Hunan Provincial Children's Hospital Review Board. Informed consent, either written or orally agreed, was obtained from the family members of infants enrolled in the study.
Patient selection and protocols
The newborns with HRF were studied in the neonatal intensive care unit (NICU) of Hunan Provincial Children's Hospital from November 2009 to February 2010. Infants were selected for study if they demonstrated characteristic physical and radiographic findings and evidence of respiratory failure requiring intratracheal intubation and mechanical ventilation using intermittent mandatory mode in the first 24 hours of their life. Control infants included preterm and term infants without diffusive lung diseases admitted to the NICU of Children's Hospital of Fudan University from May to June 2010. Infants were excluded from the study if they had major congenital anomalies.
At the enrollment, blood samples in HRF were collected from peripheral artery daily, and analyzed with a Rapidlab 348 analyzer (Bayer Corp., Medfield, MA) for blood gas. At the same time, we detected their eNO and performed respiratory monitoring for 7 days or until extubation, whichever occurred earlier. The control infants breathed spontaneously and were enrolled when they entered the NICU. They had been detected eNO and other respiratory parameters daily for 7 days or until discharge from the NICU.
Measurements of online eNO
eNO was detected according to the standard procedures for measuring eNO recommended by the American Thoracic Society and European Respiratory Society.[3] eNO was measured using a Sievers 280 Chemiluminescence analyzer (Sievers, Boulder, CO. USA). NO gas reacts with ozone, producing energy in the form of light that is proportional to the amount of NO and can be measured using a luminometer. The analyzer measured accurately to 1 part per billion (ppb) and had a 90% response time of 400 ms. Prior to each measurement, the NO analyzer was calibrated with NO-free air (filtered through an NO scavenger) and an NO calibration gas certified at 20 parts per million (ppm) (Shanghai Noventek, Shanghai, China). The analyzer had a linearity between 0-500 ppb at a sampling rate of 100 mL/min.
When making eNO measurements in ventilated infants, expired gas from the side port of the endotracheal tube was directed to the analyzer at a constant flow rate. In the control infants, a facemask was held snugly over the infant's nose and mouth. A 4 l/min bias flow of air from the wall outlet was passed through the facemask via a T-piece, this did not pressurize the circuit and hence did not result in leaks. A valve was put in the distal part of the facemask. A 6 Fr gauge catheter was fed through the leak free valve and the tip positioned so that it could lay at the infant's lips. The catheter was attached to the NO analyzer. Thirty-two measurements per second were made for 1 minute at a time. After a period of 1 minute to allow for the stabilization of eNO levels, the maximum eNO levels were calculated for 1 minute by a breath program (NO Analysis 3.21 BREATH, Sievers). The NO concentrations of the hospital compressed air was measured, and they were subtracted from the measured eNO levels, and mean values were recorded by three repeated measurements.[4]
Respiratory monitoring
When detecting eNO, we also performed respiratory monitoring on lung mechanics and gas exchange efficiency with RSS100-HR Research Pneumotach System (Hans Rudolph, Kansas City, KA, USA), coupled with a non-heated 8311B flow sensor (Flow Range: 0-10 L/min) for neonatal use. An OxiMax N-85 Portable Bedside Capnograph/Pulse Oximeter (Tyco Healthcare, CA, USA) was used to detect pulse oxygen saturation (SpO2) and end-tidal CO2 tension (PetCO2) in expired air. We also monitored the transcutaneous oxygen tension (TcPO2) and carbon dioxide tension (TcPCO2) with a TCM-3 Transcutaneous Monitoring System (Radiometer, Copenhagen, Denmark).
Evaluation of hypoxemic respiratory failure
For diagnosis of HRF, the following parameters were used: oxygen index (OI) = [mean airway pressure × fraction of inspiratory oxygen (FiO2) × 100]/ pre-ductal arterial oxygen tension (PaO2, mmHg); arterial-alveolar ratio for the oxygen tension (a/A) = PaO2/[713×FiO2-Arterial carbon dioxide tension (PaCO2)]; P/F ratio= PaO2/FiO2; and S/F ratio= SpO2/FiO2. Dead space to tidal volume ratio (VD/VT) as an index to estimate intrapulmonary shunting reflecting ventilation-perfusion mismatching was calculated following the Bohr's formula: VD/VT= (PaCO2-PaCO2)/ PaCO2.
Statistical analysis
All statistical analyses were performed by using SPSS 16.0 software (SPSS, Chicago, IL). Continuous variables are presented as means and standard deviation (SD) or median and range or interquartile range (25th to 75th percentile). Wilcoxon's Mann-Whitney U test was used to compare eNO, SpO2/FiO2 and eNO/[SpO2/(FiO2×100)] in the two groups. Pearson's correlation coefficient analysis was used to determine the relationship between eNO levels and indices of HRF.
RESULTS
Perinatal characteristics of participating infants
The clinical characteristics of the two groups of infants are shown in Table 1. There are 22 neonates in the HRF group, including 14 preterm infants and 8 term infants. All preterm infants had RDS, whereas the term infants were diagnosed with RDS (n=3), meconium aspiration syndrome (n=3), wet lung (n=1) and severe pneumonia (n=1). In the control group, there were 15 preterm and 11 term infants. All preterm infants were enrolled to the NICU for special care, whereas in the term infants, there were 4 with swallowing syndrome and 7 small-for-date infants. There were no significant differences in gestational age, birth weight and delivery modes, but there were more male infants in the HRF group, and the 1-minute and 5-minute Apgar scores of HRF were significantly lower than those in the control group.
Table 1.
Perinatal data of neonates with or without HRF
eNO level
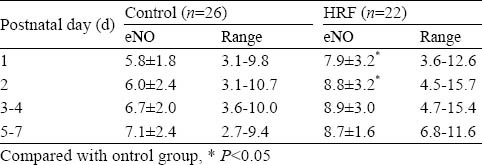
eNO concentrations of neonates with and without HRF are shown in Table 2. During the first two days of postnatal life, eNO values of HRF were higher than those of the control group (day 1, 7.9±3.2 vs. 5.8±1.8 ppb, P<0.05; day 2, 8.8±3.2 vs. 6.0±2.4 ppb, P<0.05), but there were no significant differences of eNO levels in the two groups in the following days of the first week.
Table 2.
NO concentrations in exhaled gas of neonates with or without HRF in the first week of their life (ppb, mean±SD)
Comparison of respiratory parameters
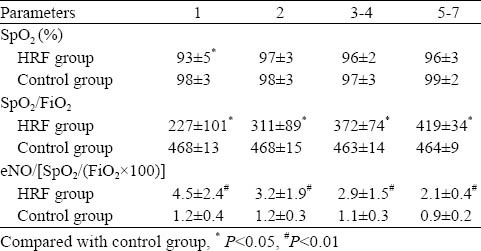
In the first postnatal day, values of SpO2 in HRF were lower than those of the control group, but no significant differences were found in the following days. After correction of SpO2 by FiO2 (SpO2/FiO2) in HRF, these values were significantly lower than those of the control group, but they increased over time consistently with improved oxygenation. Values of TcpO2 in HRF were also lower than those of the control group. There were no significant differences of PaCO2 and TcPCO2 between the two groups at all time points.
We found that values of SpO2/FiO2 were closely correlated with those of PaO2/FiO2 (r=0.719, P<0.01, regression equation: SpO2/FiO2= 160+0.73×PaO2/FiO2). The same was true for TcPO2/FiO2 and PaO2/FiO2 (r=0.562, P<0.01, regression equation: TcPO2/FiO2= 113+0.53×PaO2/FiO2). The correlation between SpO2/FiO2 and PaO2/FiO2 is shown in Figure 1.
Figure 1.
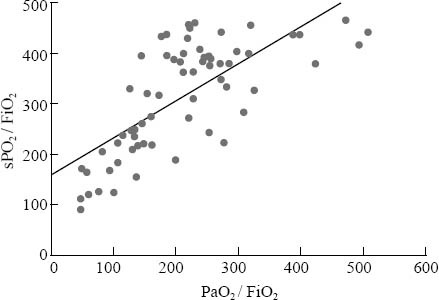
SpO2/FiO2 over PaO2/FiO2 in neonates with HRF. Correlation coefficient r=0.719, P<0.01; regression equation: SpO2/FiO2= 160+0.73×PaO2/FiO2
eNO and respiratory failure
We made the correlation analyses between eNO and several indices of HRF, including OI, a/A and PaO2/FiO2. There was no any relevance between them nor correlationship between eNO and VD/VT. We found that eNO was inversely correlated with SpO2/FiO2 (r= −0.228, P<0.05) (Figure 2). We compared eNO/[SpO2/(FiO2×100)] and found that eNO/[SpO2/(FiO2×100)] in HRF was significantly higher than that in the control group at all time points. There was a four-fold difference of eNO/[SpO2/(FiO2×100)] on day 1, and it was two-fold on days 5-7 (Table 3).
Figure 2.
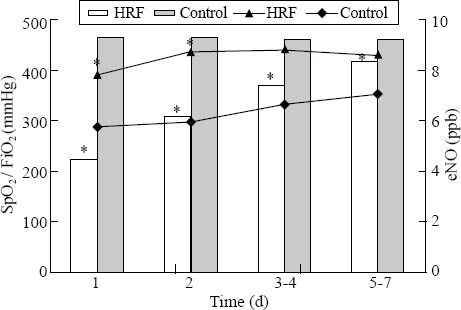
eNO was associated with SpO2 / FiO2 in neonates with or without HRF. The histogram is for SpO2 / FiO2, and the scatter gram is for eNO; *P<0.05 vs. control group.
Table 3.
oxygenation and exhaled nitric oxide ratios (SPO2/FiO2and eNO/[SpO2/(FiO2×100])in neonates with and without HRF in the first week of their life (mean±SD)
DISCUSSION
We established a method for measuring eNO of mechanically ventilated neonatal infants with HRF and non-ventilated control infants. With the non-invasive respiratory monitoring technologies, we found that eNO values of neonates with HRF were higher than those without HRF in the first two days of postnatal life, but the differences diminished with the prolonged postnatal days, reflecting pathophysiological characteristics of HRF.
eNO levels were measured using two sampling techniques, either from the side port of the endotracheal tube or, in non-ventilated infants, through a facemask. NO produced in the nose is higher than that from the lower airway, which is one of the most important factors affecting NO output;[5] however, there is no significant correlation between nasal and lower airway to eNO levels[6]. Hence we deliberately positioned the tips of the catheter so that it laid at the infant's lips. It is possible when sampling from the facemask in neonates without HRF that NO produced in the nose may have influenced the results, even though, we did find that the eNO levels of HRF neonates were still significantly higher than those of the control group.
The timing of measurements relative to the expected clinical course of the disorder is important in the interpretation of results. Our first measurement of eNO in HRF neonates was made after the intratracheal intubation, intermittent positive pressure ventilation, administration of surfactant, and confirmation of appropriate lung expansion by chest radiography. The elevated levels of eNO in neonates with HRF may reflect up-regulation of NOS (type-2) or inducible NOS (iNOS) as an spontaneous regulatory response to decrease NO production.[7] In the ventilated baboons,[8] there is deficiency of neuronal NOS and endothelial NOS, but enhanced iNOS. iNOS can be induced by proinflammatory cytokines, such as tumor necrosis factor-α, interferon δ and interleukin 1α.[9] Exogenous factors also cause transcriptional activation of iNOS, including bacterial toxins, viral infection and hypoxia.[10] In addition, HRF is characterized by significant ventilation-perfusion mismatching and intrapulmonary shunting. This pathophysiological mechanism may involve stimulation for NO production based on up-regulation of iNOS and/or eNOS in the lungs.[11]
Mechanical ventilation may also affect the NO output. Airway epithelium could produce more NO as a reaction to mechanical stretching and shear forces across the airways in the lung disease. It has been proposed that shear stress due to inappropriately high tidal volumes may increase NO production. The application of positive end-expiratory pressure has shown to increase eNO in animals,[12] but airway pressure in humans does not affect eNO plateau levels according to other reports.[3]
With regard to HRF severity and response to the early intervention, P/F ratio is considered the most stable parameter. Concerns about anemia, excessive blood draws, and a movement to minimally invasive approaches have led to fewer arterial blood gas measurements in critically ill patients, especially in neonates. Recent studies showed that SpO2/FiO2 ratios correlate with P/F ratios, and proposed that S/F can be a substitute of P/F to diagnose and monitor adult and pediatric patients with acute lung injury or acute respiratory distress syndrome;[13,14] however, the hypothesis has not been verified in studies related to neonatal HRF. We found that there is a great correlation between P/F and S/F ratios in neonatal HRF, as described by the regression equation. We investigated the relationship between eNO and SpO2/FiO2, and found that eNO in neonatal HRF decreased with oxygenation improvement, close to that in the control group. After correction of eNO by SpO2/FiO2, we found a four-fold difference of eNO/[SpO2/(FiO2×100)] on day 1, and still a two-fold difference on days 5-7, suggesting such correction is warranted for better interpretation of its clinical relevance.
Colnaghi et al[15] detected the eNO value of normal neonates in the first two days after birth, and found that there was an eNO peak in term neonates while there was no eNO peak in preterm infants, suggesting that NO participated in the postnatal respiratory adaptation. Additionally, the eNO values at 24 and 48 hours in their study were similar to our measurements at the same time points. Olsen et al[16] found that neonates with RDS had higher eNO at first 24 hours of postnatal life compared to the control group, and their eNO decreased as gas exchange improved, which is similar to our finding.
In conclusion, with the use of non-invasive eNO detection technology in ventilated neonates and spontaneously breathing controls, we found that there were differences of eNO in neonates with and without HRF, which diminished as oxygenation improved, reflecting pathophysiological characteristics of HRF. It should be considered as an alternative way in diagnosis and monitoring of neonatal HRF. Should more investigations be accomplished in this special population.
ACKNOWLEDGEMENTS
Assistance by staffs from both Hunan Provincial Children's Hospital and Children's Hospital of Fudan University is greatly appreciated. We thank Tyco Health (China) for providing OxiMax N-85 Portable Bedside Capnograph/Pulse Oximeter. We also owe thanks to Dr. Richard Hutte (GE, Boulder, CO, USA) and Radiometer (China) for their advices and technical supports.
Footnotes
Funding: This work was supported by grants from the National Natural Science Foundation of China (30872801), the Excellence in Doctorate Research Program by Ministry of Education (20090071110061), and Shanghai Rising-Star Program (09QA1400700).
Ethical approval: The protocol of investigation was approved by the Ethics Committee of Children's Hospital of Fudan University and Hunan Provincial Children's Hospital Review Board.
Conflicts of interest: The authors have no competing interests.
Contributors: Sun B designed the study, edited the manuscript with major interpretation of the clinical significance. Liu LJ performed the study, collected and analyzed the data, and drafted the manuscript. Gao XR did clinical experiment on HRF, and interpreted clinical data. Wu PP participated in the experimental part of most clinical measurements. Qian LL involved in the study design and management. Chen C arranged clinical experiment on the controls, and interpreted clinical data.
REFERENCES
- 1.Qian L, Liu C, Zhuang W, Guo Y, Chen H, Wang S, et al. Neonatal respiratory failure: a 12-month clinical epidemiologic study from 2004 to 2005 in China. Pediatrics. 2008;121:e1115–e1124. doi: 10.1542/peds.2006-2426. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ, Dweik RA, Gelb AF, Gibson PG, George SC, Grasemann H, et al. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2010;138:682–692. doi: 10.1378/chest.09-2090. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 4.May C, Williams O, Milner AD, Peacock J, Rafferty GF, Hannam S, et al. Relation of exhaled nitric oxide levels to development of bronchopulmonary dysplasia. Arch Dis Child Fetal Neonatal Ed. 2009;94:F205–209. doi: 10.1136/adc.2008.146589. [DOI] [PubMed] [Google Scholar]
- 5.Thébaud B, Arnal JF, Mercier JC, Dinh-Xuan AT. Inhaled and exhaled nitric oxide. Cell Mol Life Sci. 1999;55:1103–1112. doi: 10.1007/s000180050360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams O, Rafferty GF, Hannam S, Milner AD. Greenough Nasal and lower airway levels of nitric oxide in prematurely born infants. Early Hum Dev. 2003;72:67–73. doi: 10.1016/s0378-3782(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 7.Dweik RA, Laskowski D, Abu-Soud HM, Kaneko F, Hutte R, Stuehr DJ, et al. Nitric oxide synthesis in the lung. Regulation by oxygen through a kinetic mechanism. J Clin Invest. 1998;101:660–666. doi: 10.1172/JCI1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afshar S, Gibson LL, Yuhanna IS, Sherman TS, Kerecman JD, Grubb PH, et al. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2003;284:L749–758. doi: 10.1152/ajplung.00334.2002. [DOI] [PubMed] [Google Scholar]
- 9.Morris SM, Jr, Billiar TR. New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol. 1994;266:E829–839. doi: 10.1152/ajpendo.1994.266.6.E829. [DOI] [PubMed] [Google Scholar]
- 10.Ricciardolo FLM, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84:731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 11.Pitt BR, St Croix CM. Complex regulation of iNOS in lung. Am J Respir Cell Mol Biol. 2002;26:6–9. doi: 10.1165/ajrcmb.26.1.f224. [DOI] [PubMed] [Google Scholar]
- 12.Persson MG, Lönnqvist PA, Gustafsson LE. Positive end-expiratory pressure ventilation elicits increases in endogenously formed nitric oxide as detected in air exhaled by rabbits. Anesthesiology. 1995;82:969–974. doi: 10.1097/00000542-199504000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB, et al. Comparison of the SpO2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 14.Marraro GA. SpO2/FiO2 vs PaO2/FiO2: are we ready to establish less invasive indicators for early diagnosis of acute respiratory distress syndrome? Pediatr Crit Care Med. 2010;11:143–144. doi: 10.1097/PCC.0b013e3181b80f1e. [DOI] [PubMed] [Google Scholar]
- 15.Colnaghi M, Condò V, Pugni L, Fumagalli M, Mosca F. Endogenous nitric oxide production in the airways of preterm and term infants. Biol Neonate. 2003;83:113–116. doi: 10.1159/000067964. [DOI] [PubMed] [Google Scholar]
- 16.Olsen SL, Clark PL, Thibeault DW, Norberg M, Truog WE. Exhaled nitric oxide and tracheal endothelin-1 in preterm infants with and without RDS. Pediatr Pulmonol. 2003;36:421–426. doi: 10.1002/ppul.10371. [DOI] [PubMed] [Google Scholar]


