Abstract
BACKGROUND:
Edaravone can alleviate brain injury and improve neurological functions and symptoms. This study aimed to investigate the effect of edaravone on the p38Mitogen-activated protein kinases/Caspase-3 (p38MAPK /Caspase-3) pathway after diffuse brain injury (DBI) in rats.
METHODS:
DBI models were established according to the description of Marmarou's method. A total of 250 rats were divided (random number) into four groups: control group (CG, n=45), model group (MG, n=77), low-dose edaravone group (n=67, dosage 5 mg/kg) and high-dose edaravone group (n=61, dosage 10 mg/kg). After 1, 6, 24, 48, and 72 hours after injury, brain tissues were collected. The changes of neuron morphous in the hippocampal region were observed through Nissl staining. The expression levels of phosphorylated p38MAPK and caspase-3 were detected by immunohistochemistry and Western blotting respectively. Learning and memory function were tested with Morris water maze from the 3rd to 7th day after injury.
RESULTS:
Some neurons had histopathologic changes of necrosis and apoptosis in the model group compared with the control group. The phosphorylated p38MAPK expressions increased at 1, 6, 4, and 48 hours (P<0.05), but no significant difference was observed at 72 hours (0.54±0.19 vs. 0.40±0.14, P>0.05). Caspase-3 expressions increased at 6, 24, 48, and 72 hours respectively (P<0.05), but there was no significant difference at 1 hour (0.59±0.29 vs. 0.40±0.17, P>0.05). From the 3rd to 6th day during the Morris water maze test, the latency to find the platform was significantly prolonged (P<0.05) and times of rats crossing the platform was decreased on the 7th day (2.28±1.18 vs. 8.20±1.52, P<0.05). The phosphorylated p38MAPK expressions decreased at 6, 24 and 48 hours respectively in the low dose edaravone group compared with the model group (P<0.05), whereas no significant difference was seen at 1 hour (1.66±0.80 vs. 1.85±0.86, P>0.05). Caspase-3 expression decreased at 6, 24, 48, and 72 hours (P<0.05). The latency to find the platform was significantly shortened (P<0.05), and times of rats crossing the platform increased (4.17±1.15 vs. 2.28±1.18, P<0.05). The above mentioned parameters changed more significantly in the high-dose edaravone group than in the low-dose edaravone group.
CONCLUSION:
Edaravone can alleviate brain tissue damage after DBI, inhibit p38MAP signal activation after early injury, reduce the expression of caspase-3, and promote the recovery of neurological function in the late period.
KEY WORDS: Diffuse brain injury, Mitogen-activated protein kinases, Caspase-3, Learning-memory, Edaravone
INTRODUCTION
Mitogen-activated protein kinases (MAPKs) are serine/threonine-specific protein kinases that transfer extracellular stimuli to membrane receptor and regulate various cellular activities such as gene expression, mitosis, differentiation, proliferation, and cell survival/apoptosis. The MAPK super-family is composed of three major sets of kinases: extracellular-receptor kinases (ERKs), c-Jun N-terminal kinases (C-Jun) and p38 MAPK kinases. After being confirmed that MAPKs signal pathway activation existed in the brain ischemia model in 1993, many studies have reported that the MAPKs signal pathway plays a crucial role in the pathological process of central nervous system diseases. It has been found that the activated p38 MAPK, which participates extensively in the process of hypoxic ischemic brain damage by cascade reaction, can regulate down-stream genes or protein transcription and induce apoptosis or inflammatory reactions.[1,2] Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), as a new type of neuroprotective agents, can markedly reduce brain injury and improve neurological functions and symptoms.[3,4] Pharmacological studies have confirmed that edaravone has the effects of scavenging oxygen free radicals, anti-lipid peroxidation and anti-apoptosis.[5,6] Through establishing a rat model of diffuse brain injury, this study aimed to determine whether edaravone can decrease neurons damage and promote neurological functional recovery.
METHODS
Experimental animals
Two hundreds and fifty healthy male SD rats weighing (30±20) g were provided by Vital River Laboratories Animal Technology Co., Ltd (clean-class, certification of approval: SCXK, Beijing, 2002-003).
Reagents and instruments
Edaravone injection was purchased from Shandong Zhongke Pharmaceutical Company. Phosphorylated P38MAPK murine monoclonal antibodies were purchased from Cell Signaling Technology, Inc, USA; caspase-3 elisa was gained from Zhongshan Goldenbridge Biotechnology Co.,Ltd; and image acquisition and analysis technology were obtained from Bio-Rad Laboratories, Ltd.
Animal grouping and molding
The 250 rats were randomly divided into 4 groups: control group (CG, n=45), model group (MG, n=77), low-dose edaravone group (n=67, dosage 5 mg/kg) and high-dose edaravone group (n=61, dosage 10 mg/kg). All groups were subdivided into 5 subgroups: 1 hour, 6 hours, 24 hours, 48 hours and 72 hours after injury respectively. Five rats from each subgroup were subjected to Morris water maze test.
Diffuse brain injury (DBI) models were established according to Marmarou[7]: under etherization condition (the anaesthesia time 70-150 seconds), copper rod (diameter 18 mm, weight 450 g) fell in a vertical way and struck in a stainless gasket, which was located between the lambdoid suture and sagittal suture of rats. Rats in the control group were anesthetized but not injured. Rats in the edaravone groups were injected with edaravone immediately after injury via tail vein injection (low-dose 5 mg/kg; high-dose 10 mg/kg; q24h, d1-d3). During the modeling process, 32, 22 and 16 rats died in the model group, low and high dose groups, respectively.
Toluidine blue staining and immunochemical staining of phosphorylated p38MAPK and caspase-3
Twenty rats from each group were anesthetized with 0.4% pentobarbital sodium at 1, 6, 24, 48 and 72 hours. The chest of the rats was open, and the heart was exposed and perfused with 4% polymerisatum. Finally the rats were killed. At 1-6 mm behind the optic chiasma, coronal sections were cut, and brain tissue of the middle part was taken out, fixed with 4% paraformaldehye, embedded in paraffin, and sectioned at 5 μm. Slices were deparaffinized to hydrate and stained with toluidine blue.
Other slices were prepared, deparaffinized to hydrate, and repaired with citrate microwave. Then the slices were dripped rat monoclonal antibody phosphorylated p38MAPK (1:150) and rabbit polyclonal antibodies caspase-3 (1:150), and kept in a wet box at 4°C overnight. IgG antibody 2HRP polymer (pv two step method tests the kid, Beijing Zhongshan Goldenbridge Biotechnology Co., Ltd) was put in an incubator for 30 minutes at 37°C, stained with DAB, restained with brazilwood, dehydrated, vitrificated, and deposited. The slices were observed under a microscope.
Determination of phosphorylated p38MAPK and caspase-3 using Western blotting
Twenty rats were selected from each group and killed at 1, 6, 24, 48 and 72 hours for the removal of the hippocampus. The hippocampus was homogenized on ice after adding with denaturing solution, and centrifugated at 3000 r/min and 4°C for 5 minutes. The supernatant was collected, and the protein was quantitated using the coomassie brilliant blue method. The specimens were prepared and transferred to the membrane, which was added with antibodies (phosphorylated p38MAPK, 1:1000; caspase-3, 1:1500; β-actin, 1:2000) and incubated for 2-3 hours at room temperature. The membrane was washed and incubated with secondary antibody (phosphorylated p38MAPK, 1:1000; caspase-3, 1:1500; β-actin,1:2000), stained with ECL. The Bio-Rad system was used to measure the absorption, and the protein level was expressed as the ratio of average absorption to aim strips and internal control β-actin, which were analyzed semiquantitatively.
Learning and memory ability tests
Learning and memory ability was tested twice using the Morris water maze method at 3, 4, 5, 6 and 7 days after injury, one in the morning and the other in the afternoon according to Smith. [8] The latency for seeking the platform was recorded at 3, 4, 5 and 6 days; the platform was removed, the times for rats crossing the original platform was recorded, and the mean value was calculated.
Statistical analysis
Data were expressed as mean ±SD. ANOVA with Bonferroni's post hoc test was used to determine statistical significance as appropriate. A P value less than 0.05 was considered statistically significant.
RESULTS
Results of toluidine blue staining
In the control group, hippocampal neurons were tightly arranged, neurons were large with round nuclei and clear nucleolus, hypochromatic cytoplasm was slightly stained and uniformed, and Nissl bodies were abundant. In the model group, changes of cellular morphology in the hippocampus were seen 1 hour after injury, and cells were disorderly arranged with the extension of time. Cell body was contracted to polygon, partial cytoplasm was autolyzed, and Nissl bodies became smaller. The morphological changes of injured hippocampal neurons were alleviated in the edaravone group compared with the model group at 1, 6, 24, 48 and 72 hours, and normal neurons accounted for the majority. The above changes were more obvious in the high dose edaravone group than in the low dose edaravone group (Figure 1).
Figure 1.

Morphological changes of neurons in the hippocampal region of the rat at 72 hours after injury (Nissl staining ×400). A: control group; B: model group; C and D: low and high dose edaravone groups.
Results of phosphorylated p38MAPK and caspase-3 detection
Immunohistochemical staining showed that phosphorylated p38MAPK positive expression was mainly located in the nucleolus, and less in the cytoplasm, but caspase-3 positive expression was mainly located in cytoplasm. Fine brown particles were seen in the cytoplasm of positive cells. Positive cells in both phosphorylated 38MAPK and caspase-3 were mainly distributed in CA1and CA2 of the hippocampus, followed by CA1 and dentate gyrus.
Results of western blotting
Compared to the control group, phosphorylated p38MAPK expression in the model group was increased significantly at 1, 6, 24, and 48 hours after injury (P <0.05), but there was no significant difference between the two groups at 72 hours (P>0.05). Compared to the model group, phosphorylated p38MAPK expression in the edaravone groups was significantly decreased at 6, 24, and 48 hours after injury (P<0.05), whereas no significant difference was seen at 1 and 72 hours (P>0.05) (Table 1, Figure 2). Compared to the control group, caspase-3 expression in the model group increased significantly at 6, 24 and 48 hours after injury (P<0.05), but there was no significant difference between the two groups at 1 hour (P>0.05). Compared to the model group, caspase-3 expression in the edaravone groups decreased significantly at 6, 24 and 48 hours after injury (P<0.05), but there was no significant difference between them at 1 and 72 hours (P>0.05) (Table 2, Figure 3).
Table 1.
Comparison of phosphorylated P38MAPK protein in the hippocampal region of the rats in each group (mean ±SD)

Figure 2.
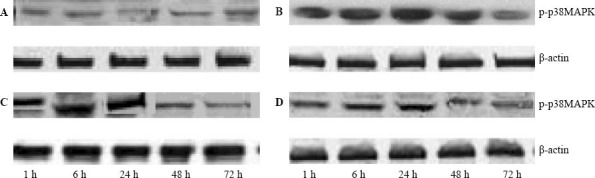
Phosphorylated p38MAPK expression in the hippocampal region of the rat by Western blotting. A: control group; B: model group; C and D: low and high dose edaravone groups.
Table 2.
Comparison of caspase-3 protein in the hippocampal region of the rats in each group (mean±SD)

Figure 3.
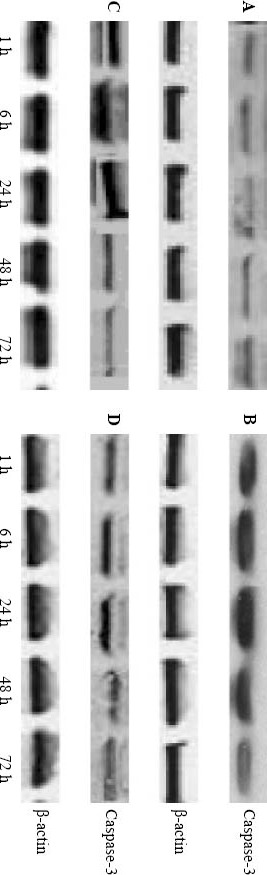
Casepase-3 espression in the hippocampal region of the rat by Western blotting A:control group;B: model group; C and D: low and high dose edaravone groups.
Results of learning and memory ability tests
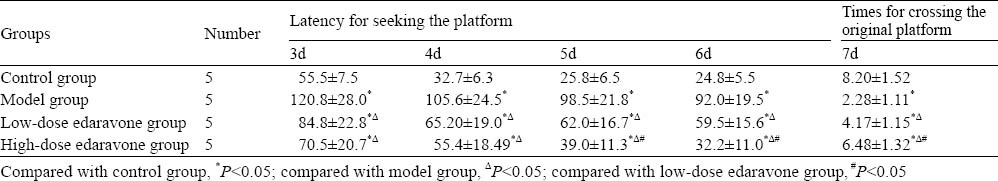
Compared to the control group, latency for seeking the platform in the model group was obviously prolonged at 3, 4, 5 and 6 days (P<0.05), and times of rats crossing the original platform markedly decreased at 7 days (P<0.05). Compared to the model group, latency for seeking the platform in the model group was evidently shortened (P<0.05), and times of rats crossing the original platform decreased obviously (P<0.05) (Table 3).
Table 3.
Results of Morris water maze in each group (mean±SD)
DISCUSSION
Diffuse brain injury (DBI), which can cause extensive damage to different parts of the brain, has already become the main cause of death in the population under 45 years old.[9] DBI may be due to the toxic effect of glutamic acid, calcium overload and inflammatory reaction, etc. Edaravone (3-methyl-1-phenyl-pyrazolin-5-one), a novel free radical scavenger, has been used clinically in the treatment of adult brain stroke to protect neuronal cells from hypoxic brain injury.[9] Edaravone provides free radicals with electrons, scavenges hydroxyl radicals, and limits hydroxyl radical-dependent lipid peroxidation,[10] thereby inhibiting radicals' hazardous activities.[11] Edaravone also affects the expression of cell-regulated genes, thus resulting in the improvement of antioxidatve ability of brain tissue, reduction of neurons apoptosis, and increase of neuroprotective function.[11,12] In this study we found that the damage of neuron structure and the study/memory ability was greatly alleviated in the high/low dose ddaravone groups compared with the model group. This finding indicates that edaravone has a good therapeutic effect on DBI.
The P38MAPK signaling pathway is a stress reaction of cells to its surroundings, which regulates the physiological and pathological processes such as differentiation, development, survival and death of cells. After cerebral ischemic injury, the p38MAPK signaling pathway plays an important role in the pathological process of inflammatory reaction, oxygen radical injury, and apoptosis of nerve cells. Pretreatment of SB202190 (P38MAPK inhibitor) brain ischemia can alleviate brain injury and promote the recovery of neurological function in cerebral ischemia rats.[13-16] This study revealed that high or low-dose edaravone can lower the level of phosphorylated p38MAPK after injury, and this effect was dose-dependent. This finding indicates that edaravone can inhibit the activation of the p38MAPK pathway after injury.
Caspase-3 is a key protease during apoptosis. The activated caspase-3 can enzymolyse specific substrates such as DNA-dependent protein kinase and sterol regulatory element binding protein, and induce apoptosis by changing its structure or affect specific signaling molecules. Caspase-3 expression and apoptosis of neural cells can be detected in the early period of traumatic brain injury, and the expression level of caspase-3 is related to the extent of brain injury.[17] p38MAPK downstream signal transduction includes the activation of caspase-3 in the process of cerebral ischemia[18] and the inhibition of P38MAPK phosphorylation can block the expression of caspase-3 and reduce neuronal apoptosis in model of neurodegenerative diseases.[19] Our study found that the distribution of P38MAPK was almost the same as that of caspase-3. After treatment with edaravone, the expression of phosphorylated P38MAPK and caspase-3 declined dose-dependently. This indicates that the protective effect of edaravone on the brain is associated with the inhibition of the neuronal apoptosis pathway of P38MAPK and caspase-3.
In conclusion, edaravone can alleviate brain damage after DBI, inhibit p38MAP signal activation after early injury, reduce the expression of caspase-3, and promote the recovery of neurological function in the late period.
Footnotes
Funding: The study was supported by a grant from Hebei Provincial Commission of Science and Technology (2009276103D-7).
Ethical approval: Not needed.
Conflicts of interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributors: Li JM proposed and wrote the study. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Zheng GY, Chen XC, Du J, Liu CY, Fang F, Zhang J, et al. Inhibitory action of propyl gallate on the activation of SAPK/ JNK a p38MAPK induced by cerebral ischemia-reperfusion in rats. Yao Xue Xue Bao. 2006;41:548–554. [PubMed] [Google Scholar]
- 2.Mayor F, Jr, Jurado-Pueyo M, Campos PM, Murga C. Interfering with MAP kinase docking interactions: implications and perspective for the p38 route. Cell Cycle. 2007;6:528–533. doi: 10.4161/cc.6.5.3920. [DOI] [PubMed] [Google Scholar]
- 3.Gao Y, Ding XS, Xu S, Wang W, Zuo QL, Kuai F. Neuroprotective effects of edaravone on early brain injury in rats after subarachnoid hemorrhage. Chin Med J (Engl) 2009;122:1935–1940. [PubMed] [Google Scholar]
- 4.Imai K, Mori T, Izumoto H, Takabatake N, Kunieda T, Watanabe M. Hyperbaric oxygen combined with intravenous edaravone for treatment of acute embolic stroke: a pilot clinical trial. Neurol Med Chir (Tokyo) 2006;46:373–378. doi: 10.2176/nmc.46.373. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Ding XS, Xu S, Wang W, Zuo QL, Kuai F. Neuroprotective effects of edaravone on early brain injury in rats after subarachnoid hemorrhage. Chin Med J (Engl) 2009;122:1935–1940. [PubMed] [Google Scholar]
- 6.Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J Neurosurg. 1994;80:291–300. doi: 10.3171/jns.1994.80.2.0291. [DOI] [PubMed] [Google Scholar]
- 7.Smith DH, Okiyama K, Thomas MJ, Claussen B, McIntosh TK. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J Neurotrauma. 1991;8:259–269. doi: 10.1089/neu.1991.8.259. [DOI] [PubMed] [Google Scholar]
- 8.Edaravone Acute Infarction Study Group. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15:222–229. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe T, Yuki S, Egawa M, Nishi H. Protective effects of MCI-186 on cerebral ischemia: possible involvement of free radical scavenging and antioxidant actions. J Pharmacol Exp Ther. 1994;268:1597–1604. [PubMed] [Google Scholar]
- 10.Nakamoto N, Tada S, Kameyama K, Kitamura K, Kurita S, Saito Y, et al. A free radical scavenger, edaravone, attenuates steatosis and cell death via reducing inflammatory cytokine production in rat acute liver injury. Free Radic Res. 2003;37:849–859. doi: 10.1080/1071576031000136586. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence MC, Jivan A, Shao C, Duan L, Goad D, Zaganjor E, et al. The roles of MAPKs in disease. Cell Res. 2008;18:436–442. doi: 10.1038/cr.2008.37. [DOI] [PubMed] [Google Scholar]
- 12.Chaparro-Huerta V, Rivera-Cervantes MC, Flores-Soto ME, Gómez-Pinedo U, Beas-Zárate C. Proinflammatory cytokines and apoptosis following glutamate-induced excitotoxicity mediated by p38 MAPK in the hippocampus of neonatal rats. J Neuroimmunol. 2005;165:53–62. doi: 10.1016/j.jneuroim.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Ipaktchi K, Mattar A, Niederbichler AD, Hoesel LM, Hemmila MR, Su GL, et al. Topical p38MAPK inhibition reduces dermal inflammation and epithelial apoptosis in burn wounds. Shock. 2006;26:201–209. doi: 10.1097/01.shk.0000225739.13796.f2. [DOI] [PubMed] [Google Scholar]
- 14.Dreixler JC, Barone FC, Shaikh AR, Du E, Roth S. Mitogen-activated protein kinase p38alpha and retinal ischemic preconditioning. Exp Eye Res. 2009;89:782–790. doi: 10.1016/j.exer.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ou ME, Tang LW, Li H. Effect and signal transduction mechanism of active components of Buyang Huanwu Decoction on vascular adhesion molecule expression. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:430–434. [PubMed] [Google Scholar]
- 16.Léveillé F, Papadia S, Fricker M, Bell KF, Soriano FX, Martel MA, et al. Suppression of the intrinsic apoptosis pathway by synaptic activity. J Neurosci. 2010;30:2623–235. doi: 10.1523/JNEUROSCI.5115-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang NF, Xu J, Xia Q, Gao Q, Shi XX, Zhou ZL, et al. Mechanism of cardioprotection induced by noninvasive limb ischemic preconditioning. Zhonghua Yi Xue Za Zhi. 2009;89:1999–2002. [PubMed] [Google Scholar]
- 18.Rácz B, Gallyas F, Jr, Kiss P, Tamás A, Lubics A, Lengvári I, et al. Effects of pituitary adenylate cyclase activating polypeptide (PACAP) on the PKA-Bad-14-3-3 signaling pathway in glutamate-induced retinal injury in neonatal rats. Neurotox Res. 2007;12:95–104. doi: 10.1007/BF03033918. [DOI] [PubMed] [Google Scholar]
- 19.Lee EJ, Park HG, Kang HS. Sodium salicylate induces apoptosis in HCT116 colorectal cancer cells through activation of p38MAPK. Int J Oncol. 2003;23:503–508. [PubMed] [Google Scholar]


