Abstract
BACKGROUND:
Glutamine (Gln) supplementation is known to decrease oxidative stress and inflammatory response, enhance resistance to infectious pathogens, shorten hospital stay, and decrease medical costs of patients. This study was undertaken to evaluate the relationship between the effect of early parenteral glutamine (Gln) supplement on acute liver injury (ALI) and heat shock protein 70 (HSP-70) expression in critical patients.
METHODS:
Forty-four patients who had been admitted to the emergency intensive care unit (EICU) of Nanjing First Hospital Affiliated to Nanjing Medical University were randomly divided into a control group (n=22) and a Gln group (n=22). The patients of the two groups received enteral and parenteral nutrition. In addition, parenteral Gln 0.4 g/kg per day was given for 7 days in the Gln group. Serum HSP-70 and Gln were measured at admission and at 7 days after admission. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBiL), serum levels of HSP-70 and Gln, mechanical ventilation (MV) time, ICU stay, peripheral blood of TNF-α, IL-6, CD3, CD4 and CD4/CD8 levels were also measured in the two groups.
RESULTS:
In the Gln group, the levels of serum HSP-70 and Gln were significantly higher after Gln treatment than those before the treatment (P<0.01). HSP-70 level was positively correlated with the Gln level in the Gln group after administration of parenteral Gln (P<0.01). The levels of serum ALT, AST, TBiL and TNF-α, IL-6 were lower in the Gln group than in the non-Gln group (P<0.01). MV time and ICU stay were significantly different between the two groups (P<0.05). The levels of CD3, CD4 and CD4/CD8 were significantly higher in the Gln group than in the control group after treatment (P<0.05).
CONCLUSION:
Parenteral Gln significantly increases the level of serum HSP70 in critically ill patients. The enhanced expression of HSP70 is correlated with improved outcomes of Gln-treated patients with acute liver injury.
KEY WORDS: Glutamine, Heat shock protein, Critically ill patients, Acute liver injury
INTRODUCTION
Systemic inflammatory response syndrome (SIRS) associated with acute liver injury is common in critically ill patients.[1] Excessive hepatic oxidative stress and increased inflammatory response may result in damage of hepatocytes. Proinflammatory cytokines like tumor necrosis factor (TNF)-α and interleukin (IL)-6 are important in the development of multiple organ dysfunction syndrome (MODS).[2] Protecting cells and tissues from injury while preserving metabolic function would presumably be beneficial to the prevention and treatment of liver injury after sepsis or other injuries.[2, 3] Glutamine (Gln) supplementation is known to decrease oxidative stress and inflammatory response, enhance resistance to infectious pathogens, shorten hospital stay, and decrease medical costs of patients.[4-6] The extent and duration of Gln depletion are proportional to the severity of patient illness.[7,8] Gln deficiency at admission to the intensive care unit is thought to be a strong predictor for hospital mortality.[9] Recently, the regulatory potential of Gln for the expression of heat shock proteins (HSPs) has been recognized.[10] HSPs are self-protective proteins that maintain cell homeostasis against protein damage and organ injury, and improve the survival of experimental models of sepsis and critical illness by decreasing neutrophil infiltration and production of cytokines and protecting cells from injury and death. These proteins are induced by a wide variety of stressors and have broad cytoprotective functions.[11-13] The 70 kDa family of HSP (HSP70), in particular, plays a vital role in cellular protection. Laboratory and clinical data of trials have shown a relationship between Gln-mediated protection and enhanced expression of heat shock protein 70 (HSP70).[14-21] However, the mechanism of this benefit has not yet been elucidated. Thus the relationship between early parenteral Gln administration and serum HSP-70 expression is not clear in critical patients with acute liver injury (ALI). The present study was undertaken to determine the effect of early parenteral Gln supplement on heat shock protein 70 (HSP-70) expression and clinical outcomes in critical patients with ALI.
METHODS
Patients
The patients included in this study were those who had been admitted to the emergency intensive care unit (EICU) of the hospital from November 2006 to September 2010. Inclusion criteria: age 18-70 years; APACHE II scores ≥15; acute liver injury diagnosed if total bilirubin level was 51 μmol/L with a two-fold increase in level of aspartate aminotransferase (AST) or alanine aminotransferase (ALT). Exclusion criteria: metabolic disease; women in pregnancy and lactation; less than 7 days of hospitalization; confounding diseases including acute viral hepatitis (hepatitis A, B, C virus, cytomegalovirus, herpes simplex virus, and Epstein-Barr virus) and other forms of liver diseases including autoimmune, metabolic liver diseases and biliary obstruction.
A total of 44 patients, 29 male and 15 female, were enrolled in this study. They were randomly divided into the two groups: a conventional treatment group (control group) and a Gln treatment group (Gln group). The study was approved by the Institutional Ethics Committee of Nanjing First Hospital and informed consent was obtained from all participants.
Nutrition
The patients of the two groups received iso-nitrogenous and isocaloric nutrition. After the recovery of intestinal function, the patients were given enteral nutrition, Peptisorb, (Nutricia S.R.L. Madrid), and increased dose was dependent on their endurance with daily calories of 25-30 kcal/kg. In the Gln group, patients were given additionally with a venous injection of Gln 0.4 g/kg per day (Ala-Gln, Dipeptiven 20%, Fresenius Kabi Co., Austria) for seven days. Meanwhile the balance between water and electrolyte was regulated, and hyperglycemia and hypoalbuminemia were treated when necessary.
Assay analysis
Venous blood (15 mL) was drawn from each patient at admission and at 7 days after treatment. Five mL blood sample was centrifuged at 1500 r/min for 10 minutes, and the serum was frozen at -80°C for later HSP-70 measurement. Serum HSP-70 was measured by Enzyme-linked Immunosorbent Assay (ELISA) following the manufacturer's instructions (EKS700, Stressgen Victoria BC, Canada, bought from Shenzhen Jingmei Biology Reagent Corporation). The other 10 mL of blood sample was put in a heparin tube, and Gln was measured by high performance liquid chromatograpy (HPLC). The levels of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBiL) were detected by an automatic biochemistry analyser. The concentrations of TNF-α, IL-6 were measured by ELISA. The concentrations of Gln and HSP-70, the rate of T-cell subsets, the duration of mechanical ventilation, and the ICU stay of the two groups were compared before and after the treatment. Meanwhile the correlation between the concentrations of Gln and HSP-70 was analyzed after the treatment.
Weaning criteria
The ventilator used was Evita 2 (Drager, Lübeck, Germany). The weaning from mechanical ventilation was attempted according to the following criteria: mechanical ventilation resolved or at least improved; body temperature below 38.5°C; hemoglobin level equal to or higher than 80 g/L; and none or a minimal dose of vasoactive or sedative drugs administered; and others including PaO2≥ 60 mmHg, SaO2≥ 90%, FiO2≤0.4, and positive end-expiratory pressure (PEEP)≤5 cmH2O. Spontaneous breath test (SBT) was performed by a 2-hour T-piece.
Statistical analysis
Continuous variables were expressed as the median (range) unless mentioned otherwise. The differences between the two groups were tested using the two-sided Fisher's exact test for categorical variables and the Wilcoxon's rank-sum test to compare variables in the cohort. P<0.05 was considered statistically significant. Statistical analysis was made using SPSS 15.0 software for Windows (SPSS Inc., Chicago, USA).
RESULTS
General data
There was no significant difference in gender, age, APACHE II score, ALT/AST, TBiL and classification of diseases between the control group and Gln group (Table 1).
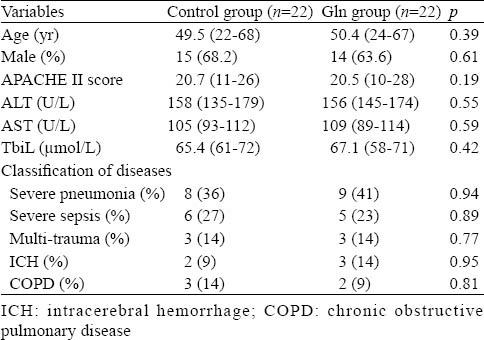
Table 1.
Baseline characteristics about patients in the two groups at admission
Changes of Gln level
Before the treatment, no significant difference was seen between the two groups (P=0.739). The level of Gln increased rapidly in the Gln group after the treatment,, and it was significantly different from that before the treatment (P=0.0003). In the control group, there was no significant change in the level of Gln before and after the treatment (P=0.053). A significant difference was observed between the two groups after the treatment (P=0.0001) (Table 2).
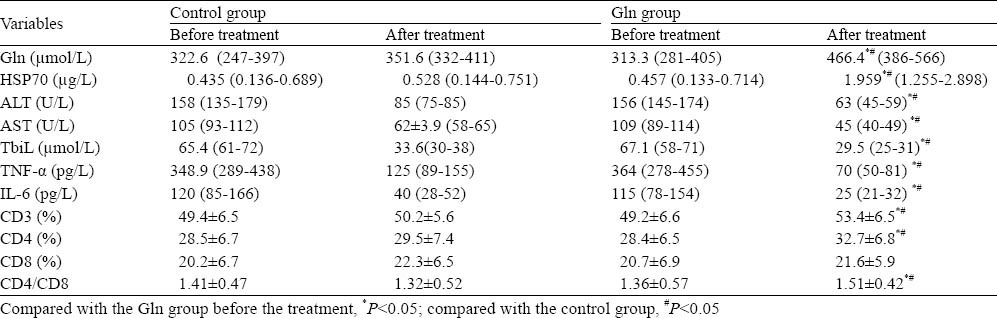
Table 2.
Change of patients' parameters between the two groups before and after the treatment
Changes of HSP70 level
Before the treatment, there was no significant difference between the two groups (P=0.853). After the treatment, the level of HSP70 increased markedly in the Gln group and it was significantly different from that before the treatment (P=0.001). The level of HSP 70 was significantly higher in the Gln group than in the control group after the treatment (P=0.002). In the control group, however, no significant change was seen before and after the treatment (P=0.392) (Table 2).
Correlation between Gln and HSP70 levels after the treatment
In the Gln group, the level of HSP70 was positively correlated with the level of glutamine after use of enriched parenteral glutamine (r=0.65, P=0.001) (Figure 1). No obvious relation was seen in the control group after conventional treatment (r=0.15, P=0.505) (Figure 2).
Figure 1.
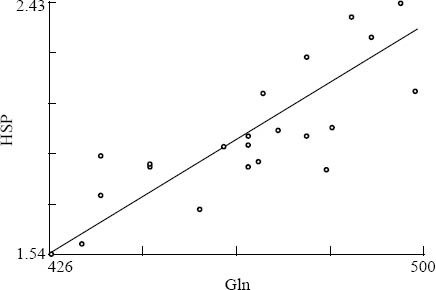
The correlation between Gln and HSP70 levels in the Gln group after the treatment.
Figure 2.
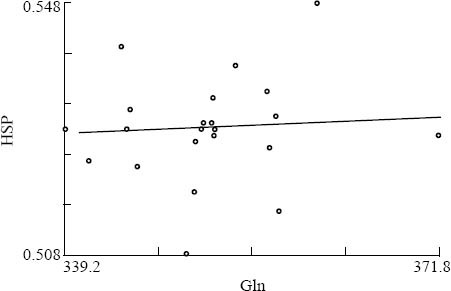
The correlation between Gln and HSP70 levels in the control group after treatment.
The levels of AST, ALT, TbiL, TNF-α, and IL-6 before and after the treatment are listed in Table 2. The differences in AST, ALT, TbiL and TNF-α, IL-6 between the two groups after the treatment were significant (P<0.05).
Changes of T cell subsets before and after the treatment
In the Gln group, there were significant changes in CD3, CD4 and CD4/CD8 after the treatment (P=0.034). But no significant change was found in T cell subsets in the control group. The difference in CD3, CD4 and CD4/CD8 was significant between the two groups after the treatment (P=0.041) (Table 2).
Mechanical ventilation (MV) time and ICU stay
MV time and ICU stay were shorter in the Gln group than in the control group (P=0.012; P=0.023, respectively) (Table 3).
Table 3.
Baseline Characteristics about patients in the two groups at admission

DISCUSSION
A number of studies have shown that parenteral Gln can improve outcomes of critically ill and injured patients.[22,23] Enhanced expression of HSP-70 can protect proteins from damage, and prevent metabolic dysfunction after injury and cellular injury and death.[23,24] It is possible that enhanced expression of HSP-70 is an important mechanism of the protective effects of Gln. This finding has been supported by laboratory data indicating that Gln can protect gut epithelial cells against injury.[25] This protection can be lost if HSP-70 expression is inhibited by either quercetin (an inhibitor of HSP-70 expression) or specific antisense inhibition of the HSP-70 gene.[25, 26] According to the correlation between Gln and HSP-70 in this study, Gln supplementation can increase the expression of HSP-70, which potentially down-regulates the inflammatory response in critical patients with liver injury. There was a significant change in AST, ALT, TbiL, TNF-α, and IL-6 between the two groups after the treatment in this study. Thus TNF-α and IL-6 play a major role in sepsis and tissue injury. It has been reported that TNF-α plays an important role in the recruitment of neutrophils to organs in lipopolysaccharide-induced sepsis.[20] Furthermore, in mice lacking functional genes for TNF-α or for the 55kDa TNF receptor, SIRS was attenuated. There is a widespread consensus that the release of TNF-α and IL-6 in cases of early sepsis is the causative factor of inflammatory response, which up-regulates the production of other cytokines. Hence, TNF-α and IL-6 play a central role in the pathologic process of SIRS initiation and organ injury.[27,28] In this study, we found that the protective effects of Gln on the liver and other organ injury can be explained: the up-regulation of HSP70 repaired injured proteins, promoted the degradation following irreparable injury, and attenuated the release of TNF-α and IL-6 in sepsis.
Our study showed that MV time and ICU stay were significantly shorter in the Gln group than in the control group. Eberle et al[29] reported that ARDS and acute liver failure were more common in the severe pneumonia group. Research demonstrated that the acute respiratory distress syndrome (ARDS) was attenuated in the Gln-supplemented animals, and this attenuation of ARDS and mortality was associated with preserved breath muscle strength and reduced tissue damage in the organs.[12,21] We believe that the administration of parenteral Gln is a good way to shorten the MV time and ICU stay in critical patients with ALI.
Our study revealed that there was a significant difference in the levels of CD3, CD4 and CD4/CD8 between the two groups at 7 days after the treatment. A recent study[30] has suggested that HSP-70 activates the immune system to induce the release of proinflammatory cytokines and anti-inflammatory cytokines. We postulate that HSP-70 may be part of an immunoregulatory response that potentially downregulates the inflammatory response. Also Gln and HSP70 may play a role in immunoregulatory function in critical diseases. T-lymphocytes, as immunoregulatory cells of the immune response, are classified into CD4 and CD8. An increased CD4/CD8 ratio may predict favorable outcome, while a decreased ratio may be a sign of unfavorable evolution.[31] A study[32] demonstrated that the supply of Gln can benefit the functioning of peripheral effector T-cells and promote leukocyte migration or adhesion in mice. Immunological and inflammatory processes are considered to be involved in the etiology and progression of critical diseases, and attention should be paid to the prospective role of high-level circulating lymphocytes and their sub-populations by Gln administration.
Our study showed that Gln enhanced HSP expression without any side-effect in critical patients. However whether this effect is dose and route dependent is not clear. Possibly low Gln levels may lead to inability of the patient to express HSP70.[33] Researchers believe that Gln is potentially ‘life-saving’ in critical illness, particularly when it is administered at a parenteral dose of over 0.3 g/kg per day.[34-36]
In conclusion, parenteral Gln administration can up-regulate serum HSP-70 concentration, decrease the levels of AST, ALT,TbiL, TNF-α, and IL-6, shorten the length of mechanical ventilation and ICU stay, and improve the ratio of CD4/CD8 in critical patients with acute liver injury. The correlation between Gln and HSP70 found in this study suggests that early parenteral glutamine administration could improve outcomes of critical patients with acute liver injury by increasing the expression of heat shock protein.
Footnotes
Funding: None.
Ethical approval: None.
Conflicts of interest: The study was approved by the Institutional Ethics Committee of Nanjing First Hospital.
Contributors: Ni HB proposed and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts. Zhang Z is the guarantor.
REFERENCES
- 1.Behrends M, Martinez-Palli G, Niemann CU, Cohen S, Ramachandran R, Hirose R. Acute hyperglycemia worsens hepatic ischemia/reperfusion injury in rats. Gastrointest Sur. 2010;14:528–535. doi: 10.1007/s11605-009-1112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singleton KD, Serkova N, Beckey VE, Wischmeyer PE. Glutamine attenuates lung injury and improves survival after sepsis: role of enhanced heat shock protein expression. Crit Care Med. 2005;33:1206–1213. doi: 10.1097/01.ccm.0000166357.10996.8a. [DOI] [PubMed] [Google Scholar]
- 3.Lu J, Wang XY, Tang WH. Glutamine attenuates nitric oxide synthase expression and mitochondria membrane potential decrease in interleukin-1beta-activated rat hepatocytes. Eur J Nutr. 2009;48:333–339. doi: 10.1007/s00394-009-0018-x. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi Y, Sawa Y, Fukuyama N, Nakazawa H, Matsuda H. Preoperative glutamine administration induces heat-shock protein 70 expression and attenuates cardiopulmonary bypass-induced inflammatory response by regulating nitric oxide synthase activity. Circulation. 2002;106:2601–2607. doi: 10.1161/01.cir.0000035651.72240.07. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- 6.De Maio A. Heat shock proteins: facts, thoughts, and dreams. Shock. 1999;11:1–12. doi: 10.1097/00024382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Plumier JC, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, et al. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest. 1995;95:1854–1860. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira GP, Oliveira MB, Santos RS, Lima LD, Dias CM, Ab’ Saber AM, et al. Intravenous glutamine decreases lung and distal organ injury in an experimental model of abdominal sepsis. Crit Care. 2009;13:R74. doi: 10.1186/cc7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pittet JF, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie RC. Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma. 2002;52:611–617. doi: 10.1097/00005373-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Weiss YG, Maloyan A, Tazelaar J, Raj N, Deutschman CS. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J Clin Invest. 2002;110:801–806. doi: 10.1172/JCI15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammarqvist F, Wernerman J, Von der Decken A, Vinnars E. Alanyl-glutamine counteracts the depletion of free glutamine and the postoperative decline in protein synthesis in skeletal muscle. Ann Surg. 1990;212:637–644. doi: 10.1097/00000658-199011000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karner J, Roth E. Alanylglutamine infusions to patients with acute pancretitis. Clin Nutr. 1990;99:43–45. doi: 10.1016/0261-5614(90)90079-8. [DOI] [PubMed] [Google Scholar]
- 14.Palmer TE, Griffiths RD, Jones C. Effect of parenteral L-glutamine on muscle in the very severely ill. Nutrition. 1996;12:316–320. doi: 10.1016/s0899-9007(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 15.Oudemans-van Straaten HM, Bosman RJ, Treskes M, van der Spoel HJ, Zandstra DF. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med. 2001;27:84–90. doi: 10.1007/s001340000703. [DOI] [PubMed] [Google Scholar]
- 16.Morlion BJ, Stehle P, Wachtler P, Siedhoff HP, Köller M, König W, et al. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery. Ann Surg. 1998;227:302–308. doi: 10.1097/00000658-199802000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin MT, Kung SP, Yeh SL, Liaw KY, Wang MY, Kuo ML, et al. Glutamine-supplemented total parenteral nutrition attenuates plasma interleukin-6 in surgical patients with lower disease severity. World J Gastroenterol. 2005;11:6197–6201. doi: 10.3748/wjg.v11.i39.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singleton KD, Wischmeyer PE. Glutamine's protection against sepsis and lung injury is dependent on heat shock protein 70 expression. Am J Physiol. 2007;292:R1839–R45. doi: 10.1152/ajpregu.00755.2006. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro SP, Villar J, Downey GP, Edelson JD, Slutsky AS. Sodium arsenite induces heat shock protein-72 kilodalton expression in the lungs and protects rats against sepsis. Crit Care Med. 1994;22:922–929. doi: 10.1097/00003246-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro SP, Villar J, Downey GP, Edelson JD, Slutsky AS. Effects of the stress response in septic rats and LPS-stimulated alveolar macrophages: Evidence for TNF-alpha post translational regulation. Am J Respir Crit Care Med. 1996;154:1843–1850. doi: 10.1164/ajrccm.154.6.8970379. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro SP, Villar J, Slutsky AS. Induction of the stress response to prevent organ injury. New Horiz. 1995;3:301–311. [PubMed] [Google Scholar]
- 22.Wischmeyer PE, Lynch J, Liedel J, Wolfson R, Riehm J, Gottlieb L, et al. Glutamine administration reduces Gram-negative bacteremia in severely burned patients: a prospective, randomized, double-blind trial versus isonitrogenous control. Crit Care Med. 2001;29:2075–2080. doi: 10.1097/00003246-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med. 2002;30:2022–2029. doi: 10.1097/00003246-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Preiser JC, Wernerman J. Glutamine, a life-saving nutrient, but why? Crit Care Med. 2003;31:2555–2556. doi: 10.1097/01.CCM.0000084863.47943.6F. [DOI] [PubMed] [Google Scholar]
- 25.Wischmeyer PE, Musch MW, Madonna MB, Thisted R, Chang EB. Glutamine protects intestinal epithelial cells: role of inducible HSP70. Am J Physiol. 1997;272:G879–G884. doi: 10.1152/ajpgi.1997.272.4.G879. [DOI] [PubMed] [Google Scholar]
- 26.Musch MW, Hayden D, Sugi K, Straus D, Chang EB. Cell-specific induction of hsp72-mediated protection by glutamine against oxidant injury in IEC18 cells. Proc Assoc Am Physicians. 1998;110:136–139. [PubMed] [Google Scholar]
- 27.Nandi D, Mishra MK, Basu A, Bishayi B. Protective effects of interleukin-6 in lipopolysaccharide (LPS)-induced experimental endotoxemia are linked to alteration in hepatic anti-oxidant enzymes and endogenous cytokines. Immunobiology. 2010;215:443–451. doi: 10.1016/j.imbio.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Liu YJ, Mao EQ, Ouyang B, Chen J, Tang YQ, Huang SW, et al. Effect of biliary tract external drainage on cytokine expression and histomorphology of intestine, liver, and lung in rats with hemorrhagic shock. Crit Care Med. 2009;37:2800–2806. doi: 10.1097/CCM.0b013e3181a59469. [DOI] [PubMed] [Google Scholar]
- 29.Eberle BM, Schnüriger B, Putty B, Barmparas G, Kobayashi L, Inaba K, et al. The impact of Acinetobacter baumannii infections on outcome in trauma patients: a matched cohort study. Crit Care Med. 2010;38:2133–2138. doi: 10.1097/CCM.0b013e3181f17af4. [DOI] [PubMed] [Google Scholar]
- 30.Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- 31.Carvounis CP. Total lymphocyte count:a promising prognostic index of mortality in patients on CAPD. Perit Dial Int. 2000;20:33–38. [PubMed] [Google Scholar]
- 32.Griveas I, Fleva A, Karanikas E, Gogos K, Sakellariou G. CD4/CD8 T-cell ratio in peritoneal dialysis effluents predicts the outcome of peritonitis in patients undergoing continuous ambulatory peritoneal dialysis. Artif Organs. 2009;33:1091–1095. doi: 10.1111/j.1525-1594.2009.00802.x. [DOI] [PubMed] [Google Scholar]
- 33.Wischmeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, Chang EB. Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J Appl Physiol. 2001;90:2403–2410. doi: 10.1152/jappl.2001.90.6.2403. [DOI] [PubMed] [Google Scholar]
- 34.Hall JC, Dobb G, Hall J, de Sousa R, Brennan L, McCauley R. A prospective randomized trial of enteral glutamine in critical illness. Intensive Care Med. 2003;29:1710–1716. doi: 10.1007/s00134-003-1937-2. [DOI] [PubMed] [Google Scholar]
- 35.Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: A systematic review of the evidence. Crit Care Med. 2002;30:2022–2029. doi: 10.1097/00003246-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Wischmeyer PE. Glutamine: role in critical illness and ongoing clinical trials. Current Opinion in Gastroenterology. 2008;24:190–197. doi: 10.1097/MOG.0b013e3282f4db94. [DOI] [PubMed] [Google Scholar]


