Abstract
BACKGROUND:
Triggering receptor expressed on myeloid cells-1 (TREM-1) in the intestine was upregulated and correlated with disease activity in inflammatory bowel diseases. Membrane-bound TREM-1 protein is increased in the pancreas, liver and kidneys of patients with severe acute pancreatitis (SAP), suggesting that TREM-1 may act as an important mediator of inflammation and subsequent extra-pancreatic organ injury. This study aimed to investigate the relationship between the expression of TREM-1 in intestinal tissue and intestinal barrier dysfunction in SAP.
METHODS:
Sixty-four male Wistar rats were randomly divided into a sham operation group (SO group, n=32) and a SAP group (n=32). A SAP model was established by retrograde injection of 5% sodium deoxycholate into the bile-pancreatic duct. Specimens were taken from blood and intestinal tissue 2, 6, 12, and 48 hours after operation respectively. The levels of D-lactate, diamine oxidase (DAO) and endotoxin in serum were measured using an improved spectro-photometric method. The expression levels of TREM-1, interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) mRNA in terminal ileum were detected by real-time reverse transcription-polymerase chain reaction (RT-PCR). Specimens of the distal ileum were taken to determine pathological changes by a validated histology score.
RESULTS:
The serum levels of D-lactate, DAO and endotoxin were significantly increased in each subgroup of SAP compared with the SO group (P<0.01, P<0.05). The expression levels of TREM-1, IL-1β and TNF-α mRNA in the terminal ileum in each subgroup of SAP were significantly higher than those in the SO group (P<0.01, P<0.05). The expression level of TREM-1mRNA was positively correlated with IL-1β and TNF-α mRNA (r=0.956, P=0.044; r=0.986, P=0.015), but the correlation was not found between IL-1β mRNA and TNF-α mRNA (P=0.133). Compared to the SO group, the pathological changes were aggravated significantly in the SAP group.
CONCLUSIONS:
The expression level of TREM-1 in intestinal tissue of rats with SAP was elevated, leading to the release of inflammatory mediators and intestinal mucosal injury. This finding indicates that TREM-l might play an important role in the development of intestinal barrier dysfunction in rats with SAP.
KEY WORDS: Severe acute pancreatitis, Triggering receptor expressed on myeloid cells-1, Intestinal barrier dysfunction, Tumor necrosis factor-α, Interleukin-1β
INTRODUCTION
Severe acute pancreatitis (SAP) is characterized by multiple complications, high mortality and complicated pathogenesis. SAP can readily progress from a localized inflammation of the pancreas to multiple organ dysfunction syndrome (MODS). The intestinal mucosal barrier plays a pivotal role in the pathophysiology of SAP, as the intestine is a primary target organ of inflammation-induced dysfunction and an initiator of MODS. A large number of studies have demonstrated that SAP is strongly associated with development of intestinal barrier dysfunction (IBD).[1] On the other hand, IBD caused by bacterial translocation, intestinal infection or endotoxin exposure can aggravate SAP, and this complicating event has been proposed as a major cause of SAP death.[2] Unfortunately, the underlying molecular mechanisms that mediate the relationship between injury of the intestinal mucosa barrier and SAP remain to be fully elucidated.
The triggering receptor expressed on myeloid cells-1 (TREM-1) protein was recently described as a diagnostic marker of inflammation, as it is induced in the presence of bacteria or endotoxin.[3,4] TREM-1 is mainly expressed on neutrophils and monocytes/macrophages, where it functions in synergy with Toll-like receptor-mediated signals to increase the release of pro-inflammatory factors, such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-lβ). Hence, TREM-1 acts as a critical amplifier of inflammatory signaling in response to lipopolysaccharide (LPS) and other microbial products.[5]
Compared with healthy people, acute pancreatitis (AP) patients have high expression of leukocyte TREM-1 mRNA.[6] Moreover, TREM-1 expression in the intestine was upregulated and correlated with disease activity in inflammatory bowel diseases.[7] Recent investigations have demonstrated that membrane-bound TREM-1 protein is increased in the pancreas, liver and kidney of patients with SAP, suggesting that TREM-1 may act as an important mediator of inflammation and subsequent extra-pancreatic organ injury in SAP.[8] Thus, in this study we sought to clarify the role of TREM-1 in the pathophysiology of intestinal barrier dysfunction in SAP.
METHODS
Animals
Male Wistar rats (weighing 250-300 g, 9 weeks old) were purchased from the Laboratory Animal Center of Jiangsu University. The rats were allowed to acclimatize to the laboratory conditions for 7 days under a 12-hour light-dark cycle at constant temperature of (21±1)°C. All of the rats were fasted for 12 hours before experiments, but allowed free access to water. The use and care of the animals for this investigation were reviewed and approved by the Institutional Animal Committee of Jiangsu University School of Clinical Medicine.
Induction of experimental SAP
The rats were randomly divided into: a sham group (SO group), and a SAP group with 32 rats in each group. Each group was further divided into 2, 6,12, and 24-hour subgroups, respectively. The rats were anesthetized by intraperitoneal injection of 50 mg/kg phenobarbital and a midline laparotomy was performed. SAP model was induced by retrograde injection of 5% sodium taurocholate (TCA; 1 mL/kg body weight) into the biliopancreatic duct in a pressure- and volume-controlled manner over 10 minutes. The rats of the SO group were subjected to isovolumetric injection of 9 g/L physiological saline using the same method.
Blood samples
Animals in each group were sacrificed at 2, 6,12 and 24 hours respectively and 3 mL of venous blood was collected. The blood sample was injected into dry test tubes and separated by centrifugation, and finally the serum was stored at -20°C until use.
Detection of the levels of D-lactate, diamine oxidase (DAO) and endotoxin by an improved spectro-photometric method
For the analysis of intestinal barrier dysfunction, the serum was quantitated for D-lactate, DAO and endotoxin concentrations using commercial kits and following the manufacturer's protocol (Genmed, China).
Analysis of TREM-1, IL-1β and TNF-α mRNAs in intestinal mucosa by RT-PCR
Total RNA was isolated from rat intestinal mucosa using TRIzol reagent (Invitrogen Corp, USA) according to the manufacturer's instructions. Complementary DNA (cDNA) was synthesized using a reverse transcription kit (Toyoba, Japan). The mRNA expression levels of the following 3 genes were quantitated (TREM-1, IL-1β, TNF-α). The sequences of PCR primer pairs used for each gene are shown in Table 1. β-actins were used as invariant housekeeping gene internal controls. PCR products were semiquantitatively analyzed on agarose gels.
Table 1.
The primer sequences and amplified fragment length

Pathological examination
Upon excision, the distal ileums (5 cm long) were promptly fixed in 4% formalin, embedded in paraffin wax and cut into serial sections (5 μm thick), following standard procedures. Paraffin-embedded tissue sections were then stained with Hematoxilin and Eosin (HE) for histological examination. The degree of mucosal damage was graded according to the standard scale of Chiu et al[9], in which: 0=normal mucosa; 1=development of subepithelial space at the tip of the villus; 2=extension of the space with epithelial lifting; 3=massive epithelial lifting; 4=denuded villi; and, 5=disintegration of the lamina propria.
Statistical analysis
All the data were expressed as mean±SD and SPSS 16.0 software was used to make one-way ANOVA and spearman's product-moment correlation coefficient analysis. Differences of grading of intestinal mucosal lesions were determined using the non-parametric Mann-Whitney U test. Statistical analysis was performed with post-hoc test. P<0.05 was considered statistically significant.
RESULTS
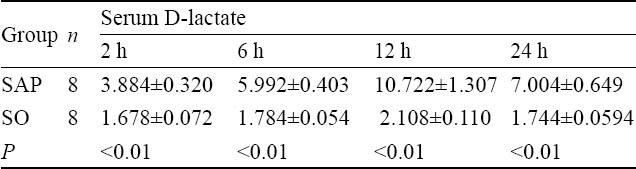
The level of D-lactate in serum
At 2 hours after injection of 50 g/L sodium taurocholate, serum D-lactate level in the samples from the mesentery vein in the SAP group was higher than that in the SO group. From 2 hours onward, a significant difference was observed between the SAP and SO groups (P<0.01, Table 2).
Table 2.
Comparison of serum D-lactate levels at different time points between the two groups (μol/ml, mean±SD)
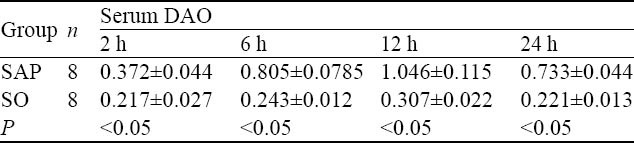
The level of DAO in serum
The levels of DAO were higher significantly in the SAP group than in the SO group at all time points, and they peaked at 12 hours (P<0.05, Table 3).
Table 3.
The comparison of serum DAO levels at different time points between the two groups (U/mg, mean±SD)
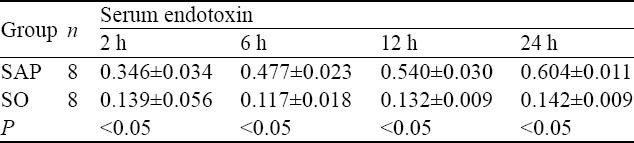
The level of endotoxin in serum
The levels of serum endotoxin in the SAP group were significantly higher than those in the SO group, and the increased levels were persistent at all time points examined (P<0.05, Table 4).
Table 4.
Comparison of serum endotoxin levels at different time points between the two groups (eu/mL, mean±SD)
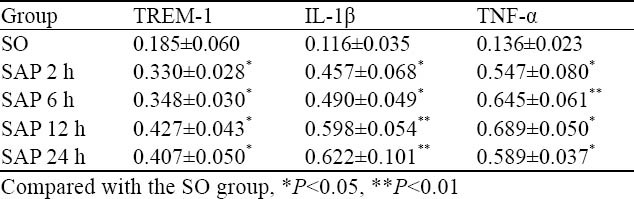
The expression levels of TREM-1, IL-1β and TNF-α mRNAs in intestinal mucosa
The expression level of TREM-1 mRNA was increased more significantly along with the time course from 2 hours in the SAP group than in the SO group (Figure 1, Table 5, P<0.05).
Figure 1.
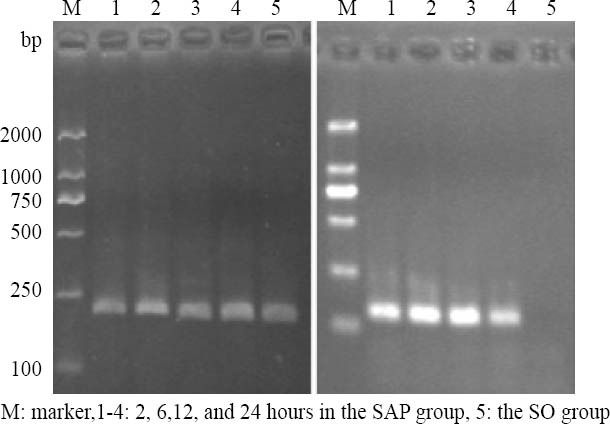
The expression of β-actin and TREM-1mRNA in rat intestine by RT-PCR.
Table 5.
Comparison of TREM-1,IL-1β and TNF-α mRNA in rat intestine at different time points between the two groups (mean±SD)
The expression levels of IL-1β and TNF-α mRNAs were increased more significantly at all time points in the SAP group than in the SO group (Figure 2, Table 5, P<0.01, P<0.05).
Figure 2.
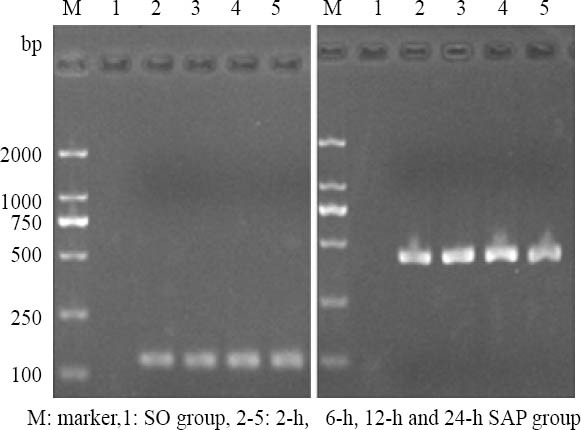
The expression of IL-1β and TNF-α mRNA in rat intestine by RT-PCR.
Correlation analysis
The expression level of TREM-1mRNA was positively correlated with IL-1β and TNF-α mRNA (r=0.956, P=0.044; r=0.986, P=0.015), but the correlation was not found between IL-1β mRNA and TNF-α mRNA (P=0.133).
Pathologic examination of intestinal mucosa
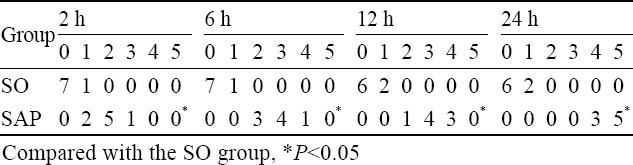
After induction of SAP, the rats exhibited characteristic pathological changes such as broadened villi, infiltration of inflammatory cells and parenchyma hemorrhage (Figure 3). The degree of intestinal pathological injury is shown in Table 6. The grades of the SAP group were significantly higher those of the SO group (P<0.05).
Figure 3.
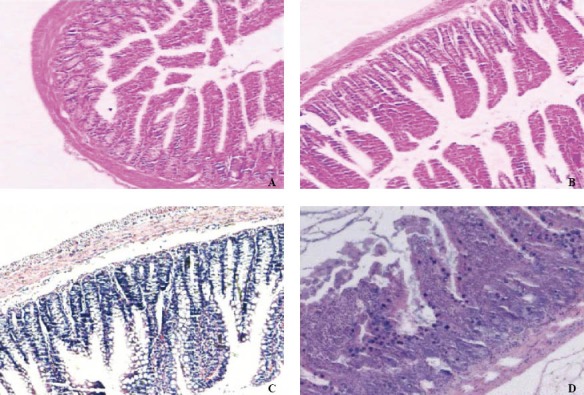
Morphological changes in intestinal mucosa after induction of SAP (HE, original magnification × 100). A: Intestinal section with normal mucosa histopathologically graded as 0; B: Intestinal section with massive epithelial lifting histopathologically graded as 3; C: Intestinal mucosa with denuded villi histopathologically graded as 4; D: Intestinal mucosa with disintegration of the lamina propria graded as 5.
Table 6.
Histological score of the ileum in each group after the induction of sever acute pancreatitis
DISCUSSION
The injury of intestinal mucosa is one of the main reasons for the accelerated aggravation of SAP. As an organ to digest and absorb nutrients, the intestine is also a unique immune organ. When SAP develops, the destruction of the intestinal mucosa barrier is an important contributing factor for the development of bacterial translocation, systemic inflammatory response syndrome (SIRS) and MODS. It is important to protect the intestinal mucosa in the treatment of SAP. TREM-1 was shown to act as an amplifier of inflammation by enhancing degranulation and secretion of pro-inflammatory mediators. Increased levels of soluble TREM-1 (sTREM-1) were found in patients or animals with infectious and non-infectious diseases, and were correlated with disease severity.[10-12] Serum sTREM-1 was found to be higher in patients with AP than in healthy volunteers.[13] Thus, it is important to investigate the role of TREM-1 in the development of SAP associated with IBD.
The results of the present study show that increased levels of serum D-lactate, DAO and endotoxin were significantly associated with the severity of SAP and IBD in rats. Moreover, the expression levels of TREM-1, IL-1β and TNF-α mRNAs in the intestinal mucosa were significantly increased in the SAP group, and TREM-1 was positively correlated with IL-1β and TNF-α. This finding suggests that TREM-1 promotes the release of inflammatory cytokines and may be involved in the pathophysiology of SAP associated with IBD.
D-lactate is a metabolic product of bacterial fermentation such as intestinal mucosa damage caused by acute intestinal hemorrhage. The intestinal bacteria produce a lot of D-lactate into the blood through the damaged intestinal mucosa. Mammals do not have an enzyme system to decompose it, so the monitoring of serum D-lactate levels can promptly reflect the extent of intestinal mucosa damage and permeability.[14] DAO is mainly in the villi of the small intestine of mammals, and its activity is closely related to villus height and mucosal cells of nucleic acids and protein synthesis.[15] DAO activity in peripheral blood can be reliably indicated by the degree of intestinal epithelial cell maturation and integrity. Thus the changes of DAO can reflect the structure and function of small intestinal mucosa.[16]
The action of pro-inflammatory cytokines is an important injury-inducing mechanism that influences the integrity of the intestinal mucosa. Excessive release of inflammatory mediators, such as IL-1β and TNF-α, can directly damage the intestinal mucosa barrier.[17 TNF-α, in particular, participates in the early steps of SAP,[18,19] and can induce expression of other inflammatory cytokines.[20] In addition, TNF-α is known to stimulate the generation of several injured substances, such as NO and oxygen free radicals, which lead to further inflammatory cascade and amplification effects.[21,22] TNF-α can also promote the permeability of intestinal mucosa barriers and break-down the tight junctions, thereby intestinal bacteria may easy transverse into the mesenteric lymph nodes and other organs.[23] Continuous invasion of intestinal bacteria and endotoxin into the body will lead to the life-threatening systemic inflammatory response syndrome (known as SIRS) and MODS. IL-1β is another classic pro-inflammatory cytokine; it can destroy endothelial barrier integrity by stimulating the release of inflammatory mediators from leukocytes and endothelial cells. On the other hand, compromised endothelial cells can enhance the synthesis and secretion of IL-1β.[24] The results of this study showed that, at all time points after operation, the expression levels of TREM-1 protein and the pathological severity scores of the intestinal mucosa of rats in the SAP group were significantly higher than those in the SO group, indicating that the mucosa damage may be related to upregulation TREM-1 protein. Reaching certain levels, injury of intestinal mucosa barrier will increase the permeability of the intestinal mucosa, cause intestinal bacteria translocation and gutorigin endotoxaemia, and result in multiple organ functional disturbance and failure.
In conclusion, there are different ways to cause function injury of the intestinal mucosa barrier during the progression of SAP. Excessive release of inflammatory mediators and intestinal bacteria translocation are main reasons to induce intestinal mucosa injury. Our data suggest that TREM-1 as an amplifier of inflammation may be involved in pancreatitis-associated IBD, and that the modulation of TREM-1 signaling may be a promising therapeutic approach for the treatment of acute pancreatitis complicated by injury of the intestinal mucosa barrier.
Footnotes
Funding: The study was supported by a grant from the National Natural Science Foundation of China (81070287).
Ethical approval: Not needed.
Conflicts of interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributors:Yin K proposed and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Nakajima T, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Sawa H, et al. Protective effects of vascular endothelial growth factor on intestinal epithelial apoptosis and bacterial translocation in experimental severe acute pancreatitis. Pancreas. 2007;34:410–416. doi: 10.1097/mpa.0b013e3180335c64. [DOI] [PubMed] [Google Scholar]
- 2.Dang SC, Zhang JX, Qu JG, Mao ZF, Wang XQ, Zhu B. Dynamic changes of IL-2/IL-10, sFas and expression of Fas in intestinal mucosa in rats with acute necrotizing pancreatitis. World J Gastroenterol. 2008;14:2246–2250. doi: 10.3748/wjg.14.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 4.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 5.Nathan C, Ding A. TREM-1: a new regulator of innate immunity in sepsis syndrome. Nat Med. 2001;7:530–532. doi: 10.1038/87846. [DOI] [PubMed] [Google Scholar]
- 6.Wang DY, Qin RY, Liu ZR, Gupta MK, Chang Q. Expression of TREM-1 mRNA in acute pancreatitis. World J Gastroenterol. 2004;10:2744–2746. doi: 10.3748/wjg.v10.i18.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schenk M, Bouchon A, Seibold F, Mueller C. TREM-1– expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest. 2007;117:3097–106. doi: 10.1172/JCI30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamei K, Yasuda T, Ueda T, Qiang F, Takeyama Y, Shiozaki H. Role of triggering receptor expressed on myeloid cells-1 in experimental severe acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17:305–312. doi: 10.1007/s00534-009-0191-6. Epub 2009 Sep 29. [DOI] [PubMed] [Google Scholar]
- 9.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 10.Park JJ, Cheon JH, Kim BY, Kim DH, Kim ES, Kim TI, et al. Correlation of serum-soluble triggering receptor expressed on myeloid cells-1 with clinical disease activity in inflammatory bowel disease. Dig Dis Sci. 2008;54:1525–1531. doi: 10.1007/s10620-008-0514-5. [DOI] [PubMed] [Google Scholar]
- 11.Tzivras M, Koussoulas V, Giamarellos-Bourboulis EJ, Tzivras D, Tsaganos T, Koutoukas P, et al. Role of soluble triggering receptor expressed on myeloid cells in inflammatory bowel disease. World J Gastroenterol. 2006;12:3416–3419. doi: 10.3748/wjg.v12.i21.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Roldán N, Ferat-Osorio E, Aduna-Vicente R, Wong-Baeza I, Esquivel-Callejas N, Astudillo-de la Vega H, et al. Expression of triggering receptor on myeloid cell 1 and histocompatibility complex molecules in sepsis and major abdominal surgery. World J Gastroenterol. 2005;11:7473–7479. doi: 10.3748/wjg.v11.i47.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferat-Osorio E, Wong-Baeza I, Esquivel-Callejas N, Figueroa-Figueroa S, Duarte-Rojo A, Guzmán-Valdivia-Gómez G, et al. Triggering receptor expressed on myeloid cells-1 expression on monocytes is associated with inflammation but not with infection in acute pancreatitis. Crit Care. 2009;13:R69. doi: 10.1186/cc7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morencos FC, de las Heras Castaño G, Martín Ramos L, López Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1995;40:1252–1256. doi: 10.1007/BF02065533. [DOI] [PubMed] [Google Scholar]
- 15.Kitanaka J, Kitanaka N, Tsujimura T, Terada N, Takemura M. Expression of diamine oxidase (histaminase) in guinea-pig tissues. Eur J Pharmacol. 2002;437:179–185. doi: 10.1016/s0014-2999(02)01302-x. [DOI] [PubMed] [Google Scholar]
- 16.Peng X, Yan H, You Z, Wang P, Wang S. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns. 2004;30:135–139. doi: 10.1016/j.burns.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X-p, Zhang J, Song Q-l, Chen HQ. Mechanism of acute pancreatitis complicated with injury of intestinal mucosa barrier. J Zhejiang Univ Sci B. 2007;8:888–895. doi: 10.1631/jzus.2007.B0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereda J, Sabater L, Cassinello N, Gómez-Cambronero L, Closa D, Folch-Puy E, et al. Effect of simultaneous inhibition of TNF-alpha production and xanthine oxidase in experimental acute pancreatitis: the role of mitogen activated protein kinases. Ann Surg. 2004;240:108–116. doi: 10.1097/01.sla.0000129343.47774.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strobel O, Wachter D, Werner J, Uhl W, Müller CA, Khalik M, et al. Effect of a pneumoperitoneum on systemic cytokine levels, bacterial translocation, and organ complications in a rat model of severe acute pancreatitis with infected necrosis. Surgical Endoscopy. 2006;20:1897–903. doi: 10.1007/s00464-005-0417-x. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 21.Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24(Suppl 1):45–51. doi: 10.1097/01.shk.0000191413.94461.b0. [DOI] [PubMed] [Google Scholar]
- 22.Mole DJ, Taylor MA, McFerran NV, Diamond T. The isolated perfused liver response to a ‘second hit’ of portal endotoxin during severe acute pancreatitis. Pancreatology. 2005;5:475–485. doi: 10.1159/000086614. [DOI] [PubMed] [Google Scholar]
- 23.Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Yang K, et al. Intestinal damage mediated by Kupffer cells in rats with endotoxemia. World J Gastroenterol. 2002;8:923–927. doi: 10.3748/wjg.v8.i5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leveau P, Wang X, Sun Z, Börjesson A, Andersson E, Andersson R. Severity of pancreatitis-associated gut barrier dysfunction is reduced following treatment with the PAF inhibitor lexipafant. Biochemical Pharmacology. 2005;69:1325–1331. doi: 10.1016/j.bcp.2005.01.023. [DOI] [PubMed] [Google Scholar]


