Abstract
BACKGROUND:
Pulmonary stretch reflex plays an important role in regulation of respiratory movement. This study aimed to evaluate the effect of pulmonary stretch reflex on lung injury in rabbits with acute respiratory distress syndrome (ARDS).
METHODS:
ARDS rabbits were given intratracheal infusion of hydrochloric acid and ventilated with neurally adjusted ventilatory assistance (NAVA) with a tidal volume (VT) of 6 mL/kg and the electrical activity of diaphragm (EAdi)-determined positive end expiratory pressure. After isolation of the bilateral vagus nerve trunk, the rabbits were randomized into two groups: sham operation (SHAM) group (n=5) and bilateral vagotomy (VAG) group (n=5). Gas exchange and respiratory mechanics were detected at baseline, after lung injury and 1, 2, and 3 hours after ventilation respectively. Pulmonary permeability index, pathological changes and inflammatory response were also measured.
RESULTS:
Compared with the SHAM group, PaO2/FiO2 in the VAG group decreased significantly 2 and 3 hours after ventilation (P<0.05). There was no significant difference in PaCO2 between the SHAM and VAG groups (P>0.05), and the VAG group had a high VT, peak pressure (Ppeak), and mean pressure (Pm) compared with the SHAM group 1, 2, 3 hours after ventilation (P<0.05). Compared to the SHAM group, dead space fraction (VD/VT) and respiratory system elastance (Ers) in the VAG group increased (P<0.05) and static pulmonary compliance (Cst) decreased markedly (P<0.05) after ventilation for 3 hours. Lung wet/dry weight ratio (W/D) (8.4±1.2 vs. 6.6±1.0), lung injury score (6.3±1.8 vs. 3.8±1.3), tumor necrosis factor-α (TNF-α) (779±372 pg/mL vs. 355±130 pg/mL) and interleukin-8 (IL-8) (169±21 pg/mL vs. 118±17 pg/mL) increased significantly in the VAG group compared with the SHAM group (P<0.05).
CONCLUSION:
Lung injury is aggravated after bilateral vagotomy, demonstrating that pulmonary stretch reflex may have protective effect on the lung.
KEY WORDS: Pulmonary stretch reflex, Vagus nerve, Lung injury, Acute respiratory distress syndrome, Electrical activity of diaphragm, Mechanical ventilation
INTRODUCTION
Pulmonary distention or deflation can stimulate pulmonary stretch receptors to induce pulmonary stretch reflex (PSR). PSR is conducted from the vagus nerves to the respiratory center, and can regulate respiratory movement by changing the frequency and intensity of the diaphragmatic nerve and the electrical activity of the diaphragm (EAdi). PSR can prevent hypoventilation or hyperventilation of pulmonary alveoli.[1,2]
The small tidal volume (VT) ventilatory strategy (6 mL/kg) has been the only measure to improve the prognosis in patients with acute respiratory distress syndrome (ARDS).[3] However, as a clinical symptom complex, it is inappropriately used as the same or fixed level of ventilatory support in different ARDS patients at different stages, because hypoventilation or hyperventilation may aggravate lung injury caused by ARDS.[4,5] PSR plays an important role in the respiratory regulation when the small VT ventilatory strategy is used in ARDS.
We hypothesized that maintaining intact PSR would protect the lung in ARDS due to the respiratory regulation. In this study we studied the effects of PSR on lung injury in ARDS rabbits after administration of acid to the lung.
METHODS
Animal preparation
The present experiment was approved by the Institutional Animal Use and Care Committee of our university. Ten New Zealand white rabbits, 2.2-3.0 kg, were supplied by the Laboratory of Experimental Animals of the Institute of Animal Sciences, Jiangsu Academy of Agricultural Sciences. The rabbits were anesthetized with an intramuscular bolus of ketamine hydrochloride (35 mg/kg) and xylazine (10 mg/kg), followed by continuous intravenous infusion of ketamine hydrocloride (10 mg/kg per hour) and xylazine (2 mg/kg per hour). Normal saline was administered at 5-6 mL/kg per hour through an ear vein to maintain a mean arterial pressure> 60 mmHg. Mean arterial pressure, heart rate, and arterial blood gases were obtained from an ear artery. Body temperature was measured with a rectal probe and was maintained between 36 °C and 39.0 °C using a heated blanket.
The rabbits were tracheostomized and ventilated by a Servo-i ventilator (Maquet Critical Care, Sweden). Baseline ventilation was done in a volume-controlled mode with a VT of 6 mL/kg, a respiratory rate of 35 breaths/min, a fraction of inspired oxygen (FiO2) of 0.5, and positive end expiratory pressure (PEEP) 2 cmH2O.
The bilateral vagus nerve was isolated from the vagina carotica at the first tracheal ring. An 8-Fr catheter (8 Fr/100 cm, Maquet Critical Care, Sweden) with an array of ten micro-electrodes for recording of EAdi was passed through the mouth and positioned so that the electrode array was in the esophagus at the level of the crural diaphragm. Hydrochloricacid (HCl, pH 1.5) was administered intratracheally (2.0 mL/kg),[6] followed by a 5-second recruitment maneuver with continuous positive airway pressure of 20 cmH2O. ARDS was defined as oxygenation index (PaO2/FiO2) < 150 mmHg.[7]
Principles of neurally adjusted ventilatory assistance (NAVA)
NAVA collected EAdi signals through the electrode catheter positioned at the diaphragm at the bottom of the esophagus. Through sensors, the collected EAdi signals were transferred to ventilators installed with NAVA software. According to these signals, ventilators began ventilating. The delivered airway pressure during inspiration was determined by the multiplication product of EAdi (unit:μv) and NAVA level (unit: cmH2O/μv) every 16 ms.[8]
Parameters of NAVA
Optimal PEEP selected by EAdi
After lung recruitment (25 cmH2O PEEP for 10 seconds in the mode of continuous positive airway presssure), PEEP decreased 5 cmH2O every 3 minutes from 20 cmH2O progressively during NAVA in order to monitor EAdi and blood pressure. When PEEP decreased, tonic EAdi obviously increased and phasic EAdi decreased, recruitment maneuver was performed again, and PEEP should be adjusted to the level before the increase of tonic EAdi.[9]
NAVA level
During pressure support ventilation, VT was set at 6 mL/kg by adjusting PSR. With the use of preview tool, NAVA level was set to PSR level, and VT maintained at 6 mL/kg.[8,10]
Experimental protocol
The rabbits were randomly divided into two groups: sham operation (SHAM) group (n=5), with intact vagus; and bilateral cervical vagotomy (VAG) group (n=5), bilateral vagotomy. All rabbits were sacrificed with an overdose of anesthesia 3 hours after NAVA ventilation.
Measurement of hemodynamics, gas exchange and respiratory mechanics
Mean arterial pressure and heart rate were monitored continuously (Spacelab, Model 1500, American) and recorded, at baseline, after acid aspiration injury, and every hour during NAVA ventilation. Arterial blood gases were measured using an automated blood gas analyzer (Nova, Waltham, MA). VT, peak airway pressure (Ppeak), mean airway pressure (Pm) and PEEP were obtained from the ventilator. At baseline, after acid aspiration injury and 3-hour ventilation, dead space fraction (VD/VT) was measured with Douglas bag using a rebreathing method, and respiratory system elastance (Ers) was calculated as the ratio between the airway pressure corresponding to the volume of 50% of inspiratory capacity and the volume itself which described by Franco et al.[11] Static pulmonary compliance (Cst) was equal to 1/Ers.
Estimation of pulmonary edema
Pulmonary edema was estimated by the ratio of lung wet to dry weight (W/D), which was usually used to indicate the severity of pulmonary edema. Briefly, the lung zones were removed and cleared of all extrapulmonary tissues. The lung was weighed before drying and then dried at 80 °C until the weight was constant.
Histopathological assay
Observation by scanning electron microscope (SEM)
Tissue taken from the lobus intermedius of the right lung was fixed in 2.5% glutaraldehyde, and longitudinally cut to examine parenchymal structures. All tissue blocks were impregnated with 2.5% tannic acid for 2 days. A counter fixation in 2% OsO4 for 4 hours was followed by dehydration in ethanol and dried in a critical point dryer. The preparation was coated with gold and examined with a SEM (S-300N, HITACH, Japan).
Observation by light microscope
The tissue of the right lung was stored in 10% PBS buffered formalin, paraffin embedded and cut into 5 μm sections. The sections were stained with hematoxylin and eosin, and pathological changes were observed by a pathologist blinded to the groups. Lung injury was determined by findings in 10 randomly selected low-power fields (×100) for each section. Edema, inflammation of alveoli and interstitial tissue, hemorrhage, atelectasis, necrosis, and hyaline membrane formation were scored on a 0-4 point scale: point 0, no injury; point 1, injury in 25% of the field; point 2, injury in 50%; point 3, injury in 75%; and point 4, injury throughout the field. The injury of the lung was finally scored by the sum of above scores.[12]
Cytokine assessment
Tumor necrosis factor alpha (TNF-α) and interleukin-8 (IL-8) in the bomogenate serumrom of lung tissue were analyzed in a blinded fashion using ELISA kits (R&D Systems, American). The sensitivities of these kits for TNF-α and IL-8 were 16 pg/mL and 15 pg/mL respectively, and both were specific for rabbits. The absorbance of each well was read at 450 nm with a microplate reader (Bio-Rad, Model 680, Japan).
Statistical analysis
Statistical analyses were made using the SPSS13.0 software package. Continuous data were expressed as means ± standard deviation. Hemodynamics, gas exchange and lung mechanics were compared using analysis of variance (ANOVA). One-way ANOVA was used to determine statistical differences in W/D, lung injury score, TNF-α and IL-8. A multiple comparison procedure (S-N-K test) was used after ANOVA yielded significant results. A significant difference was defined as P<0.05.
RESULTS
Before acid aspiration, there were no significant differences in mean arterial pressure, PaO2/FiO2, PaCO2 and Ppeak (P>0.05). After acid injury (before the division of rabbits into two groups) in each group, PaO2/FiO2 was 133.9± 28.2 mmHg in the SHAM group and 134.4 ±26.1 mmHg in the VAG group; and Cst was significantly reduced by 56%±11% in the SHAM group and 47%±6% in the VAG group respectively. There were no significant differences in PaO2/FiO2 and Cst between the two groups (P>0.05).
Effects of blocking the pulmonary stretch reflex on hemodynamics, gas exchange and lung mechanics
After randomization and allocation to the two groups, mean arterial pressure was higher in the VAG group than in the SHAM group, but there was no significant difference between the two groups (P>0.05) (Table 1).
Table 1.
Effect of blocking of the pulmonary stretch reflex on hemodynamics and gas exchange in ARDS (mean±SD, n=5)
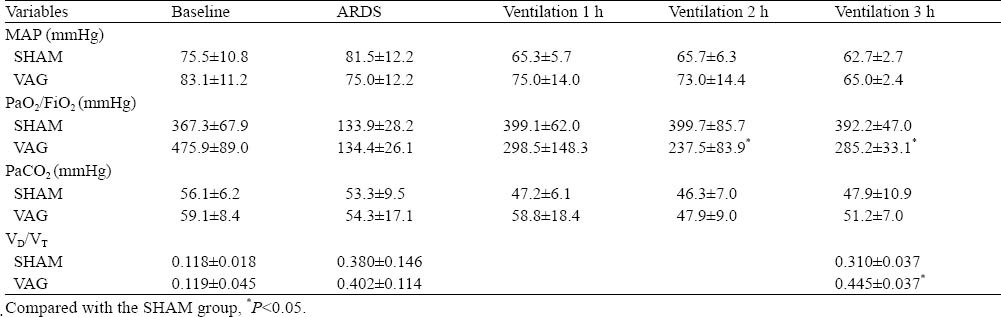
At 2, 3 hours after ventilation, PaO2/FiO2 decreased more significantly in the VAG group than in the SHAM group (P<0.05), and there was no significant difference in PaCO2 between the two groups during 3 hours of ventilation (P>0.05). VD/VT in the SHAM group was obviously lower than that in the VAG group after 3 hours of ventilation (0.310±0.037 vs. 0.445±0.037, P<0.05) (Table 1).
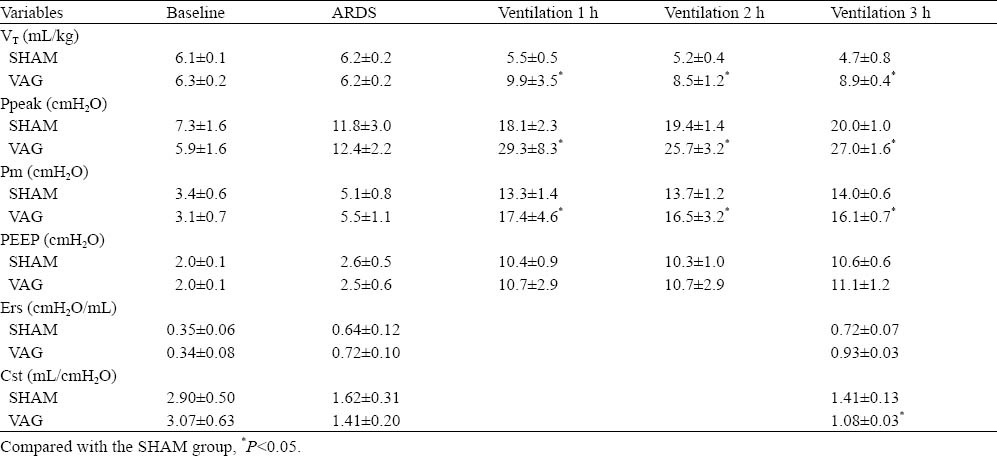
Ppeak was higher in the VAG group than in the SHAM group (P<0.05) and there was no significant difference of PEEP between the two groups (P>0.05) at 1, 2, 3 hours. Compared with the SHAM group, the VAG group had a higher Ers and a lower Cst at 3 hours of ventilation (P<0.05) (Table 2).
Table 2.
Effect of blocking of the PSR on pulmonary mechanics in ARDS rabbits (mean±SD, n=5)
Effect of blocking PSR on pulmonary edema
The VAG group had a higher W/D than the SHAM group (8.4±1.2 vs. 6.6±1.0, P<0.05) (Table 3).
Table 3.
Effect of blocking of the PSR on W/D, lung injury score and mediators of inflammation in ARDS rabbits (mean±SD, n=5)

Effect of blocking the PSR on pulmonary histopathological change
Histopathological examination showed that the lung in the VAG group was damaged seriously and the alveoli structure was in disorder with extensive alveolar septum rupture and formation of the bullae lung, alveoli wall thickness, defluxion of pulmonary superficial cells, pulmonary edema, alveoli and interstitial hemorrhage, and diffused interstitial infiltration of mononuclear cells and granulocytes (Figure 1). Histologic examination of the SHAM group revealed clearly demarcated alveoli as honeycomb appearance, alveoli wall thinning and uniform alveoli space (Figure 1). Edema, hemorrhage and infiltration in alveoli and interstitial tissue were improved obviously in the VAG group compared with the SHAM group. The lung injury score was higher in the VAG group than in the SHAM group (6.3±1.8 vs. 3.8±1.3, P<0.05) (Table 3).
Figure 1.
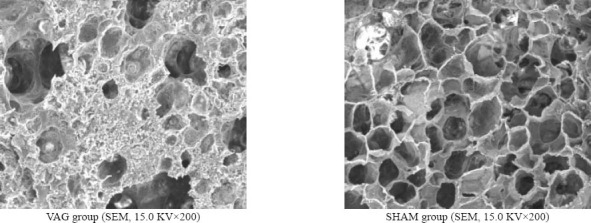
Effect of blocking of the PSR on pathological changes in ARDS rabbits observed with SEM.
Effect of blocking the PSR on mediators of inflammation
The concentrations of TNF-α and IL-8 were increased more significantly in the VAG group than in the SHAM group (P<0.05) (Table 3).
DISCUSSION
In our study, the results showed that VAG could increase dead space ventilation and pulmonary elastance, decrease pulmonary compliance, and aggravate pulmonary edema, lung pathological injury and inflammatory response of ARDS rabbits ventilated with the small VT.
PSR plays an important role in adjustment of ARDS ventilation, and EAdi amplitude can show the intensity of PSR to some degree. There are four types of lung sensors:[13] slowly adapting receptors (SARs), rapidly adapting receptors (RARs), high-threshold Aδfibre receptors (HTARs) and C fibre receptors (HTARs). The first two are mechanosensitive and the last two are chemosensitive. When these sensors are stimulated, the excitation signal will be conducted to the respiration center through the common afferent fibers-vagus nerve. The distention or deflation of the lung mainly stimulate SARs, trigger PSR, and transmit to the respiration center through the vagus nerve. This can change the discharge of the diaphragmatic nerve and EAdi, and adjust the respiratory movement on feedback.[14, 15]
After blocking PSR, the amplitude of EAdi and the ventilatory support level significantly increased. The respiratory rate of rabbits decreased and the amplitude was characterized by decreased EAdi frequency and increased EAdi amplitude after bilateral vagotomy. In NAVA, the pressure delivered above PEEP during inspiration was determined by the multiplication product of EAdi and NAVA level.[9] There was no significant difference in NAVA level between the two groups, but VT and Ppeak were higher in the VAG group than in the SHAM group because of the increased EAdi amplitude. Since the afferent nerve of PSR, the vagus nerve involves the conversion of inspiration and respiration and the adjustment of the depth and frequency of respiration.[1] Hence, bilateral vagotomy in this study resulted in the weakened physiological functions of PSR and the deepened inspiration due to increased VT and Ppeak. If PSR was intact and NAVA was extremely high, the amplitude of EAdi and the ventilatory support also declined. Ventilatory support in NAVA was the self-selected small VT ventilation that was not constant though the incipient VT was set at 6 mL/kg in this study. This was more suitable for different pulmonary pathophysiologic characteristics in ARDS rabbits and might have lung protective effect.
Lung injury in ARDS rabbits was aggravated significantly when bilateral vagotomy was performed. Permeability and pneumonedema of the lung increased after cutting off the vagus nerve.[16] This finding was not consistent with the reported:[17] there were no significant differences in the physiochemical features of alveolar surfactant and the pulmonary histopathological findings between the VAG group and the SHAM group. W/D and lung injury score were higher in the VAG group than in the SHAM group, and the alveoli structure in the VAG group was severely damaged by extensive alveolar septum rupture, pulmonary edema, alveoli and interstitial hemorrhage, and infiltration of a number of inflammatory cells. We speculated that although bilateral vagotomy might lead to the lung injury of ARDS rabbits, increased Ppeak and VT predominated in the lung injury through over-stretching the alveolar epithelium and capillary endothelium. Lowered V/Q, diffusion disorder, alveoli collapse, lung edema, and alveolar surfactant abnormality [17] resulted in lowered oxygenation and lung compliance in the VAG group. High Ppeak and VT of the VAG group caused alveoli over-distension, which compressed alveoli blood capillaries and reduced capillary blood flow. Thus, dead space ventilation and VD/VT increased in the VAG group and the SHAM group. In the early stage of mechanical ventilation in ARDS rabbits, enhanced respiratory movement kept adequate alveolar ventilation, and caused comparable PCO2 between the two groups.[17]
Interruption of PSR aggravated the lung inflammatory reaction of ARDS, which was characterized by significantly increased concentration of TNF-α and IL-8 in lung tissue. The vagal-cholinergic nerve system took part in the regulation of systemic inflammatory reaction.[18,19,20] Hence we excluded the effect of bilateral vagotomy on elevated cytokines, which interrupted the cholinergic anti-inflammatory pathway.
As a result, EAdi could to some extent reflect the intensity of PSR. If the ventilatory parameters are set up according to PSR, they would be helpful to find the proper ventilatory support for different ARDS patients. This might provide a new way for selecting the proper ventilatory support for ARDS patients.
In conclusion, lung injury in ARDS rabbits was aggravated after bilateral vagotomy, and this indicates that PSR might be protectively effective to the lung.
Footnotes
Funding: This study was supported by the Outstanding Medical Academic Leader Program of Jiangsu Province (2007), Natural Science Foundation of Jiangsu Province grant (Bk2008298), and the Medical Science and Technology Development Foundation of Jiangsu Province (BS2007045).
Ethical approval: The present experiment was approved by the Institutional Animal Use and Care Committee of Southeast University.
Conflicts of interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributors: Wu XY proposed the study, and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Widdicombe J. Reflexes from the lungs and airways: historical perspective. J Appl Physiol. 2006;101:628–634. doi: 10.1152/japplphysiol.00155.2006. [DOI] [PubMed] [Google Scholar]
- 2.Cross BA, Jones PW, Guz A. The role of vagal afferent information during inspiration in determining phrenic motoneurone output. Respir Physiol. 1980;39:149–167. doi: 10.1016/0034-5687(80)90042-0. [DOI] [PubMed] [Google Scholar]
- 3.Malhotra A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med. 2007;357:1113–1120. doi: 10.1056/NEJMct074213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175:160–166. doi: 10.1164/rccm.200607-915OC. [DOI] [PubMed] [Google Scholar]
- 5.Katherine J, Peter C. Mechanical ventilation in ARDS: one size does not fit all. Crit Care Med. 2005;33:1141–1143. doi: 10.1097/01.ccm.0000162384.71993.a3. [DOI] [PubMed] [Google Scholar]
- 6.Greenfield LJ, Singleton RP, McCaffree DR, Coalson JJ. Pulmonary effects of experimental graded aspiration of hydrochloric acid. Ann Surg. 1969;170:74–86. doi: 10.1097/00000658-196907000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, et al. Injurious mechanical ventilation and end-Organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 8.Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, et al. Neural control of mechanical ventilation. Nat Med. 1999;5:1433–1436. doi: 10.1038/71012. [DOI] [PubMed] [Google Scholar]
- 9.Allo JC, Beck JC, Brander L, Brunet F, Slutsky AS, Sinderby CA. Influence of neurally adjusted ventilatory assist and positive end-expiratory pressure on breathing pattern in rabbits with acute lung injury. Crit Care Med. 2006;34:2997–3004. doi: 10.1097/01.CCM.0000242520.50665.9F. [DOI] [PubMed] [Google Scholar]
- 10.Brander L, Leong-Poi H, Beck J, Brunet F, Hutchison SJ, Slutsky AS, et al. Titration and implementation of neurally adjusted ventilatory assist in critically ill patients. Chest. 2009;135:695–703. doi: 10.1378/chest.08-1747. [DOI] [PubMed] [Google Scholar]
- 11.Valenza F, Guglielmi M, Maffioletti M, Tedesco C, Maccagni P, Fossali T, et al. Prone position delays the progression of ventilator-induced lung injury in rats: does lung strain distribution play a role? Crit Care Med. 2005;33:361–367. doi: 10.1097/01.ccm.0000150660.45376.7c. [DOI] [PubMed] [Google Scholar]
- 12.Smith KM, Mrozek JD, Simonton SC, Bing DR, Meyers PA, Connett JE, et al. Prolonged partial liquid ventilation using conventional and high-frequency ventilatory techniques: gas exchange and lung pathology in an animal model of respiratory distress syndrome. Crit Care Med. 1997;25:1888–1897. doi: 10.1097/00003246-199711000-00030. [DOI] [PubMed] [Google Scholar]
- 13.Lin S, Walker J, Xu L, Gozal D, Yu J. Behaviours of pulmonary sensory receptors during development of acute lung injury in the rabbit. Exp Physiol. 2006;92:749–755. doi: 10.1113/expphysiol.2006.036673. [DOI] [PubMed] [Google Scholar]
- 14.Zhang JW, Walker JF, Guardiola J, Yu J. Pulmonary sensory and reflex responses in the mouse. J Appl Physiol. 2006;101:986–992. doi: 10.1152/japplphysiol.00161.2006. [DOI] [PubMed] [Google Scholar]
- 15.Wong KA, Bano A, Rigaux A, Wang B, Bharadwaj B, Schürch S, et al. Pulmonary vagal innervation is required to establish adequate alveolar ventilation in the newborn lamb. J Appl Physiol. 1998;85:849–859. doi: 10.1152/jappl.1998.85.3.849. [DOI] [PubMed] [Google Scholar]
- 16.Berry D, Ikegami M, Jobe A. Respiratory distress and surfactant inhibition following vagotomy in rabbits. J Appl Physiol. 1986;61:1741–1748. doi: 10.1152/jappl.1986.61.5.1741. [DOI] [PubMed] [Google Scholar]
- 17.Lalani S, Remmers JE, Green FH, Bukhari A, Ford GT, Hasan SU. Effects of vagal denervation on cardiorespiratory and behavioral responses in the newborn lamb. J Appl Physiol. 2001;91:2301–2313. doi: 10.1152/jappl.2001.91.5.2301. [DOI] [PubMed] [Google Scholar]
- 18.Thomas R, Bernik MD, Steven G. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 19.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, et al. Pharmacological stimulation of the cholinergic antiinflammotory pathway. J Exp Med. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]


