Abstract
BACKGROUND:
The study aimed to investigate the clinical characteristics of acute renal failure (ARF) caused by oral acyclovir.
METHODS:
A 45-year-old Chinese male patient with acyclovir-induced ARF suffered from abdominal pain for one day. The pain was extended to the epigastric area from the right lower quadrant. Transient oliguria was seen in addition to microscopic hematuria and proteinuria. The serum creatinine concentration was 304 μmol/L. Eight days before the occurrence of ARF, the patient took oral acyclovir for facial neuritis.
RESULTS:
His renal function was restored completely following the discontinuation of acyclovir, with continuous renal replacement therapy for 54 hours and some symptomatic treatment.
CONCLUSION:
The presentation of acute renal failure caused by acyclovir can be diverse, but the prognosis is good after active treatment.
KEY WORDS: Acute renal failure, Acyclovir, oral, Continuous vein-vein hemofiltration
INTRODUCTION
Acute renal failure (ARF) is defined as an abrupt or rapid decline in renal filtration. This condition is usually marked by a rise in serum creatinine concentration or by azotemia [a rise in blood urea nitrogen (BUN) concentration]. A rise in the creatinine level can result from medications (eg, cimetidine, trimethoprim) that inhibit the tubular secretion of the kidney. A rise in the BUN level can occur without renal injury, resulting instead from such sources as GI or mucosal bleeding, steroid use, or protein loading, so a careful inventory must be taken before determining whether renal injury is present. In this study, we reported a patient with ARF caused by oral acyclovir.
Case report
A 45-year-old male patient, weight 60 kg, complained of mild and non-radiating abdominal pain, which started without any obvious cause for one day. The next day, he suffered from more severe abdominal pain, and the pain extended to the waist and the epigastric area from the right lower quadrant. But plain abdominal radiograph and abdominal ultrasound revealed no abnormalities in the urinary tract at a local hospital. And the urine decreased dramatically to 400 mL/24 hours. Then he was transferred to our hospital. At admission, blood and urinary amylase concentrations were 248 U/L and 1487 U/L, respectively. Blood analysis revealed elevated levels of serum creatinine (304 μmol/L) and BUN (12.33 mmol/L). CT revealed a possible slight enlargement of the pancreas. Abdominal ultrasound indicated the presence of a small amount of fluid in the hepatorenal recess and around both kidneys. In addition, his serum creatinine increased to 368 μmol/L and BUN to 15.44 mmol/L. According to these results, this patient was initially diagnosed with severe acute pancreatitis and acute renal failure, and he was admitted to ICU.
After admission to ICU, he received somatostatin to reduce secretion of pancreatin, omeprazole to reduce gastric acid secretion, imipenem with cilastatin (Tienam) to treat infection, and continuous renal replacement therapy (continuous vein-vein hemofiltration, CVVH). At the same time, he had taken acyclovir tablets, 3.5 mg/ kg bodyweight every 12 hours for 8 days for a herpes zoster infection. The latest health examination revealed that his renal function was normal before the use of acyclovir. Abdominal CT on the day of admission to ICU and the third day after treatment showed edema of the right renal fascia and perirenal fat capsule. Repeated abdominal CT scans on the day of admission to ICU and the third day after treatment showed no swelling of the pancreas or fluid around it (Figure 1). Blood and urine amylase concentrations returned to normal (119 U/L and 34 U/L, respectively). Because of insufficient evidence to support the initial diagnosis of severe acute pancreatitis, the patient was finally diagnosed with acute renal failure caused by acyclovir. Therefore, initial treatments such as somatostatin, omeprazole, and cilastatin stopped, but CVVH was continously used for 54 hours.
Figure 1.
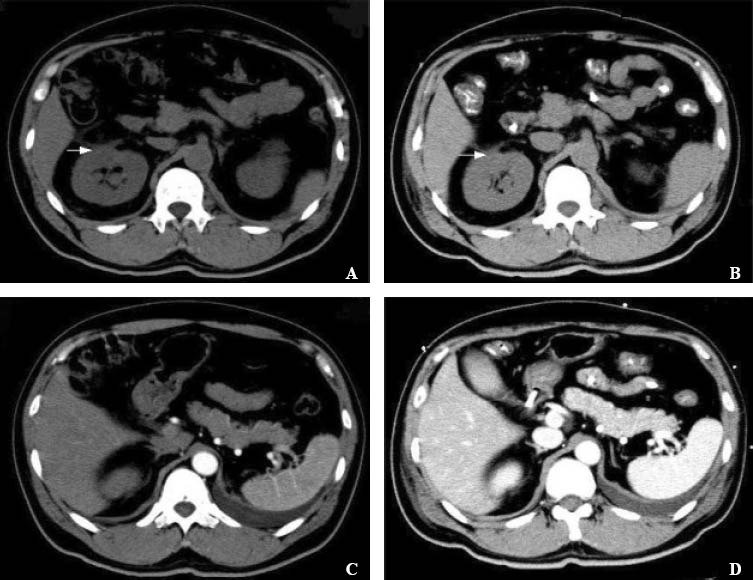
Abdominal CT and repeated abdominal CT scanson. A and B: abdominal CT on the day of admission to ICU (A) and the third day after treatment (B) showed edema of the right renal fascia and perirenal fat capsule; C and D: repeated abdominal CT scans on the day of admission to ICU (C) and the third day after treatment (D) showed no swelling of the pancreas or fluid around it.
During the course of treatment, the serum creatinine concentration decreased from 368 to 69 μmol/L and BUN from 15.44 to 4.14 mmol/L (Figure 2) until the patient was discharged. In addition, microscopic hematuria, leukocyte levels, proteinuria and occult blood were improved.
Figure 2.
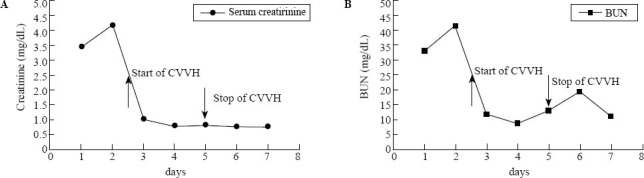
Concentrations of serum creatinine and BUN before and after hemodialysis.
DISCUSSION
In this patient, diagnosis was made of severe acute pancreatitis (SAP) and acute renal failure (ARF) according to his symptoms and signs including abdominal pain, increased levels of blood, urine amylase, serum creatinine and blood urea nitrogen as well as slight swelling of the pancreas on abdominal ultrasonograph. ARF was due to SAP. However, the pain disappeared very soon, blood and urine amylase concentrations returned to normal after 1 day, and abdominal CT showed a normal pancreas without effusion. We thought that the initial diagnosis of SAP and ARF may be wrong. The increased levels of blood and urine amylase may be related to the decrease in metabolic clearance of amylase because of renal failure.[1] Thus we considered that the signs and symptoms of the patient were probably related to the renal impairment. It is known that acyclovir can cause changes of retinol-binding protein and β2-microglobulin, indicating injury to the proximal renal tubule. In our patient, retinol-binding protein and β2-microglobulin were not normalized after treatment, thus the injury to the renal proximal tubule may be related to acyclovir, and the recovery needs more time. Acyclovir-induced ARF may be caused by acute tubular interstitial nephritis.
Acyclovir, a nucleoside, antiviral, macromolecular compound, has oral bioavailability of 15% to 30% and a plasma half-life of about 2.5 hours. It is distributed in various tissues and body fluids, but accumulates especially in the lung and kidney. It is mainly excreted by the kidney, with 60% to 90% by renal tubules and a small amount by glomeruli. Both intravenous and oral acyclovir can induce ARF. Most of the agent is excreted in its original form after injection, and this is barely soluble in urine and therefore tends to be deposited as crystals in the lumens of the distal convoluted renal tubules.[2] In case of oral administration, acyclovir is mainly excreted via glomerular filtration and tubular secretion, and only 14% of the unchanged agent is excreted in the urine. Acyclovir crystal formation in the renal duct can induce blockage of nephrons, edema, bleeding of the renal interstitium, and reduce glomerular filtration rate, thus leading to ARF after a single injection of 50 mg acyclovir into the peritoneum.[3] The pathogenesis of acyclovir-induced ARF is thought to be renal obstruction associated with microcrystal deposition.[4,5] Therefore, microscopic hematuria and proteinuria induced by acyclovir may also be related to renal obstruction.
The risk factors for acyclovir-related ARF include use of high-dose bolus injection,[6] low blood volume, inadequate hydration,[2] elderly patients, high peak plasma levels of the agent,[7] and pre-existing renal insufficiency. The most common risk factor for drug-induced ARF is the increasing age, and this could also be found by the administration of acyclovir.[8] Acyclovir 5-10 mg/kg is given intravenously three times a day, and it should be infused slowly over 1 hour. The dosage of oral acyclovir is 200 mg, four times a day. The maximum daily dosage is 30 mg/kg or 1.5 g/m2. The amount of acyclovir that induces ARF is usually more than 1000 mg/d for adults. The most important and common risk factor for acyclovir-induced ARF is the high-dose bolus injection. However, our patient was only 45-year-old, and a relatively low dose (3.5 mg/kg bodyweight) of acyclovir tablets was taken orally. We conclude that acyclovir-induced ARF is not limited to the elderly population, but likely to occur in younger patients, which is perhaps associated with individual predisposition or immunity.[9]
Renal side-effects of acyclovir have been well documented, and reversible ARF, with or without oliguria, could be found in 12%-48% of the patients who have received this agent.[2,10] The underlying pathogenetic mechanisms of ARF are obstructive nephropathy[6] because of crystallization of the agent into the renal tubules and tubulointerstitial nephritis.[10] Renal biopsy following acyclovir-induced ARF shows interstitial renal edema, eosinophil infiltration in the renal interstitium, and loss of brush borders from the proximal tubules. Epithelial cells of the proximal and distal tubules are flat or lose their nuclei, and some of the tubules become necrotic.[11] In our patient, it was hard to make a definite diagnosis because he refused a kidney biopsy.
In a retrospective analysis of 41 patients treated with intravenous acyclovir, none of the eight patients who developed ARF required dialysis.[12] But continuous renal replacement therapy contributed to the recovery of renal function in our patient, leading to the rapid restoration of serum creatinine and blood urea nitrogen levels. As mentioned above, the prognosis for acyclovir-induced ARF depends on early discovery and timely withdrawal of acyclovir. Monitoring of urine volume, renal function and acyclovir dose may prevent the development of acyclovir-induced ARF. In particular, it is important to calculate the dosage of acyclovir appropriately and to avoid rapid intravenous infusion of high-dose acyclovir in patients who are elderly or have renal impairment.[4] Active treatment, in some cases, continuous renal replacement therapy should be given to those with suspected acyclovir-induced ARF.
Acute renal failure induced by oral acyclovir is uncommon, especially in younger patients and its clinical presentation can be varied. Active treatment, including continuous renal replacement therapy, can restore acute reversible oliguric or non-oliguric acyclovir-induced renal failure.
ACKNOWLEDGMENTS
We thank Elizabeth (Liz) Wagel for revising the manuscript. We also thank the nursing staff of both intensive care and nephrology departments for their major contributions in the daily care of this patient.
Footnotes
Funding: None.
Ethical approval: Not needed.
Conflicts of interest: The authors have no commercial associations or sources of support that might pose a conflict of interest.
Contributors: Meng JB proposed the study and wrote the first draft. All authors contributed the design and interpretation of the e study and to further drafts..
REFERENCES
- 1.Pieper-Bigelow C, Strocchi A, Levitt MD. Where does serum amylase come from and where does it go? Gastroenterol Clin North Am. 1990;19:793–810. [PubMed] [Google Scholar]
- 2.Sawyer MH, Webb DE, Balow JE, Straus SE. Acyclovir induced renal failure. Clinical course and histology. Am J Med. 1988;84:1067–1071. doi: 10.1016/0002-9343(88)90313-0. [DOI] [PubMed] [Google Scholar]
- 3.Perazella MA. Crystal-induced acute renal failure. Am J Med. 1999;106:459–465. doi: 10.1016/s0002-9343(99)00041-8. [DOI] [PubMed] [Google Scholar]
- 4.Izzedine H, Launay-Vacher V, Deray G. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis. 2005;45:804–817. doi: 10.1053/j.ajkd.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Delluc A, Mocquard Y, Latour P, Goas JY. Encephalopathy and acute renal failure during acyclovir treatment. Rev Neurol (Paris) 2004;160:704–706. doi: 10.1016/s0035-3787(04)71022-x. [DOI] [PubMed] [Google Scholar]
- 6.Tucker WE, Jr, Macklin AW, Szot RJ, Johnston RE, Elion GB, de Miranda P, et al. Preclinical toxicology studies with acyclovir: acute and subchronic test. Fundam Appl Toxicol. 1983;3:573–578. doi: 10.1016/s0272-0590(83)80107-9. [DOI] [PubMed] [Google Scholar]
- 7.Brigden B, Rosling AE, Woods NC. Renal function after acyclovir intravenous injection. Am J Med. 1982;73:182–185. doi: 10.1016/0002-9343(82)90087-0. [DOI] [PubMed] [Google Scholar]
- 8.Naughton CA. Drug-induced nephrotoxicity. Am Fam Physician. 2008;78:743–750. [PubMed] [Google Scholar]
- 9.Giustina A, Romanelli G, Cimino A, Brunori G. Low dose acyclovir and acute renal failure. Ann Intern Med. 1988;108:312. doi: 10.7326/0003-4819-108-2-312_1. [DOI] [PubMed] [Google Scholar]
- 10.Rashed A, Azadeh B, Abu Romeh SH. Acyclovir-induced acute tubulo-interstitial nephritis. Nephron. 1990;56:436–438. doi: 10.1159/000186190. [DOI] [PubMed] [Google Scholar]
- 11.Becker BN, Fall P, Hall C, Milam D, Leonard J, Glick A, et al. Rapidly progressive acute renal failure due to acyclovir: case report and review of the literature. Am J Kidney Dis. 1993;22:611–615. doi: 10.1016/s0272-6386(12)80939-5. [DOI] [PubMed] [Google Scholar]
- 12.Pacheco LR, Tavares HM, Moysés Neto M, Dantas M, Rocha LS, Ribeiro KM, et al. Acute renal failure related to intravenous acyclovir. Rev Assoc Med Bras. 2005;51:275–278. doi: 10.1590/s0104-42302005000500019. [DOI] [PubMed] [Google Scholar]


