Abstract
BACKGROUND:
Infection-induced thrombocytopenia (TCP) is an independent risk factor for death of patients with sepsis, but its mechanism is unknown. This study aimed to explore the underlying mechanism of TCP based on the relationship between TLR4 expression and platelet activation in septic patients.
METHODS:
A total of 64 patients with sepsis were prospectively studied. Platelet count (PC), mean platelet volume (MPV), platelet distribution width (PDW), platelet TLR4 expression, platelet PAC-1 expression, sCD40L and TNF-α concentrations were compared between the healthy control group (15 volunteers) and sepsis group (64 patients) at admission and on the 3, 5, and 9 days after admission. The changes of MPV and PDW in the TCP and non-TCP subgroups of sepsis before and after treatment were recorded. Prognostic index was analyzed.
RESULTS:
PC was lower in the sepsis group (P=0.006), and MPV and PDW were higher in the sepsis group than those in the healthy control group (P=0.046, P=0.001). Platelet TLR4 and PAC-1 expressions, and sCD40L and TNF-α levels increased more significantly in the sepsis group (P<0.001). PAC-1 expression and TNF-α level were higher in the TCP group than in the non-TCP group before and after treatment (P=0.023, P=0.011). sCD40L concentration and platelet TLR4 expression were significantly higher in the treated TCP group than in the non-TCP group (P=0.047, P=0.001). Compared to the non-TCP group, the rate of bleeding was higher (P=0.024) and the length of ICU stay was longer (P=0.013). The APACHE II score and the 28-day mortality were higher in the TCP group (P<0.01, P=0.048).
CONCLUSIONS:
The elevation of platelet TLR4 expression in sepsis along with platelet activation is closely related to the incidence of thrombocytopenia. The occurrence of TCP is a sign of poor prognosis in sepsis patients.
KEY WORDS: Sepsis, Thrombocytopenia, Toll-like receptor, Platelet activation, Glycoprotein IIb/IIIa, Soluble CD40 ligand, β-Thromboglobulin, Tumor necrosis factor-α, Interleukin-8
INTRODUCTION
Infection-induced thrombocytopenia (TCP) is an independent risk factor for death of patients with sepsis. Studies have shown that 35%-59% of septic patients may develop TCP, and that the progressive decline of platelet count in critically ill patients often indicates a serious condition.[1-4] If the mechanism of TCP is clarified, it will be helpful to improve the prognosis of septic patients. This
study aimed to explore the underlying mechanism of TCP based on the relationship between TLR4 expression and platelet activation in septic patients.
METHODS
Patients
In this prospective, controlled clinical study, a total of 64 septic patients, 43 males and 21 females, were enrolled. They were at age of (62.1±10.9) years, with an APACHE II score of 17.96±8.26. The patients were admitted to ICU of the First Center Hospitial, Tianjin, China, from May 2008 to December 2009. Fifteen healthy volunteers, 10 males and 5 females, aged (58.3±14.2) years, served as a control group. Microbial culture was positive in 43 patients, Gram-negative (G-) bacilli in 26, Gram-positive (G+) bacteria in 9, and mixed infection in 8.
Diagnosis and treatment
The diagnosis of sepsis was dependent on the Diagnostic Criteria for Sepsis set at the International Conference on Sepsis Definition in 2001.[5] If the patients met the criteria and were admitted to ICU more than once during the observation period, they were enrolled one time at the first admission to ICU. The exclusion criteria included cancer; immune system diseases; seriously injured hematopoietic system, serious injury to and malignant tumors of the hematopoietic system; age <14 years or >75 years; post cardiopulmonary resuscitation; end-stage of the liver or renal failure; death within 24 hours after admission. Treatment was given according to the Guide for Sepsis Treatment issued at Saving Sepsis Campaign Conference in 2003.[6]
Parameters
Peripheral blood was collected at admission, and at 6:00 am on the 3rd, 5th and 9th day after admission. The parameters measured were as follows: platelet count (PC), mean platelet volume (MPV), platelet distribution width (PDW), platelet TLR4-positive expression rate, platelet PAC-1 positive expression rate, plasma sCD40L, and TNF-α concentration. At the same time, APACHE II score, ICU length of stay, bleeding events and 28-day mortality were recorded.
Groups
The study group comprised 64 septic patients with and 15 healthy volunteers served as controls. The septic patients were subdivided into two groups: TCP group, 27 patients, platelet count less than 150×109/L at least for one time;[7] and non-TCP group, 37 patients. We compared the parameters selected at admission and on the 9th day after admission between the sepsis group and the control group and between the TCP group and the non-TCP group before and after treatment.
Methods of detection
Flow cytometry was used to determine the positive rates of TLR4 and PAC-1 in platelet surface: FITC PAC-1/PAC-1 + RGDS, PE TLR 4/IgG2a, Percp CD61 (Becton, Dickinson and Company, USA). sCD40L, TNF-α concentrations in serum were measured with ELISA.
Statistical analysis
Statistical analyses were performed with SPSS 11.5, and the data were expressed as mean ± standard deviation. The data of time-based measurements between the groups were compared using one-way ANOVA. Student's t test was used for comparison of parameters between the sepsis group and the healthy control group, and between the TCP group and the non-TCP group. P<0.05 was considered statistically significant.
RESULTS
Platelet parameters Platelet count
At admission, PC in the sepsis group was significantly lower than that in the control group (P=0.006). After the treatment, PC in the sepsis group gradually increased, and at admission, PC on the 9th day was significantly higher (P=0.043), but it was still lower than that in the control group (P=0.031). During the period of observation, PC decreased to less than 150×109/L at least one time in 27 patients and the occurrence rate of TCP was 42.19% (Table 1).
Table 1.
Platelet parameters in the healthy control group and the sepsis group (mean ±SD)

Mean platelet volume (MPV)
At admission, MPV in the sepsis group was significantly higher than that in the control group (P=0.046). After the treatment, MPV in the sepsis group gradually declined. On the 3rd day, no significance was observed between the two groups (P=0.073). At admission, MPV in the 27 patients with TCP was significantly higher than that in the patients with non-TCP (11.88±1.27 fL vs. 10.17±0.89 fL, P<0.01) (Table 1).
Platelet distribution width (PDW)
At admission, PDW was increased more significantly in the sepsis group than in the control group (P<0.001). After the treatment, PDW in the sepsis group gradually decreased and on the 3rd day it was increased compared to the control group. On the 5th day no significance was observed between the two groups (P=0.059). PDW on the 9th day was significantly decreased (P=0.004) before the treatment. At admission, PDW was significantly increased in the 27 patients with TCP compared with those with non-TCP (18.97±1.17 fL vs. 17.42±1.69 fL, P=0.04) (Table 1).
Correlation analysis
MPV was moderately negatively correlated with PC (r =-0.517, P=0.007), and PDW highly negatively correlated with PC (r=-0.702, P=0.008) in sepsis patients.
Comparison of parameters between the sepsis group and control group
The positive rates of platelet TLR4 and PAC-1 in the sepsis group were higher than those in the control group at admission (P<0.001), but they were significantly lower on the 9th day after treatment than those at admission (P<0.001). TLR4 in the sepsis group was still significantly higher than that in the control group on the 5th day (P0-3<0.001, P5=0.003), but there was no difference on the 9th day between the two groups (P=0.052). PAC-1 in the sepsis group was higher than that in the control group during the whole observation period (P=0.042). The levels of sCD40L and TNF-α in the sepsis group was higher than those in the control group at admission (P<0.001). sCD40L was decreased more significantly on the 9th day than at admission (P<0.001), but it was still higher than that in the control group (P=0.013). TNF-α was significantly higher than that in the control group on the 9th day (P<0.001), but it was significantly lower than that at admission (P<0.01) (Table 2).
Table 2.
Platelet TLR4, PAC-1 expression, sCD40L and TNF-α concentrations in the control group and the sepsis group (mean ±SD)
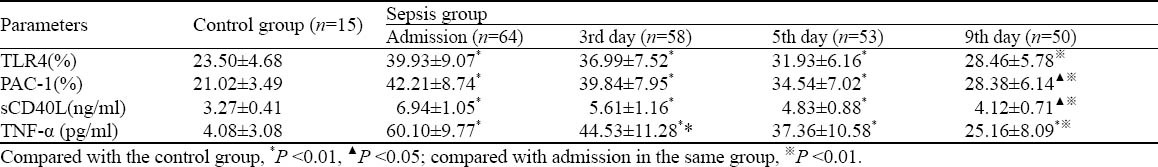
PAC-1 was moderately negatively correlated with PC (r=-0.409, P<0.001) and moderately positively correlated with PDW (r=0.318, P<0.001), platelet TLR4 expression (r=0.341, P<0.001), and sCD40L (r=0.519, P<0.001), whereas sCD40L was moderately positively correlated with TNF-α (r=0.542, P<0.001) in sepsis patients.
Comparison of parameters between the TCP group and non-TCP group
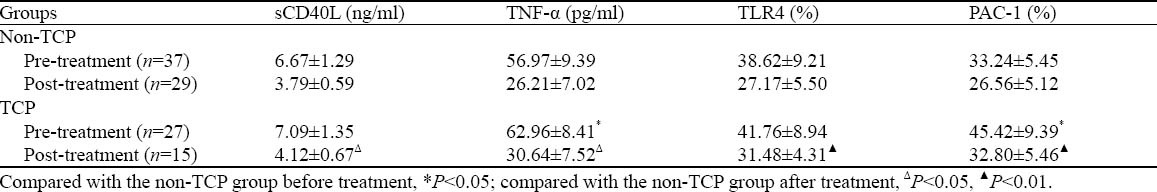
The incidence of TCP was 42.19% throughout the observation period. No statistical difference was observed in sCD40L between the TCP group and the non-TCP group before treatment (P=0.201). The level of sCD40L decreased after treatment in both groups, but the level of sCD40L was higher in the TCP group than in the non-TCP group (P=0.047). sCD40L was moderately negatively correlated with PC in the TCP group (r=-0.343, P=0.001). Compared with the non-TCP group, the TCP group had a higher level of TNF-α before and after treatment (Ppre=0.011, Pafter=0.018). Before treatment, there was no statistical difference in the pre-treatment platelet TLR4 expression between the TCP group and the non-TCP group (P=0.177); after treatment, the platelet TLR4 expression declined in both groups, but it was significantly higher in the TCP group than in the non-TCP group (P=0.001). PAC-1 expression in the TCP group was higher than that in the non-TCP group before and after treatment (Ppre=0.023, Pgfter<0.001) (Table 3).
Table 3.
Platelet TLR4, PAC-1 expression, and concentrations of sCD40L and TNF-α in the TCP and non-TCP groups (mean±SD)
Comparison of prognosis between the TCP group and the non-TCP group
Compared with the non-TCP group, the TCP group had a shorter ICU stay (7.11±2.26 days vs. 9.31±2.48 days, P=0.013), a lower bleeding rate (10.81% vs. 48.15%, P=0.024), a higher APACHE II score (15.37±7.47 vs. 22.66±8.13 points, P<0.01) at admission, and a higher 28-day mortality (44.44% vs. 21.62%, P=0.048).
DISCUSSION
Platelet count (PC) can accurately and sensitively predict the condition and prognosis of critically ill patients.[8] In this study, lower PC, higher MPV, and increased PDW were noted in the sepsis group compared with the control group. Correlation analysis showed that PDW and MPV could respond to the changes of platelet function better in patients with sepsis.
In patients with sepsis, increased destruction and consumption of platelets are the main causes of thrombocytopenia.133 sCD40L is a serum marker of platelet activation in vivo, and about 95% of circular sCD40L comes from platelets.[9,10] PAC-1 is the exposed fibrinogen binding site after the activation of GPIIb, and it is an early membrane marker of platelet activation.[11,12] Toll-like receptor is considered the first defence against the invasion of pathogenic microbials.[13] Platelets can also express TLR;[14] however, there are many controversies over the biological functions of platelet TLR4 receptor.
In this study, platelet TLR4 and PAC-1 expressions increased in patients with sepsis at admission. This finding indicates that TLR4 expression increased adaptively to sepsis, and there was a correlation between the high expression of platelet TLR4 and platelet activation. The TLR4 expression in this study was different from the in vitro result reported by Cognasse et al[15,16], i.e. after stimulation with LPS in vitro, the platelet TLR4-positive rate decreased significantly. Thus in the in vitro experiment, the platelets were concentrated solution of the separated-purified des-white platelets, and after stimulation with LPS without the influence of other immune cells, such platelets were unable to increase the expression of TLR4 alone.
The levels of sCD40L and TNF-α increased significantly in septic patients and they were positively correlated. It has been proved that inflammatory response and platelet activation interact with each other, and they participate in the inflammatory response and contribute to the pathogenesis of TCP.
GPIIb/IIIa expression, plasma levels of TNF-α in TCP patients and non-TCP patients in the present study suggested that platelet activation and inflammation in the TCP patients were higher than those in the non-TCP patients. Although there was no significant difference in the pre-treatment plasma sCD40L and platelet TLR4- positive rate between the TCP and non-TCP groups, sCD40L and platelet TLR4-positive rate in the TCP group were higher than those in the non-TCP group after treatment. They were possibly due to destroyed coagulation system, reduced platelet count, and inhibited up-regulation of platelets after stimulation of endotoxin or, in some TCP patients, to the low sCD40L level in
circulation caused by decreased PC. With the increase of PC and the activation of newborn platelets after treatment, TLR4 expression in platelets and sCD40L level were increased, or they were higher than those in the non-TCP group. Compared with the non-TCP group, the TCP group had a higher incidence of hemorrhage, a longer ICU stay and a higher 28-day mortality. Thrombocytopenia may imply the serious condition of septic patients and predict their poor prognosis.
Footnotes
Funding: None.
Ethical approval: Not needed.
Conflicts of interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributors: Wang YQ designed the research, analyzed the data, and wrote the first draft. All authors contributed to the interpretation of the study and to further drafts.
REFERENCES
- 1.Levi M, Lowenberg EC. Thrombocytopenia in critically ill patients. Seminars in Thrombosis & Hemostasis. 2008;34:417–424. doi: 10.1055/s-0028-1092871. [DOI] [PubMed] [Google Scholar]
- 2.Vandijck DM, Blot SI, De Waele JJ, Hoste EA, Vandewoude KH, Decruyenaere JM. Thrombocytopenia and outcome in critically ill patients with bloodstream infection. Heart Lung. 2010;39:2126. doi: 10.1016/j.hrtlng.2009.07.005. Epub 2009 Oct 15. [DOI] [PubMed] [Google Scholar]
- 3.Guo YL, Liu DQ, Bian Z, Zhang CY, Zen K. Down-regulation of platelet surface CD47 expression in Escherichia coli O157:H7 infection-induced thrombocytopenia. PLoS One. 2009;4:e7131. doi: 10.1371/journal.pone.0007131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference 1. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 5.Torgersen C, Dünser MW, Schmittinger CA, Pettilä V, Ruokonen E, Wenzel V, et al. Current approach to the haemodynamic management of septic shock patients in European intensive care units: a cross-sectional, self-reported questionnaire-based survey. Eur J Anaesthesiol. 2010 Nov 17; doi: 10.1097/EJA.0b013e3283405062. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, Hahn EG. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002;30:1765–1771. doi: 10.1097/00003246-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 8.Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, Hahn EG. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002;30:1765–1771. doi: 10.1097/00003246-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Inwald DP, Faust SN, Lister P, Peters MJ, Levin M, Heyderman R, et al. Platelet and soluble CD40L in meningococcal sepsis. Intensive Care Med. 2006;32:1432–1437. doi: 10.1007/s00134-006-0250-2. Epub 2006 Jun 30. [DOI] [PubMed] [Google Scholar]
- 10.Vanderschueren S, De Weerdt A, Malbrain M, Vankersschaever D, Frans E, Wilmer A, et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med. 2000;28:1871–1876. doi: 10.1097/00003246-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Nijsten MW, ten Duis HJ, Zijlstra JG, Porte RJ, Zwaveling JH, Paling JC, et al. Blunted rise in platelet count in critically ill patients is associated with worse outcome. Crit Care Med. 2000;28:3843–3846. doi: 10.1097/00003246-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Stéphan F, Hollande J, Richard O, Cheffi A, Maier-Redelsperger M, Flahault A. Thrombocytopenia in a surgical ICU. Chest. 1999;115:1363–1370. doi: 10.1378/chest.115.5.1363. [DOI] [PubMed] [Google Scholar]
- 13.Von Hundelshausen P, Weber C. Platelets as immune cells bridging inflammation and cardiovascular disease. Circulation Research. 2007;5:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 14.McEver RP. P-selectin/PSGL-1 and other interactions between platelets, leukocytes, and endothelium. In: Michelson AD, editor. Platelet. New York: Academic/Elsevier Science; 2002. pp. 139–155. [Google Scholar]
- 15.Ogura H, Kawasaki T, Tanaka H, Koh T, Tanaka R, Ozeki Y, et al. Activated platelets enhance microparticle formation and platelet-leukocyte interaction in severe trauma and sepsis. J Trauma. 2001;50:801–809. doi: 10.1097/00005373-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 16.André P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: The switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–899. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 17.Inwald DP, Faust SN, Lister P, Peters MJ, Levin M, Heyderman R, et al. Platelet and soluble CD40L in meningococcal sepsis. Intensive Care Med. 2006;32:1432–1437. doi: 10.1007/s00134-006-0250-2. Epub 2006 Jun 30. [DOI] [PubMed] [Google Scholar]
- 18.Furman MI, Krueger LA, Linden MD, Barnard MR, Frelinger AL, 3rd, Michelson AD. Release of soluble CD40L from platelets is regulated by glycoprotein IIb/IIIa and actin polymerization. J Am Coil Cardiol. 2004;43:2319–2325. doi: 10.1016/j.jacc.2003.12.055. [DOI] [PubMed] [Google Scholar]
- 19.Larkin D, Murphy D, Reilly DF, Cahill M, Sattler E, Harriott P, et al. ICln, a novel integrin alphaIIbbeta3-associated protein, functionally regulates platelet activation. J Biol Chem. 2004;279:272–286. doi: 10.1074/jbc.M402159200. [DOI] [PubMed] [Google Scholar]
- 20.Bennett JS, Berger BW, Billings PC. The structure and function of platelet integrins. Thromb Haemost. 2009;7:200–205. doi: 10.1111/j.1538-7836.2009.03378.x. [DOI] [PubMed] [Google Scholar]
- 21.Mitsios JV, Tambaki AP, Abatzis M, Biris N, Sakarellos-Daitsiotis M, Sakarellos C, et al. Effect of synthetic peptides corresponding to residues 313-332 of the alphaIIb subunit on platelet activation and fibrinogen binding to alphaIIbbeta3. Eur J Biochem. 2004;271:855–862. doi: 10.1111/j.1432-1033.2004.03990.x. [DOI] [PubMed] [Google Scholar]
- 22.Mangiafico S, Cellerini M, Nencini P, Gensini G, Inzitari D. Intravenous glycoproteinGPIIb/IIIa inhibitor (tirofiban)followed by intra-arterial urokinase and mechanical thrombolysis in stroke CJ3. Am J Neuroradiol. 2005;26:2595–2601. [PMC free article] [PubMed] [Google Scholar]
- 23.McGuire K, Jones M, Werling D, Williams JL, Glass EJ, Jann O. Radiation hybrid mapping of all 10 characterized bovine Toll-like receptors. Anim Genet. 2006;37:47–50. doi: 10.1111/j.1365-2052.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein DR. Toll-like receptors and other links between innate and acquired alloimmunity. Curr Opin Immunol. 2004;16:538–544. doi: 10.1016/j.coi.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol. 2001;166:1148–1155. doi: 10.4049/jimmunol.166.2.1148. [DOI] [PubMed] [Google Scholar]
- 26.Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, et al. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 27.Cognasse F, Hamzeh H, Chavarin P. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Bio. 2005;83:196–198. doi: 10.1111/j.1440-1711.2005.01314.x. [DOI] [PubMed] [Google Scholar]
- 28.Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, et al. Platelet-mediated modulation of adaptive immunity A communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Ebihara I, Shoji H, Ushiyama C, Suzuki S, Koide H. Treatment with polymyxin B-immobilized fiber reduces platelet activation in septic shock patients: decrease in plasma levels of soluble P-selectin, platelet factor 4 and beta-thromboglobulin. Inflamm Res. 1999;48:171–175. doi: 10.1007/s000110050442. [DOI] [PubMed] [Google Scholar]
- 30.Montrucchio G, Bosco O, Del Sorbo L, Fascio Pecetto P, Lupia E, Goffi A, et al. Mechanisms of the priming effect of low doses of lipopoly-saccharides on leukocyte-dependent platelet aggregation in whole blood. Thromb Haemost. 2003;90:872–881. doi: 10.1160/TH03-02-0085. [DOI] [PubMed] [Google Scholar]
- 31.Ward JR, Bingle L, Judge HM, Brown SB, Storey RF, Whyte MK, et al. Agonists of Toll-like receptor (TLR)2 and TLR4 are unable tomodulate platelet activation by adenosine diphosphate and platelet activation factor. Thromb Haemost. 2005;94:831–834. [PubMed] [Google Scholar]
- 32.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Delezay O, Pozzetto B, McNicol A, et al. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol. 2008;141:84–91. doi: 10.1111/j.1365-2141.2008.06999.x. [DOI] [PubMed] [Google Scholar]
- 33.Buduneli N, Ozçaka O, Nalbantsoy A. Salivary and Plasma Levels of Toll-like Receptor 2 and Toll-Like Receptor 4 in Chronic Periodontitis. J Periodontol. 2010 Dec 7; doi: 10.1902/jop.2010.100467. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Yang FL, Li CH, Hsu BG, Tsai NM, Lin SZ, Harn HJ, et al. The reduction of tumor necrosis factor-alpha release and tissue damage by pentobarbital in the experimental endotoxemia model. Shock. 2007;28:309–316. doi: 10.1097/SHK.0b013e31803dd04d. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto N, Kawabe T, Imaizumi K, Hara T, Okamoto M, Kojima K, et al. CD40 plays a crucial role in lipopolysaccharide induced acute lung injury. Am J Respir Cell Mol Biol. 2004;30:808. doi: 10.1165/rcmb.2003-0197OC. [DOI] [PubMed] [Google Scholar]


