Abstract
BACKGROUND:
Ventilator induced lung injury (VILI) is a serious complication in the treatment of mechanical ventilating patients, and it is also the main cause that results in exacerbation or death of patients. In this study, we produced VILI models by using glucocorticoid in rats with high tidal volume mechanical ventilation, and observed the content of macrophage inflammatory protein-1α (MIP-1α) in plasma and bronchoalveolar lavage fluid (BALF) and the expression of MIP-1α mRNA and nuclear factor-kappa B (NF-κB) p65 mRNA in the lung so as to explore the role of glucocorticoid in mechanical ventilation.
METHODS:
Thirty-two healthy Wistar rats were randomly divided into a control group, a ventilator induced lung injury (VILI) group, a dexamethasone (DEX) group and a budesonide (BUD) group. The content of MIP-1α in plasma and BALF was measured with ELISA and the level of MIP-1α mRNA and NF-κBp65 mRNA expressing in the lung of rats were detected by RT-PCR. The data were expressed as mean±SD and were compared between the groups.
RESULTS:
The content of MIP-1α in plasma and BALF and the level of MIP-1α mRNA and NF-KBp65 mRNA in the lung in the DEX and BUD groups were significantly lower than those in the VILI group (P<0.001). Although the content of MIP-1α in plasma and BALF and the level of MIP-1α mRNA and NF-κBp65 mRNA in the lung in the BUD group were higher than those in the DEX group, there were no significant differences between them (P>0.05).
CONCLUSIONS:
Glucocorticoid could down-regulate the expression of MIP-1α by inhibiting the activity of NF-κB in the lung and may exert preventive and therapeutic effects on VILI to some extent. The effect of local use of glucocorticoid against VILI is similar to that of systemic use, but there is lesser adverse reaction.
KEY WORDS: Mechanical ventilation, Lung injury, Macrophage inflammatory protein-1α, Nuclear factor-kappa B, Glucocorticoid, Inflammation
INTRODUCTION
Ventilator induced lung injury (VILI) is a serious complication in the treatment of mechanical ventilating patients, and it is also the main cause resulting in exacerbation or death of patients.™ Richard et al[2] reported that inflammatory reaction played an important role in the occurrence and development of VILI. In this study we produced VILI models by using glucocorticoid in rats with high tidal volume mechanical ventilation and observed the content of macrophage inflammatory protein -1α (MIP-Ia) in plasma and bronchoalveolar lavage fluid (BALF) and the expression of MIP-Ia mRNA and nuclear factor-kappa B (NF-κB) p65 mRNA in the lung so as to explore the role of glucocorticoid in mechanical ventilation.
METHODS
Groups and treatment
Thirty-two healthy male wistar rats, weighing 300 ± 20 g, were bought from the Animal Experiments Center of Shanxi Medical University. They were randomly divided into four groups: control group without mechanical ventilation but given an intraperitoneal injection of physiological saline at a dose of 0.1 mL/kg per day for 7 days; VILI group given an intraperitoneal injection of physiological saline at a dose of 0.1 mL/kg per day and ventilation for 7 days; DEX group given an intraperitoneal injection of dexamethasone at a dose of 0.1 mL/kg per day and ventilation for 7 days;[3] BUD group placed in the Plexiglas box (40 cm×30 cm×20 cm), given aerosol inhalation of budesonide (1 mg budesonide dissolved in 5 mL physiological saline), two times per day, 20 minutes one time with a 980 type ultrasonic atomizer (Shanghai Medicine Professional Mechanical Factory), and then ventilated in 7 days later.[4]
Sample collection and preparation
The rats were anesthetized by an intraperitoneal injection of 25% urethan (4 mL/kg), and then tracheotomized. Except the rats in the control group, those received mechanical ventilation by connection with respirator, and the ventilation parameters were set at respiratory rate 60/min, inspiration/expiration 1/3, tidal volume 40 mL/kg,[5] oxygen concentration 21%, ventilation time four hours. After the ventilation, 2-3 mL blood was collected from the abdominal aorta, anti-coagulated at 3000 r/min for 10 minutes, and kept in an EP tube for freeze-storage. Then the rats were killed, and upper-middle lung tissue was removed from the right lung, fixed in formalin, routinely embedded, and stained with HE. Lung injury was observed under a light microscope. The lower lung tissue was removed from the right lung, and put into an EP tube. Bronchoalveolar lavage fluid was collected from the left lung (3 mL×4), anti-coagulated at 3500 r/min for 10 minutes, and kept in an EP tube for freeze-storage.
Measurement of MIP-1α in plasma and BALF by ELISA
The content of MIP-1α in plasma and BALF was measured with ELISA according to the instructions of the kit, which was purchased from Nanjing Jiancheng Bio-engineering Institute.
Measurement of MIP-1α mRNA and NF-κB p65 mRNA in the lung by RT-PCR
The lung tissue with wet weight 0.2 g was collected, and sample error was less than ±0.02 g. The total RNA was extracted by Trizol, then reversely transcribed into cDNA. The upstream and downstream fragments of MIP-1α (synthetized by Beijing Aoke Bio-technology Company) were 5’-GAGCTGGAACTAAATGCCTGA-3’, 5’-TCACCAAACACAGTGTGAGCA-3’ respectively, and the amplified fragment length was 326 bp. The upstream and downstream fragments of NF-κBp65 (synthetized by Beijing Aoke Biotechnology Company) were 5’-GACCTGGCATCTGTGGACAAC-3’, 5’-TCCGCAATGGAGGAGAAGTCT-3’ respectively, and the amplified fragment length was 221 bp. The upstream and downstream fragments of β-actin (synthetized by Shanghai CZECH Gene Technology Limited Company) were 5’-GATGGTGGGTATGGGTCAGAAGGAC-3’, 5’-GCTCATTGCCGATAGTGATGACCT-3’ respectively, and the amplified fragment length was 630 bp. Thirty cycles were used at 95 °C for 2 minutes, 94 °C for 50 seconds, 58 °C for 50 seconds, 72 °C for 30 seconds, and 72 °C for 10 minutes; twenty cycles were used at 63 °C in β-actin. After PCR reaction, the relative mRNA expression was scanned by the GIS-1000 image analysis system from Shanghai Tannon Technology Limited Corporation. PCR product relative expression intensity = aiming product grayscale value / β-actin grayscale value.
Statistical analysis
All data were expressed as mean±standard deviation. The data were compared using SNK’s method after the variance equality test. P<0.05 was considered statistically significant. The data were processed by SPSS11.0.
RESULTS
Pathological changes of lung tissues
Under a light microscope, alveoli didn’t show any inflammatory cell infiltrates in the control group, and the alveolar interval was normal. While in the rats of the VILI group, diffuse pulmonary interstitial edema and inflammatory cell infiltration were found, part of alveoli was ruptured and mixed together, and bleeding appeared in the alveolar space. The rats in the DEX and BUD groups had slightly inflammatory cell infiltration and mild alveolar interstitial edema (Figure 1).
Figure 1.
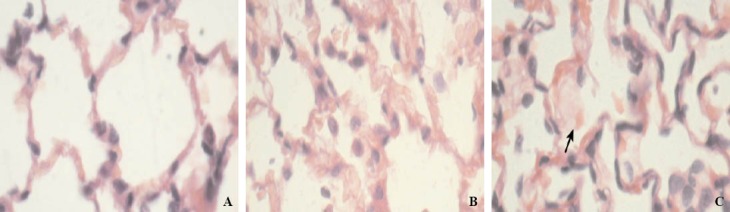
Lung tissue (HE original magnification ×400). A: in the control group, the alveolar interval was normal, and there was no inflammatory cell; B: in the BUD group, slightly inflammatory cell infiltration and mild alveolar interstitial edema were found; C: in the VILI group, part of alveoli were ruptured, and there were diffuse pulmonary interstitial edema and inflammatory cell infiltration.
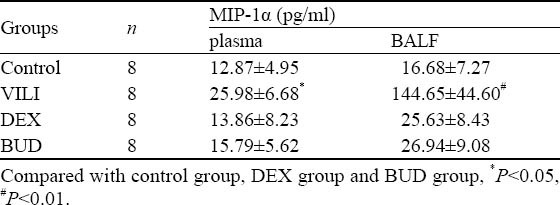
Content of MIP-1α in plasma and BALF
The content of MIP-1α in plasma and BALF in the VILI group was higher than that in the control group, DEX group and BUD group (P<0.05, respectively). Although the content of MIP-1α in plasma and BALF in the BUD group was higher than that of the DEX group, there were no significant differences between them (P>0.05) (Table 1).
Table 1.
Content of MIP-1α in plasma and BALF of rats in different groups (mean±SD)
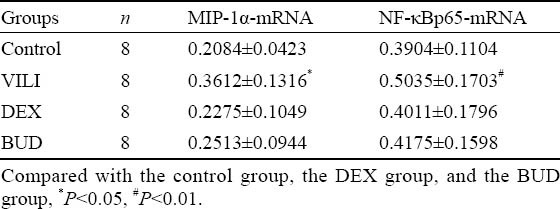
Expression of MIP-1α mRNA and NF-κB p65 mRNA in the lung
In the VILI group, the expression of MIP-1α-mRNA and NF-κBp65-mRNA was higher than that in the control group. The expression of MIP-1α-mRNA and NF-κBp65-mRNA was higher in the BUD group than in the DEX group, but there was no statistical significance between the two groups (Table 2).
Table 2.
Expression of MIP-1α-mRNA and NF-κBp65-mRNA in the lung of rats in different groups (mean±SD)
DISCUSSION
Mechanical ventilation is an important method for treating respiratory failure, and VILI is its major complication with an incidence rate of 15%.[6] Except mechanical injury such as barotrauma, volutrauma and atelectasis, VILI can also induce inflammatory cell activation and produce lots of inflammatory mediators and cytokines, leading to further injury to the lung tissue.[7,8] There are many inflammatory cells and cytokines involved in the generating VILI, and their relationship is complex. Previously we found that MIP-1α and NF-κB played an important role in the pathogenesis of VILI. Mechanical ventilation can cause inflammatory cells to release MIP-1α by activating NF-κB,[9] which causes inflammatory injury to the lung.[10] Therefore it might be a new direction for preventing VILI to interrupt NF-κB activation and decrease MIP -1α releasing.
Animal experiments showed that dexamethasone inhibited NF-κB activation in the lung of ventilated rats with a high tidal volume, and the expression of inflammatory cytokines in BALF such as TNF-α, IL-6, and MIP-1α was significantly reduced. This indicated that glucocorticoid exerted preventive effect on VILI.[11] However, it was controversial over systemic use of glucocorticoid in ALI/ ARDS because there were many adverse reactions, which might inhibit immuno-functions and cause secondary serious infection.
In recent years, local inhaling glucocorticoid has attracted great attention. Wang et al[12] reported that treatment of chlorine gas lung injury with nebulized budesonide or intravenous betamethasone had similar positive effects on recovery of lung function. Jansson et al[13] reported that pretreatment with budesonide totally prevented the formation of lung edema at the low concentration of LPS and had partial effects on acute lung injury and leukocyte influx at the high concentration. In our study the content of MIP-1α in plasma and BALF and the expression of MIP-1α mRNA and NF-κB p65 mRNA were lower than those in the DEX group compared with the VILI group. The result was similar to Held’s result. This indicated that glucocorticoid can control the inflammatory reaction in mechanical ventilation of VILI rats. The results of HE staining showed that there were only some inflammatory cells infiltrating in the DEX and BUD groups, and alveolar interstitial edema was significantly less in these groups than in the VILI group. Dexamethasone may restrain an activation of NF-κB and reduce the expression of MIP-1α in the lung of rats. We also found that the content of MIP-1α in plasma and BALF and the expression of MIP-1α mRNA and NF-κB p65 mRNA were lower in the BUD group than those of the DEX group, but there was no statistical significance between the two groups. There was a similar protective function between the BUD group (aerosolization) and DEX group (abdominal injection). Hence inhalation of moderate glucocorticoid may control non-specific inflammatory reaction of mechanical ventilation in lung tissue, and have preventive and therapeutic effects on VILI.
Footnotes
Funding: None.
Ethical approval: Not needed.
Conflicts of interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributors: Liu ZH proposed the study, collected and analyzed the data, and wrote the first draft. All authors contributed to the intellectual context and approved the fi nal version.
REFERENCES
- 1.Dreyfuss D, Soler P, Saumon G. Mechanical ventilation-induced pulmonary edema. Interaction with previous lung alterations. Am J Respir Crit Care Med. 1995;151:1568–1575. doi: 10.1164/ajrccm.151.5.7735616. [DOI] [PubMed] [Google Scholar]
- 2.Richard JD, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Eur Respir J Suppl. 2003;42(Suppl):2–9. doi: 10.1183/09031936.03.00420103. [DOI] [PubMed] [Google Scholar]
- 3.Balsley MA, Malesevic M, Stemmy EJ, Gigley J, Jurjus RA, Herzog D, et al. A cell-impermeable cyclosporine A derivative reduces pathology in a mouse model of allergic lung inflammation. J Immunol. 2010;185:7663–7670. doi: 10.4049/jimmunol.1001707. Epub 2010 Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parmar JJ, Singh DJ, Hegde DD, Lohade AA, Soni PS, Samad A, et al. Development and evaluation of inhalational liposomal system of budesonide for better management of asthma. Indian J Pharm Sci. 2010;72:442–448. doi: 10.4103/0250-474X.73916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang XR, Du YC, Jiang HY, Xu JY, Xu YJ. Experimental study of acute lung injury induced by different tidal volume ventilation in rats. Chin Med J (Engl) 2005;118:777–780. [PubMed] [Google Scholar]
- 6.Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol. 2000;89:1645–1655. doi: 10.1152/jappl.2000.89.4.1645. [DOI] [PubMed] [Google Scholar]
- 7.Wolpe SD, Davatelis G, Sherry B, Beutler B, Hesse DG, Nguyen HT, et al. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988;167:570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imanaka H, Shimaoka M, Matsuura N, Nishimura M, Ohta N, Kiyono H. Ventilator-induced lung injury is associated with neutrophil infiltration, macrophage activation, and TGF-beta 1 mRNA upregulation in rat lungs. Anesth Analg. 2001;92:428–436. doi: 10.1097/00000539-200102000-00029. [DOI] [PubMed] [Google Scholar]
- 9.Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affect local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:109–116. doi: 10.1164/ajrccm.160.1.9803046. [DOI] [PubMed] [Google Scholar]
- 10.Altemeier WA, Matute-Bello G, Frevert CW, Kawata Y, Kajikawa O, Martin TR, et al. Mechanical ventilation with moderate tidal volume synergistically increases lung cytokine response to systemic endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004;287:L533–L542. doi: 10.1152/ajplung.00004.2004. [DOI] [PubMed] [Google Scholar]
- 11.Held HD, Boettcher S, Hamann L, Uhlig S. Ventilation-induced chemokine and cytokine release is associated with activation of nuclear factor-B and is blocked by steroids. Am J Respir Crit Care Med. 2001;163:711–716. doi: 10.1164/ajrccm.163.3.2003001. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Winskog C, Edston E, Walther SM. Inhaled and intravenous corticosteroids both attenuate chlorine gas-induced lung injury in pigs. Acta Anaesthesiol Scand. 2005;49:183–190. doi: 10.1111/j.1399-6576.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 13.Jansson AH, Eriksson C, Wang X. Effects of budesonide and N-acetylcysteine on acute lung hyperinflation,inflammation and injury in rats. Vascul Pharmacol. 2005;43:101–111. doi: 10.1016/j.vph.2005.03.006. [DOI] [PubMed] [Google Scholar]


