Abstract
BACKGROUND:
Despite a large amount of resuscitation research, the survival rate after cardiac arrest remains low, and brain injury is the key issue. Neuroglobin (NGB) is an oxygen-binding heme protein found in the brain with a protection role against ischemic-hypoxic brain injury. Hemin is an effective activator of neuroglobin. This study was undertaken to assess the effect of hemin on expression of neuroglobin (NGB) in the cerebral cortex, neuro-deficit score (NDS) and pathological changes after cardiopulmonary resuscitation (CPR) in rats.
METHODS:
A total of 120 male Sprague-Dawley (SD) rats were randomly divided into a control group (A), a CPR group (B) and a Hemin group (C). The animal model of cardiac arrest (CA) induced by asphyxia and CPR was established. NGB expression in the cerebral cortex with immunohistochemistry, NDS and pathological changes in the cerebral cortex were examined at 3, 6, 12, 24 hours after recovery of spontaneous circulation (ROSC) in each group. Experimental data were treated as one-factor analysis of variance and the Tukey test.
RESULTS:
In comparison with group A, NGB expression was increased significantly at 12 and 24 hours after ROSC (P<0.05 or P<0.01), NDS was decreased significantly at each time point after ROSC (P<0.01), and pathological changes were severe at each time point after ROSC in group B. In comparison with group A, NGB expression was increased significantly at 6, 12, 24 hours after ROSC (P<0.05 or P<0.01), NDS was decreased significantly at 3, 6, 12 hours after ROSC (P<0.01) in group C. In comparison with group B, NGB expression was increased significantly at 12 and 24 hours after ROSC, NDS was increased significantly at 12 and 24 hours after ROSC, and pathological changes were milder in group C.
CONCLUSION:
There were increased NGB expression in the cerebral cortex, decreased NDS, and severe pathological changes after CPR in rats. Hemin treatment up-regulated expression of NGB, improved NDS, mitigated pathological changes, and alleviated cerebral injury after CPR.
KEY WORDS: Cardiopulmonary resuscitation, Neuroglobin, Neurodeficit score, Hemin, Cerebral injury, Rats
INTRODUCTION
Prevention and treatment of brain injury after cardiac arrest was an important part of cardiopulmonary resuscitation. In recent years, some index of early detection of brain injury had been used for prediction, treatment, and outcome assessment in experimental and clinical application studies. Neuroglobin is a third oxygen-carrying globin localized in neurons.[1] Recent studies have suggested that NGB is closely related to ischemic-hypoxic brain damage.[2] Hemin is an activator of neuroglobin, and can resist ischemic-hypoxic injury.[3] To investigate the brain damage and the possible protection mechanism after CPR, we established in this study the animal models of asphyxial cardiac arrest (ACA) by clamping an endotracheal tube at expiration and observed the expression of NGB in the cerebral cortex, neuro-deficit score (NDS), and pathological changes.
METHODS
Animals, reagents and instrument
Adult Sprague-Dawley (SD) male rats, weighing 250-350 g, were provided by the Experimental Animal Center of Wenzhou Medical College, and the animal license number was SCXK (Shanghai) 2007-0005.
Hemin was bought from Sigma-Aldrich, Inc., USA, and first antibody (rabbit anti-rat NGB polyclonal antibody) and secondary antibody (the anti-goat IgG antibody) were purchased from the Beijing Institute of Radiation Medicine, and Zhongshan Goldbridge Biotelhnology Co., Ltd, Beijing, respectively.
DW-2000-type animal respirator and Olympus BX41 microscope were purchased from Shanghai JiaPeng Technology Co., Ltd. and Olympus Corporation, Japan, respectively.
Groups
A total of 120 male SD rats were randomly divided into a control group (A), a CPR group (B), and a Hemin group (C), 40 rats in each group. After intubation (group A), at 0.5, 3, 6, 12, 24 hours after ROSC (groups B, C), 8 rats were killed at every time point in each group, respectively.
Preparation of animal models
The animal model of asphyxial cardiac arrest (CA) was made by clamping a tracheostomy tube:[4] CPR started at 3 minutes after cardiac arrest (chest compression, epinephrine therapy and mechanical ventilation). Within 2 minutes after return of spontaneous circulation (ROSC), the rats were intraperitoneally injected with 10 mL/kg saline (groups A and B) or 10 mL/kg 9 mmol/L hemin (group C) at the beginning of resuscitation. The rats in the group A were anesthesized and tracheostomized, and received vascular puncture without choking or CPR. At the corresponding time after surgery, the rats were killed after anesthesia.
According to the Utstein standard,[5] cardiac arrest was defined when mean arterial pressure declined to 25 mmHg and ECG pattern showed ventricular fibrillation, asystole or pulseless electrical activity. ROSC was defined when there were mean arterial blood pressure over 60 mmHg, self-ECG rhythm and arterial pulse wave. Resuscitative efforts stopped if ROSC didn’t occur within 10 minutes after chest compression.
HE staining and NGB immunohistochemistry of the cerebral cortex
At the end points, the rats were anesthesized by injecting 5% chloral hydrate (7 mL/kg) into the abdominal space. Thoraxes of the rats were opened, and blood samples were washed away by transcardiac puncture of the left cardiac ventricle by warm saline and 0.1 mol/L PBS–buffered paraformaldehyde. The brain tissue was removed and placed in 0.1 mol/L PBS-buffered paraformaldehyde for 24 hours. An area at the frontal lobe of the left brain was obtained and embedded in paraffin. A series of consecutive secions (4 μm in thickness) were prepared from paraffin-embedded tissues for conventional HE staining and immunocytochemistry. Three photos were taken from each slice in the same location. Image analysis software Image pro plus 6.0 was used to count the number of positive cells in each photo.
Neuro-deficit score
According to Geocadin’ Neurodeficit score method,[6] neurologic deficit scoring consisted of five components including level of consciousness, cranial nerve function, breathing pattern, motor and sensory function, and behavior. A score of 80 was assigned to each category; 0 indicates brain death and 80 for normal function.
Statistical analysis
Data were presented as mean ± SD. Significant differences between treatments were determined by one-factor analysis of variance using the SPSS 13.0. Differences between the 3 groups were compared using the Tukey test. P<0.05 was considered statistically significant.
RESULTS
General data for each group
No significant differences were obvserved in weight and MAP at baseline between the 3 groups (P>0.05). During CPR, there was no significant difference in cardiac arrest time, adrenaline dose, time for chest compression, and arterial pressure (MAP) between group B and group C (P>0.05).
Changes of the NGB expression in the cerebral cortex
The expression of NGB at the protein level was determined at 0.5, 3, 6, 12, 24 hours after ROSC by immunohistochemistry. In group A, the number of NGB-immunoreactive (NGB-IR) neurons cells in the cerebral cortex steadily expressed, and there was no significant difference at different time points (P>0.05). The number of NGB-IR cells gradually increased in groups B and C; compared with the same time point in group A, the number of NGB-IR neurons was significantly higher at 12 and 24 hours after ROSC in group B (P<0.01); and compared with group C, it was higher at 6, 12 and 24 hours after ROSC in group B (P<0.05 or P<0.01) (Table 1, Figure 1).
Table 1.
The changes of NGB-IR neurons in the cerebral cortex in rats (mean±SD, n=8)
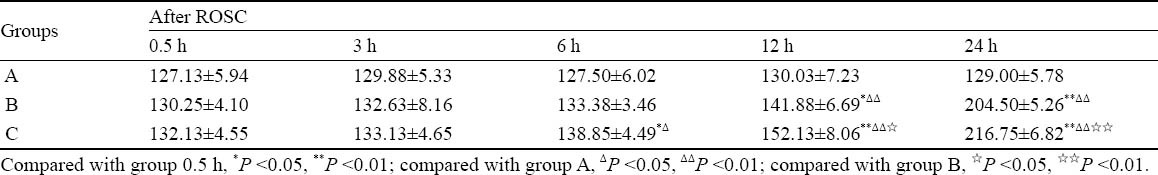
Figure 1.
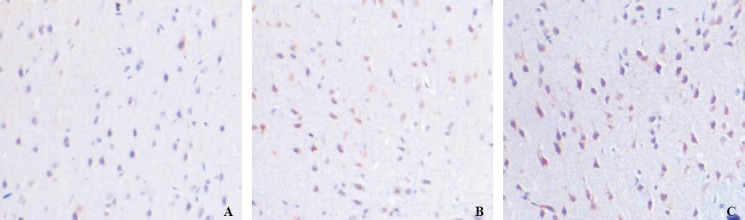
NGB immunohistochemistry staining in the cerebral cortex (original magnification×200). A: at 24 hours in group B; B: at 12 hours in group C; C: at 24 hours in group C.
Comparison of neuro-deficit score
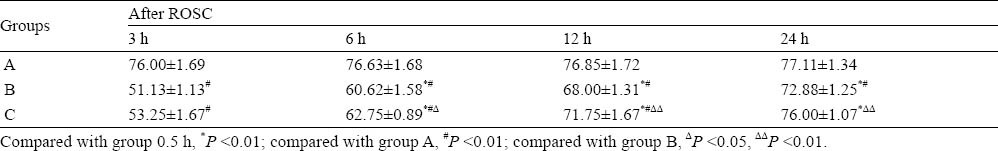
The NDS decreased to the lowest at 3 hours after ROSC in group B and group C, and then gradually increased. Compared with the same time point in group A, the NDS in group B increased obviously after ROSC (P <0.01); the NDS in group C at 3, 6 and 12 hours after ROSC increased obviously (P<0.01). Compared with the same time point in group B, the NDS in group C at 6, 12 and 24 hours after ROSC increased dramatically (P <0.05 or P <0.01) (Table 2).
Table 2.
Neuro-deficit score (NDS) of rats (mean±SD, n=8)
Histopathologic changes in the cerebral cortex
The distribution of neuronal damage was evaluated by HE staining. There were no obvious ischemic cell changes in the brain of rats in group A (Figure 2A). In group B, at the beginning of ROSC, neurons demonstrated very slight ischemic changes. After hypoxia/ischemic-reperfusion injury, however, ischemic damage increased over time such as neuron-generalized edema, local cell damage, cell morphological change, dispersed cell arrangement, cell shrinkage, increased shell area of surrounding tissue between the cells, necrosis, and dark nucleus. At 6 hours after ROSC, edema and necrosis reached the most serious stage, and normal neuronal cells were found, proliferation of glial cells was extensive and was slightly improved at 12 hours after ROSC (Figure 2B, D). In group C there was a mild ischemic edema in the brain. Neuronal degeneration, neuronal necrosis, cavitation phenomenon, and gliosis proliferation were found. These pathological changes were less in group C than in group B (Figure 2C, E). ABC
Figure 2.
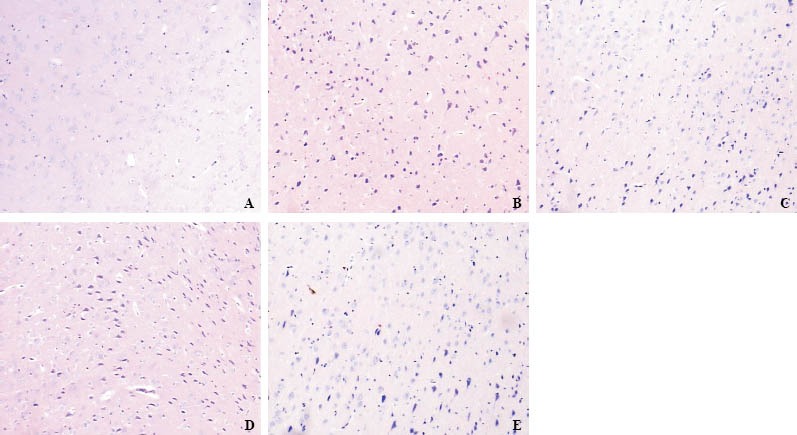
The results of HE staining in the cerebral cortex at 6 hours (original magnification× 100). A: at 6 hours in group A; B: at 6 hours in group B; C: at 12 hours in group C; D: at 24 hours in group B; E: at 24 hours in group C.
DISCUSSION
NGB, expressed in the neurons system, is a newly discovered and the third generation of oxygen-carrying globin. Its functions include binding, storing and transporting oxygen, scavenging reactive species, and maintaining the ability of cells.[7, 8] NGB may be closely related to ischemic-hypoxic brain injury, and it was a safety factor for defending against ischemic-hypoxic injury.[9,10] Immunocytochemistry showed that NGB expression didn’t change with the time in the control group in the present study. This indicated that under the non-hypoxic condition, NGB expression was very stable. The expression of NGB was significantly increased in the cerebral cortex at 12 hours after ROSC, and still increased at 24 hours. This indicated that NGB may be an intrinsic protection factor of cortical neurons under hypoxia. NGB was upregulated in the cerebral cortex after ischemia-hypoxia injury during cardiac arrest. This
study showed that with increased expression of NGB, NDS and pathological changes in the cerebral cortex were improved after ROSC. Shang et al[11] reported that the expression of NGB in ischemia-reperfusion brain injury was similar to the result of this study, suggesting that increased NGB expression may be one of the mechanisms of inhibiting brain injury caused by ischemia-hypoxia injury.
Many factors affected the time and characteristics of brain dysfunction after CPR, but still some are unknown.[12,13] In this study, NDS reached the lowest at 3 hours after ROSC in group B, and then gradually increased, but was still significantly lower than that in the control group. This finding showed that after ROSC, nerve function was severely impaired in the early recovery period. In group B, pathological changes were observed in the cerebral cortex, and were severe at 6 hours after ROSC; necrosis of neurons and glial cell proliferation were found alone with the brain damage.[14] The severity of pathological changes was consistent with NDS, which suggested that NDS can promptly reflect the severity of brain dysfunction.
Hemin is an effective activator of neuroglobin generation, which can induce neuroglobin gene transcription and translation and provide heme and iron to assembe NGB. In their in vitro studies of NGB neuronal expression, Zhu et al[15] found that the induction of NGB in mRNA and protein by hemin was concentration- and time-dependent. In this study, the cortical expression of NGB in group C increased at 6 to 24 hours after ROSC, and was significantly higher than that in group B and the control group. With the increase of NGB expression, NDS significantly increased, and pathological changes in the cerebral cortex were significantly improved in group C than in group B. This finding suggested that on the recovery of brain damage, hemin has protective effect on the cerebral cortex by up-regulating the expression of NGB.
Footnotes
Funding: This work was supported by grants from Wenzhou Science and Technology Bureau (Y20080084, Y20090241).
Ethical approval: This work was approved by the Institutional Ethics Committee of Wenzhou Medical College.
Conflicts of interest: All authors declare that there are no competing interests involving this work.
Contributors: He AW and Chen SQ wrote the first draft of this paper. All authors read and approved the final version.
REFERENCES
- 1.Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 2.Burmester T, Hankeln T. What is the function of neuroglobin? J Exp Biol. 2009;212:1423–1428. doi: 10.1242/jeb.000729. [DOI] [PubMed] [Google Scholar]
- 3.Lakkisto P, Csonka C, Fodor G, Bencsik P, Voipio-Pulkki LM, Ferdinandy P, et al. The heme oxygenase inducer hemin protects against cardiac dysfunction and ventricular fibrillation in ischaemic/reperfused rat hearts: role of connexin 43. Scand J Clin Lab Invest. 2009;69:209–218. doi: 10.1080/00365510802474392. [DOI] [PubMed] [Google Scholar]
- 4.Cheng SQ, Li ZP, Wang SS, Murao Y. Factors influencing asphyxial cardiac arrest. Emerg Med J China. 2005;14:814–817. [Google Scholar]
- 5.Idris AH, Becker LB, Ornato JP, Hedges JR, Bircher NG, Chandra NC, et al. Utstein-style guidelines for uniform reporting of laboratory CPR research. Circulation. 1996;94:2324–2336. doi: 10.1161/01.cir.94.9.2324. [DOI] [PubMed] [Google Scholar]
- 6.Geocadin RG, Malhotra AD, Tong S, Seth A, Moriwaki G, Hanley DF, et al. Effect of acute hypoxic preconditioning on qEEG and functional recovery after cardiac arrest in rats. Brain Research. 2005;1064:146–154. doi: 10.1016/j.brainres.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 7.Hua S, Antao ST, Corbett A, Witting PK. The significance of neuroglobin in the brain. Current Medicinal Chemistry. 2010;17:160–172. doi: 10.2174/092986710790112611. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Yin G, Huang F, Dewilde S, Hay-Schmidt A. Neuroglobin expression in the rat suprachiasmatic nucleus: colocalization, innervation, and response to light. J Comp Neurol. 2010;518:1556–1569. doi: 10.1002/cne.22290. [DOI] [PubMed] [Google Scholar]
- 9.Jin K, Mao XO, Xie L, John V, Greenberg DA. Pharmacological induction of neuroglobin expression. Pharmacology. 2011;87:81–84. doi: 10.1159/000322998. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin K, Mao Y, Mao X, Xie L, Greenberg DA. Neuroglobin expression in ischemic stroke. Stroke. 2010;41:557–559. doi: 10.1161/STROKEAHA.109.567149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang AJ, Zhou DB, Wang LH, Gao Y, Fan M, Wang X, et al. Increased neuroglobin levels in the cerebral cortex and serum after ischemia-reperfusion insults. Brain Research. 2006;1078:219–226. doi: 10.1016/j.brainres.2006.01.064. [DOI] [PubMed] [Google Scholar]
- 12.Schneider A, Bottiger BW, Popp E. Cerebral resuscitation after cardiocirculatory arrest. Anesth Analg. 2009;108:971–979. doi: 10.1213/ane.0b013e318193ca99. [DOI] [PubMed] [Google Scholar]
- 13.Menzebach A, Bergt S, von Waldthausen P, Dinu C, Nöldge-Schomburg G, Vollmar B. A comprehensive study of survival, tissue damage, and neurological dysfunction in a murine model of cardiopulmonary resuscitation after potassium-induced cardiac arrest. Shock. 2010;33:189–196. doi: 10.1097/SHK.0b013e3181ad59a3. [DOI] [PubMed] [Google Scholar]
- 14.Du HG, Yin LC, He M, Zhang GJ, Tian Y, Wang C, et al. Application of ulinastatin in severe craniocerebral injuries. Chin J Traumatol. 2005;8:236–239. [PubMed] [Google Scholar]
- 15.Zhu Y, Sun Y, Jin K, Greenberg DA. Hemin induces neuroglobin expression in neural cells. Blood. 2002;100:2494–2498. doi: 10.1182/blood-2002-01-0280. [DOI] [PubMed] [Google Scholar]


