Abstract
BACKGROUND:
Lidocaine can promote the apoptosis of eosinophils, which is normally delayed by IL-5; it has a good effect on serious steroid resistant asthma (SRA). The study aimed to explore the effect of nebulized lidocaine inhalation on asthma.
METHODS:
It was a randomized, double-blind, placebo-controlled and prospective study. A total of 36 patients with acute asthma were divided into groups A1, A2, B1 and B2, with 9 patients in each group. The patients of groups A1 and A2 had steroid resistant asthma (SRA) and those of groups B1 and B2 had steroid sensitive asthma (SSA). Patients in groups A2 and B1 were administered nebulized lidocaine in addition to routine treatment, while patients in groups A1 and B2 were given nebulized normal saline apart from routine treatment and served as placebo-controlled groups.
RESULTS:
There were significant differences in heart rate, respiratory rate, and peak flow rate and forced expiratory volume in one second between the experimental groups and the placebo-controlled groups. There was no significant difference between groups A2 and B1, and between A1 and B2.
CONCLUSION:
Inhaled lidocaine is beneficial to asthma patients, especially those with steroid-resistant asthma.
KEY WORDS: Asthma, Lidocaine, Treatment
INTRODUCTION
In recent years, lidocaine has been used to treat asthma. A report showed that both venoclysis and nebulized inhalation of lidocaine can attenuate bronchial hypersensitivity (BHS) in asthma patients.[1] Evidence indicated that lidocaine can promote the apoptosis of eosinophils, which is normally delayed by IL-5,[2] and that it has a good effect on serious steroid resistant asthma (SRA).[3] The present study was undertaken to determine the effect of nebulized inhalation of lidocaine on various types of asthma after an acute onset.
METHOD
Patients
Thirty-six patients with acute asthma, 17 females and 19 males, aged from 18 to 70 years (mean 41.9±15.4 years), were enrolled in the study.[4] According to their sensibility to hormone, the patients were divided into steroid sensitive asthma (SSA) and steroid resistant asthma (SRA). Since there is no consistent definition of SRA, dosage or treatment time, we adopted the well-recognized definition made by Woolcock,[5] but didn’t further divide SRA into types I and II.
Methods
It was a prospective study based on the principle of randomization, placebo control and double blindness. SRA patients were classified as group A, and SSA patients were classified as group B. Eighteen patients were randomly selected from each group; 18 patients from each group were randomly subdivided into groups A1, A2, and B1, B2, 9 patients in each group. All these patients received the routine treatment (ultrasonic nebulizer, YiNiao 402, Shanghai; particle diameter: 1-5 μm; inhalation speed 1-2 mL/min). Patients in the groups A1 and B2 were added with 5 mL normal saline, and served as placebo-controlled groups; patients in the groups A2 and B1 were added with 5 mL lidocaine (concentration 2%). Patients, who had too much sputum and needed endotracheal intubation or tracheotomy, were excluded from the study.
Variables
A Spirometer (DFM-86II, made in China) was used to measure the parameters of lung function, and a multi-function monitor (MT6000 made in America) was used for the detection of temperature, pulse, electrocardiogram, heart rate, respiration rate, oxygen saturation of blood, etc. Parameters were collected at 10 minutes after the onset of acute asthma but before inhalation, 10, 20, 30 minutes after inhalation, respectively.
Statistical analysis
The data were analyzed using SPSS version 13.0. A P value less than 0.05 was considered statistically significant. Differences in multi-sample means were compared using the F test and the q test, and differences between the experimental groups and the placebo-controlled groups were compared using Student's t test. Index values were expressed by mean±SD.
RESULTS
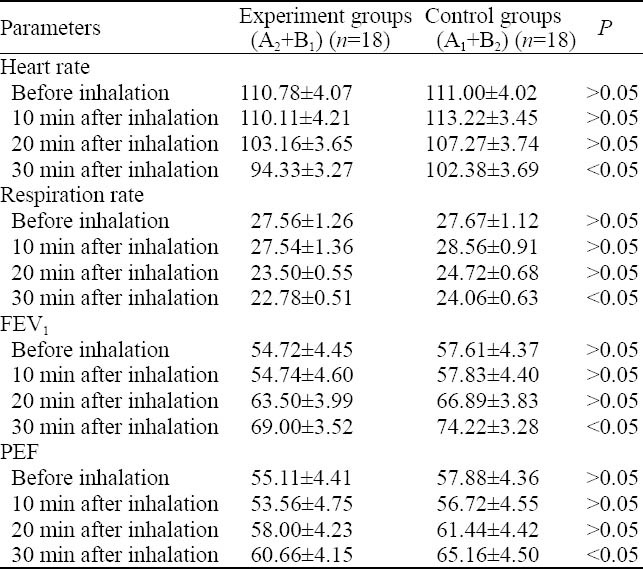
There were differences in heart rate, respiration rate, forced expiratory volume in one second (FEV1), and peak expiratory flow (PEF) between the experimental groups and the placebo-controlled groups at 10 and 20 minutes, but without statistically differences. However, significant differences were observed between these groups at 30 minutes (P <0.05, Table 1).
Table 1.
Comparison of heart rate, respiration rate, FEV1 and PEF before and after lidocaine inhalation between the experimental and control groups (mean±SD)
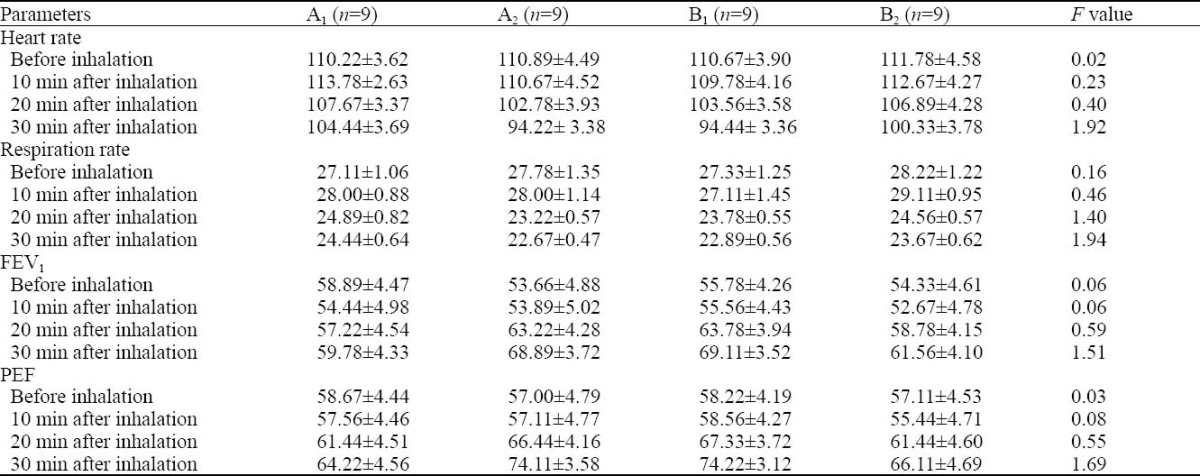
Analysis of variance revealed no significant differences between groups A1, A2, B1 and B2 (P >0.05, Table 2).
Table 2.
The changes of heart rate, respiration rate, FEV1 and PEF during an acute attack of different types of asthma before and after lidonaine inhalation (mean±SD)
DISCUSSION
In this study, no statistical significance was observed at each time point between groups A1 and A2, and between groups B1 and B2 despite different treatments. Analysis of variance found that there were no significant differences at different time points between the four groups (A1, A2, B1, and B2); however there was significant difference between the experimental groups and the placebo-controlled groups (P<0.05). This finding indicated that different treatment may exert different effect on the patients. The F test showed that there was no significant difference between the groups. The result may be due to the limited number of patients in each group. The lung function of patients in groups A1 and B2 (normal saline) decreased at first and then increased, but it increased significantly in group B2; the lung function of patients in groups A2 and B1 (lidocaine) increased at first, but increased more significantly than in groups A1 and B2. The results indicated that lidocaine is effective in treating any type of asthma during an acute attack.
FEV1 and PEF are the most important variables among all parameters because they objectively reflect the degree of airway stenosis. Previous studies showed that after lidocaine inhalation, FEV1 and PEF decreased at first and increased subsequently, presenting a V-like trend.[1-6] Gao et al[7] reported that FEV1 and PEF of patients declined at 5 and 10 minutes after inhalation of lidocaine one time, slightly increased at 20 minutes, and greatly increased at 45-60 minutes, which also presented a V-like trend. Hunt et al[3] reported that FEV1 and PEF were not decreased after inhalation of lidocaine apart from the routine treatment. In the present study, FEV1 and PEF didn’t change at 10 minutes after routine treatment plus lidocaine inhalation in patients with different types of asthma, and they slightly increased at 20 minutes; at 30 minutes, FEV1 and PEF in the experimental groups were higher than those in the placebo-controlled groups (P<0.05). Groeben et al reported that intravenous lidocaine and oral mexiletine blocked reflex bronchoconstriction in asthmatic subjects. Single doses of inhaled lignocaine were well tolerated in subjects with mild to moderate asthma and any tendency to bronchoconstriction can be prevented with salbutamol pretreatment.[6] In the present study, after lidocaine inhalation at 10 minutes after routine treatment in patients with acute asthma, FEV1 and PEF showed a tendency of ‘no change at first, and then an increase’. This result indicated that such administration is safe and effective.
At present, the mechanism of lidocaine effect on asthma is not clear. Studies suggest that the mechanism may include two aspects: (1) Lidocaine can directly inhibit the constriction of the airway smooth muscle. Because of the special structure of the airway, like a tree, the contraction of the airway smooth muscle directly affects the bronchostenosis. The inhaled lidocaine adheres to the surface of the endobronchial tube and exists at a high concentration, thus inhibiting the constriction of the smooth muscle. The bronchioles with a diameter <1 mm are easily to be narrow and short when the smooth muscle contracts because such bronchiols are arrayed longitudinally and their wall is not supported by cartilage. The inhaled lidocaine makes the spiral and constrictive smooth muscle in a relaxed and paralysed status, and thus bronchostenosis can be alleviated and asthma can be controlled. (2) The nerve may be blocked. The chronic inflammation of the airway causes airway injury, and the airway needs to be restored and reshaped. Bronchial smooth muscle is hyperplastic, resulting in change of basilar membrane structure and persistent high reaction of the airway. The inhaled lidocaine acts directly on nerve receptor, inhibits the vagus nerve from transmitting, and thus attenuates airway response.
Footnotes
Funding: None.
Ethical approval: Not needed.
Conflicts of interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributors: LV ZM wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Groeben H, Silvanus MT, Beste M, Peters J. Both intravenous and inhaled lidocaine attenuate reflex bronchoconstiriction but at different plasma concentrations. Am J Respir Crit Care Med. 1999;159:530–535. doi: 10.1164/ajrccm.159.2.9806102. [DOI] [PubMed] [Google Scholar]
- 2.Okada S, Hagan JB, Kato M, Bankers-Fulbright JL, Hunt LW, Gleich GJ, et al. Lidocaine and its analogues inhibit II. 5 mediated survival and activation of human eosinophils. J Immunol. 1998;160:4010–4017. [PubMed] [Google Scholar]
- 3.Hunt LW, Swedlund HA, Gleich GJ. Effect of nebulizded Lidocaine on severe glucocorticoid-dependent asthma. Mayo Clin Proc. 1996;71:361–368. doi: 10.4065/71.4.361. [DOI] [PubMed] [Google Scholar]
- 4.Asthma Group in the Society of Respiratory Disease of Chinese Medical Association. Guide for treating bronchial asthma. Chin J Tubere Respir Dis. 1997;20:261–267. [Google Scholar]
- 5.Woolcock. Corticosteroid resistant asthma. Am J Respir Crit Cane Med. 1996;154:845–848. doi: 10.1164/ajrccm/154.2_Pt_2.S45. [DOI] [PubMed] [Google Scholar]
- 6.Harrison TW, Tattersfield AE. Effect of sigle doses of inhaled Lignocaine on FEV1, and bronchial reactivity in asthma. Respir Med. 1998;1992:1359–1363. doi: 10.1016/s0954-6111(98)90142-1. [DOI] [PubMed] [Google Scholar]
- 7.Gao ZY, Li PK, Zhao JZ, Jiang RF, Yang BJ, Zhang MH, et al. Effects of airborne fine particulate matter on human respiratory symptoms and pulmonary function. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2010;28:748–751. [PubMed] [Google Scholar]


