Abstract
BACKGROUND:
In cases of severe sepsis and septic shock, a series of pathophysiological changes lead to multiple organ dysfunction syndrome. This study aimed to investigate the expression of glucocorticoid receptor mRNA in the rat lung following endotoxin (LPS) induced shock.
METHODS:
Totally 56 SD rats were randomly divided into 4 groups: LPS shock group (n=16), LPS+vasoactive intestinal peptide group(VIP) group, (n=16), LPS+VIP+ glucocorticoid (GC) group, (n=16),and control group (n=8). LPS shock was induced by intravenous injection of LPS (10 mg/kg) in rats. Within 15 minutes after LPS injection, rats in the treatment groups received VIP (5 nmol/kg) or VIP and methylprednisolone (3 mg/kg). The control group was given normal saline instead of LPS. The rats of the four groups were sacrificed at 6 hours,24 hours after injection respectively, and the lung tissues were collected. Pathological changes of the lungs were examined by light microscopy and electron microscopy. GRmRNA expression in the lung tissues was evaluated by RT-PCR.
RESULTS:
In the LPS shock group, lung histopathology demonstrated destruction of the alveolar space,widening of the inter-alveolar space, inflammatory cell infiltration and interstitial edema. However,pathological changes in the LPS+ VIP group and LPS+ VIP+GC group were milder than those in the LPS shock group. Six hours after LPS injection, GR mRNA expression was down-regulated in the LPS group (0.72± 0.24) and LPS+ VIP group (0.88±0.27) (P<0.05) as compared with the control group (1.17±0.22). The LPS shock group showed a more significant down-regualtion than the LPS+VIP group, but the difference was not statistically significant (P>0.05). In contrast, GRmRNA expression in the LPS+ VIP+GC group was significantly up-regulated at 6 hours and further at 24 hours (1.45±0.32 and 1.91±0.46 respectively) (P<0.05).
CONCLUSION:
GrmRNA expression decreased in LPS induced lung injury in rats. Combined treatment with VIP and GC mitigated lung injury ang inflammation. The mechanism may be related to up-regulation of GR mRNA expression.
KEY WORDS: Glucocorticoid, GRmRNA, Vasoactive intestinal peptide, LPS, Shock, Inflammation, Lung injury, Rat
INTRODUCTION
In the event of severe sepsis and septic shock, a series of pathophysiological changes including uncontrolled inflammatory response, endothelial cell damage, coagulation and fibrinolysis disruption, micro-thrombosis, and adrenal dysfunction lead to multiple organ dysfunction syndrome (MODS). The lung is one of the first involved and most damaged organs.[1] Glucocorticoid (GC) known to modulate immune and inflammatory responses has been used as an adjuvant therapy for septic shock and MODS.[2] Glucocorticoid receptor (GR) is a key member of the hormonal nuclear receptor family and also an important nuclear transcription factor. Upon conjugating with the ligand in the cytoplasm, the GC-GR complex enters the nucleus and functions in transcriptional regulation. The GC-GR complex regulates the inflammatory process of sepsis, thus the expression of GR can affect the role of GC. Vasoactive intestinal peptide (VIP) has a strong immunomodulatory function. It achieves a protective effect by inhibiting the release of tumor necrosis factor (TNF-α), interleukin (IL-1) by the lung-activated macrophages and promoting the generation of anti-inflammatory factor IL-10 in septic shock and multiple organ dysfunction.[3]
In this study, we used the endotoxin (LPS)-induced shock rat model to assess the GRmRNA expression in the lung tissue and its regulation by GC and VIP in an attempt to provide evidence for their clinical use in the treatment of sepsis and septic shock.
METHODS
Experimental animals and groups
Six to eight weeks old male SD rats of clean grade weighing 150-200 g were obtained from the Shanghai Experimental Animal Center of the Chinese Academy of Sciences and were randomly divided into a LPS shock group (16 rats), a LPS+VIP shock group (16), a LPS+VIP+GC shock group (16), and a control group (8).
Reagents and instruments
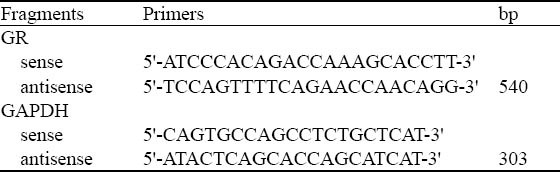
The following reagents and instruments were used: LPS (E Coli O55B5 from Sigma Corporation); methylprednisolone (Belgium Pfizer Manufacturing Belgium NV's batch number P11267); VIP (Switzerland Novabiochem Corporation); Trizol reagent, RT-PCR Kit, Premix Ex Taq enzyme (TaKaRa Company); Blood Pressure Monitor (Virdia 24C, the United States, HP); PCR instrument (BIO-RAD Inc., USA); and the sequences of specific PCR primers (Shanghai Health Industry Co., Ltd. Synthetic) (Table 1).
Table 1.
GRmRNA RT-PCR primers
Model making
The rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.3 ml/100 g). Carotid artery catheter was inserted to monitor changes of mean arterial pressure (MAP) and heart rate (HR). The shock model was produced by tail vein injection of refined LPS (E Coli O55B5) 10 mg/kg (LPS group). Within 15 minutes after LPS injection, the treatment groups were injected by tail vein with either VIP 5 nmol/kg (LPS+VIP group) or VIP 5nmol/kg+methylprednisolone 3 mg/kg (LPS+VIP+GC group). The control group was injected with the same volume of normal saline instead of LPS. MAP reduction by 50% was used as a marker for shock modeling success.[3]
Specimen collection
The rats were sacrificed at designated time points by bleeding. The chest was opened and a small specimen of lung tissue taken from the right side was snap frozen in a freezer at -80°C for RNA extraction. The specimen for electron microscopy was retained at the same time. The whole left lung was preserved in 4% paraformaldehyde for light microscopy.
General conditions and hemodynamic changes
The rats were observed for apathy, chills, abdominal distention, diarrhea and hair erection. Their MAP was monitored continuosly, and their survival was calculated at 6 and 24 hours respectively.
RT-PCR for detecting GRmRNA expression
Total RNA was extracted by Trizol reagent from 100 mg of lung tissue of each rat as directed by manufacturer's instructions. 0.5μg of total RNA was reverse-transcribed with primer Oligo-dT in a total reaction volume of 10 μl. One μl of template RT product was amplified by PCR with specific primers (Table 1). GAPDH mRNA was amplified by 30 cycles to serve as an internal control. 5 μl of PCR product was electrophorased in 1.5% agarose gel. The value of GRmRNA expression was calculated.
Pathological observation of lung tissue
Light microscopy and electron microscopy were used for pathological observation of lung tissue.
Statistical analysis
Results were expressed as mean±SD. SPSS11.0 software was used for analysis of variance (ANOVA). Pair groups were compared with the least-significant-difference (LSD) test and Student's t test. P<0.05 was considered statistically significant.
RESULTS
General situation and survival rate
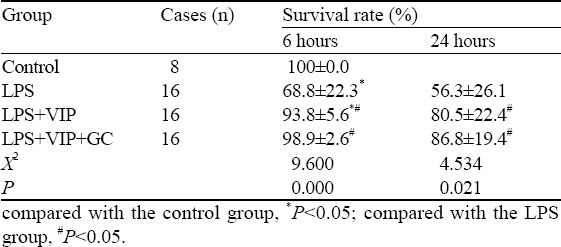
The LPS group displayed gradual emergence of vertical hair, apathy, diarrohea, tremor, rapid breathing, and significantly reduced eating and drinking, in addition to reduced number of activities before dying from convulsion, double incontinence, deep breathing, rigid limbs. These symptoms in the LPS+VIP group and the LPS+VIP+GC group were relatively mild. The survival rates at 6 and 24 hours are shown in Table 2.
Table 2.
The survival rates of the four groups
Pathological changes
Light microscopy
HE staining showed that the inter-alveolar space was diffusely widened in the LPS group. There was prominent bleeding, exudate, capillary congestion, interstitial edema, and increased number of phagocytic cells in the alveolar cavity. The LPS+VIP group showed mild focal alveolar changes. Only a few red blood cells, inflammatory cell infiltration, and capillary congestion were seen in the alveolar cavity. In the LPS+VIP+GC group there was no significant alveolar exudation or hemorrhage, infiltrated by a small amount of inflammatory cells. The alveolar interval widened slightly with a small amount of inflammatory cells infiltrated in the alveolar wall (Figure 1).
Figure 1.
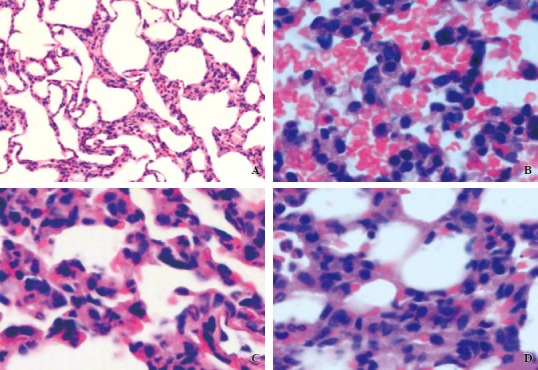
The histopathological changes of lung by a light microscope. A: the control group (original magnification × 400): normal alveolar spaces, alveolar septum. B: the LPS group (original magnification × 400): the diffuse alveolar septa widened with a lot of red blood cells and inflammatory cell infiltration. C: the LPS + VIP group (original magnification × 400): the focal widened alveolar septum and cavity with a small amount of red blood cells, inflammatory cell infiltration. D: the LPS + VIP + GC group (original magnification × 400): light alveolar hemorrhage, a small amount of inflammatory cells in the alveolar wall.
Electronic microscopy Compared with the control group, the LPS group showed alveolar capillary wall thickening, II alveolar epithelial cell necrosis, lamellar body vacuolization, and inflammatory cell infiltration. The lesions were less severe in the LPS+VIP group and the LPS+VIP+GC group (Figure 2).
Figure 2.
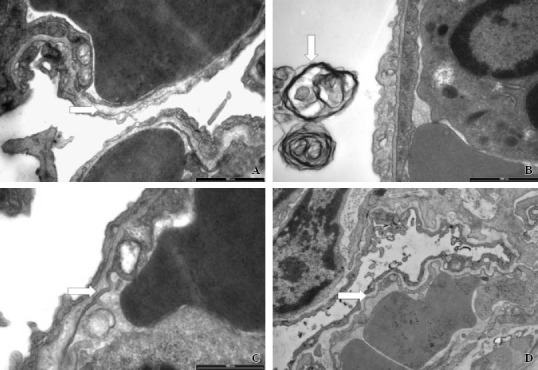
Histopathological changes shown by a light electronic microscope. A: the normal alveolar capillary wall in the control group (original magnificationx3300). B: capillary wall thickening, II-type necrosis, lamellar body vacuolization, inflammatory cell infiltration in the LPS group (original magnification×3300). C: no obvious capillary wall thickening in the alveolar cavity and slightly swollen mitochondria in the LPS + VIP group (original magnification×3300). D: no obvious capillary wall thickening in the alveolar cavity in the LPS + VIP + GC group (original magnification×3300).
GRmRNA expression in lung tissue
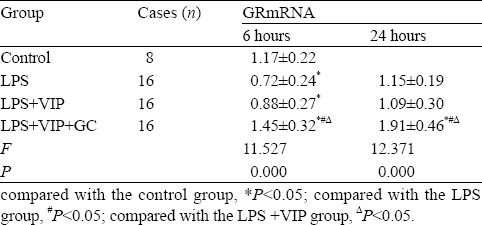
GRmRNA expression changed significantly at 6 hours and 24 hours after LPS injection. GRmRNA expression decreased more significantly in the LPS group than in the control group at 6 hours (P<0.05) and it returned to normal at 24 hours. GRmRNA expresssion was lower in the LPS+VIP group than in the control group (P<0.05) and slightly higher in the LPS shock group at 6 hours (P>0.05). GRmRNA expression was not significantly different in the LPS shock group, LPS+VIP group and control group at 24 hours (P>0.05). GRmRNA expression in the LPS+VIP+GC group increased more significantly than in the other three groups at 6 hours and 24 hours (P<0.05) (Table 3).
Table 3.
GRmRNA expression in lung tissue
DISCUSSION
In our study LPS was found to decrease GRmRNA expression in lung tissue, whereas GC intervention enhanced GRmRNA expression in the injured lung. GR belongs to the nuclear receptor superfamily, which has a molecular weight of about 94 kDa and is present in the lung, liver, brain, gastrointestinal smooth muscle, skeletal muscle, lymphoid tissues and thymus. It has two subtypes, α and β.[4] GRα is responsible for ligand-binding, which, upon conjugating with GC, regulates the expression of GC responsive genes. GRβ can not bind GC and has no transcription activation. However it can reduce the sensitivity of GC. By activating GRα and forming a GC-GR complex in the cytoplasm, GC is transferred into the nucleus
in order to play a transcriptional regulatory role: inhibiting a variety of inflammatory cytokines including IL-1, IL-6, TNF-α.[5’6] Animal studies have shown that the reduction of GR may lead to adrenal insufficiency[7] Da et al[8] reported that in the porcine endotoxin shock model, GR expression in the lung, liver and kidney tissue was markedly reduced, accompanied with upregulation of inflammatory mediators such as activated protein-1 (AP-1), TNF-α, nuclear transcription factor (NF-κB) 6 hours after intravenous injection of endotoxin. In the present study significant downregulation of GRmRNA by RT-PCR was observed 6 hours after shock induced by LPS, demonstrating that LPS may interfere with the anti-inflammatory and immune regulatory roles of endogenous GC.
VIP is a 28 amino acids biologically active peptide and one of the most abundant lung neuropeptides. It has a wide range of regulatory functions in various cell types in the lung and exerts protective effect on septic shock and organ damage.[3,9] Exogenous application of VIP may be therapeutic for shock,[10] and helps to restore lung function.[3,11] In the current study, intravenous VIP reduced injury to rat lung tissue indicating that exogenous VIP has some protective effect on LPS-mediated lung injury in rats. VIP may affect the inhibition of GRmRNA expression in lung tissue by LPS.
In recent years, replacement therapy by low doses of GC has been effective in the management of septic shock.[2] In the current study the rats were injected with 3 mg/kg of methylprednisolone, which is considered as a moderate-low dose. In the event of severe infection and septic shock, GC by downregulating GR expression alleviates inflammatory response and inhibits the excessive expression of inflammatory factors.[12,13] Some researchers[14] found that a low-dose of dexamethasone eased LPS-induced inflammatory response to pneumonia and pulmonary fibrosis in rats, increased the expression of GR, increased GR protein expression in particular, and promoted the transcription of GR protein. Kamiyama et al,[15] however, reported that high doses of methylprednisolone (40 mg/kg) given intraperitoneally at the same time with LPS in guinea pigs significantly reduced the expression of GRα, thus leading to a significant reduction of GRα gene transcription in the nucleus. In our study, VIP alone mildly relieved inflammatory reaction, but a low-dose methylprednisolone in conjunction with VIP interfered with the LPS-induced lung injury, resulting in considerable amelioration of pathological changes in the injured lung, correlating with a significantly increased GR gene expression. These results indicate that low GC in combination with VIP can help to resist LPS-induced lung injury and inflammatory reactions. Its mechanism may be closely related to the promotion of GR gene transcription.
In conclusion, in the rat model of LPS-induced shock, GRmRNA expression in lung tissue is decreased. Combined intervention with VIP and GC may reduce lung injury but its mechanism is closely related to the promotion of GR transcription. Moderately low-dose of GC in conjunction with VIP can be effective in the management of septic shock, but the timing of medication, dosage and clinical results require further study.
Footnotes
Funding: The study was supported by grant from Shanghai Mnuicipal Health Bureau Foundation (050241).
Ethical approval: None.
Competing interest: None.
Contributors: All authors contributed to the study. Zhang YM is the guarantor.
REFERENCES
- 1.Lemaout C, Gonzalez H, Aboab J, Annane D. Pathophysiology of septic shock. Presse Med. 2006;35:521–527. doi: 10.1016/s0755-4982(06)74628-0. [DOI] [PubMed] [Google Scholar]
- 2.Jacobi J. Corticosterroid replacement in critically ill patients. Crit Care Clin. 2006;22:245–253. doi: 10.1016/j.ccc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Zhang YC, Yang LP, Tang DH. Protective effects of vasoactive intestinal peptide on intestinal lesions induced by endotoxic shock in rat. Chin J Pediatr. 2006;44:369–373. [PubMed] [Google Scholar]
- 4.Smoak KA, Cidlowski JA. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev. 2004;125:697–706. doi: 10.1016/j.mad.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Gougat C, Jaffuel D, Gagliardo R, Henriquet C, Bousquet J, Demoly P, et al. Overexpression of the human glucocorticoid receptor alpha and beta isoforms inhibits AP-1 and NF-kappaB activities hormone independently. J Mol Med. 2002;80:309–318. doi: 10.1007/s00109-001-0302-6. [DOI] [PubMed] [Google Scholar]
- 6.Russcher H, Smit P, van Rossum EF, van den Akker EL, Brinkmann AO, de Heide LJ, et al. Strategies for the characterization of disorders in cortisol sensitivity. J Clin Endocrinol Metab. 2006;91:694–701. doi: 10.1210/jc.2005-2212. [DOI] [PubMed] [Google Scholar]
- 7.Schaaf MJ, Cidlowski JA. Molecular mechanisms of glucocorticoid action and resistance. J Streoid Biochem Mol Biol. 2002;83:37–48. doi: 10.1016/s0960-0760(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 8.Da J, Chen L, Hedenstierna G. Nitric oxide up-regulates the glucocorticoid receptor and blunts the inflammatory reaction in porcine endotoxin sepsis. Crit Care Med. 2007;35:26–32. doi: 10.1097/01.CCM.0000250319.91575.BB. [DOI] [PubMed] [Google Scholar]
- 9.Groneberg DA, Springer J, Fischer A. Vasoactive intestinal polypeptide as mediator of asthma. Pulm Pharmacol Ther. 2001;14:391–401. doi: 10.1006/pupt.2001.0306. [DOI] [PubMed] [Google Scholar]
- 10.Gololobov G, Noda Y, Sherman S, Rubinstein I, Baranowska-Kortylewicz J, Paul S. Stabilization of vasoactive intestinal peptide by Lipids. J Pharmacol Exp Ther. 1998;285:753–758. [PubMed] [Google Scholar]
- 11.Groneberg DA, Rabe KF, Wagner U, Fischer A. Vasoactive intestinal polypeptide in the respiratory tract: physiology and pathophysiology. Pneumology. 2004;58:330–338. doi: 10.1055/s-2004-818352. [DOI] [PubMed] [Google Scholar]
- 12.Buttgereit F, Scheffold A. Rapid glucocorticoid effects on immune cells. Steroids. 2002;67:529–534. doi: 10.1016/s0039-128x(01)00171-4. [DOI] [PubMed] [Google Scholar]
- 13.Werner A, Braun M, Kietzmann M. Isolation and cultivation of canine corneal cells for in vitro studies on the anti-inflammatory effects of dexamethasone. Vet Ophthalmol. 2008;11:67–74. doi: 10.1111/j.1463-5224.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang XQ, Zhou X, Zhou Y, Rong L, Gao L, Xu W. Low-dose dexamethasone alleviates lipopolysaccharide inducedacute lung injury in rats and upregulates pulmonary glucocorticoid receptors. Respirology. 2008;13:772–780. doi: 10.1111/j.1440-1843.2008.01344.x. [DOI] [PubMed] [Google Scholar]
- 15.Kamiyama K, Matsuda N, Yamamoto S, Takano K, Takano Y, Yamazaki H, et al. Modulation of glucocorticoid receptor expression, inflammation, and cell apoptosis in septic guinea pig lungs using methylprednisolone. Am J Physiol Lung Cell Mol Physiol. 2008;295:998–1006. doi: 10.1152/ajplung.00459.2007. [DOI] [PubMed] [Google Scholar]


