Abstract
BACKGROUND:
Uncontrolled hemorrhage is responsible for over 50% of all trauma-related deaths within the first 48 hours after admission. Clinical observations together with recent research resulted in an appreciation of the central role of coagulopathy in acute trauma care. A synopsis is presented of different retrospective analyses based upon datasets from severe multiply injured patients derived from the TR-DGU database (Trauma Registry of the Deutsche Gesellschaft fur Unfallchirurgie (DGU)/ German Society of Trauma Surgery) with respect to frequency, risk stratification and therapeutic options of acute traumatic coagulopathy (ATC).
METHODS:
The synopsis of different analyses based upon the datasets from severe multiply injured patients derived from the TR-DGU database and development/validation of a scoring system (TASH-score = Trauma Associated Severe Hemorrhage) that allows an early and reliable estimation for the probability of massive transfusion as a surrogate for life-threatening hemorrhage after severe multiple injuries.
RESULTS:
The high frequency of ATC upon emergency room admission is associated with significant morbidity and mortality in multiply injured patients. The TASH-score is recognized as an easy-to-calculate and valid scoring system to predict the individual's probability for massive transfusion and thus ongoing life-threatening hemorrhage at a very early stage after severe multiple injuries.
CONCLUSION:
An early aggressive management of ATC including a more balanced administration of blood products to improve outcome is advocated.
KEY WORDS: Coagulopathy, Epidemiology, Management, Risk stratification, Trauma
INTRODUCTION
Trauma is the leading cause of death in persons aged 5 to 44 years[1] and accounts for approximately 10% of all deaths in general.[2] Despite substantial improvement in acute trauma care, uncontrolled haemorrhage is responsible for over 50% of all trauma-related deaths within the first 48 hours after admission.[3] These clinical observations together with recent research resulted in a new appreciation of the central role of coagulopathy in acute trauma care. Current literature suggests that acute traumatic coagulopathy (ATC) is multifactorial with certain mechanisms being predominant whereas others manifest only in specific clinical states[4] (Figure 1). To date, six key initiators of coagulopathy in trauma have been described as tissue trauma, shock, hemodilution, hypothermia, acidemia, and inflammation.[4] Most recently, Brohi et al[5] emphasized the role of hypoperfusion for the initiation of ATC. As each abnormality itself may substantially exacerbate the other, a downward spiral is initiated rapidly and accelerates to death.[6] However, the adverse outcomes from uncontrolled non-surgical hemorrhage and disturbed hemostasis are not restricted to mortality only but also include organ dysfunction and loss due to prolonged hemorrhagic shock as well as the early termination of surgical procedures in order to save life.[6] Thus, early recognition accompanied by adequate and aggressive management of ATC would substantially reduce mortality and improve outcomes in severely injured patients.[7] A comprehensive review of the mechanisms underlying ATC has been published.[4]
Figure 1.
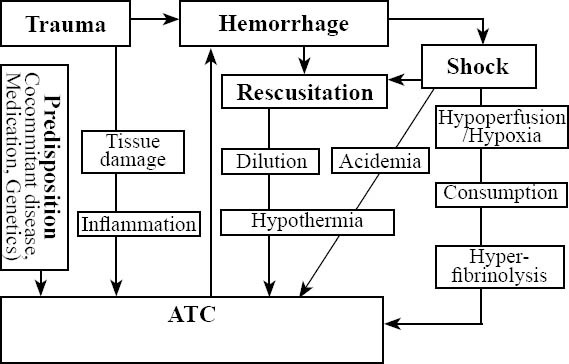
Potential mechanisms underlying ATC. Besides dilutional coagulopathy, hemorrhage may also induce shock followed by acidemia and hypothermia, further triggering coagulopathy to form the so-called “lethal triad”. Trauma with shock, hypoperfusion and hypoxia can also cause ATC associated with further consumption and hyperfibrinolysis. The clinical importance of inflammation for the development of ATC has not yet been fully understood.[4]
The present study has three purposes. First, the clinical impact of the problem is emphasized by providing actual frequency rates of ATC upon emergency room (ER) admission. Second, as early identification of patients at risk for severe bleeding requiring massive transfusion (MT) is rather difficult in the acute clinical setting but may substantially influence therapeutic strategies towards a more aggressive stabilization of the disturbed hemostatic system, a simple scoring system allowing an early and reliable estimation for the probability of MT as a surrogate for life-threatening hemorrhage after severe multiple injuries are presented. Third, key issues are considered during acute care of the bleeding trauma patient including novel approaches towards a more balanced transfusion therapy.
METHODS
The data were collected from different analyses of datasets from severe multiple injured patients derived from the Trauma Registry of the Deutsche Gesellschaft fur Unfallchirurgie (TR-DGU) database/ Arbeitsgemeinschaft Scoring of the German Society of Trauma Surgery(DGU).[8]
TR-DGU
The TR-DGU database/Arbeitsgemeinschaft Scoring of the DGU [8] which was founded in 1993 is run by a small steering group from different trauma centers in Germany (Working Group on Polytrauma/ AG Polytrauma). It is a prospective, multicenter, standardized and anonymous documentation of multiply injured trauma patients at four consecutive post-trauma stages from injury to hospital discharge: pre-hospital phase; emergency room and initial surgery (until admission to the intensive care unit (ICU)); ICU; and outcome status at discharge and description of injuries and procedures. The registry contains detailed information on demographics, injury pattern, co-morbidities, pre- and in-hospital management, time course, relevant laboratory findings including data on transfusion, and outcome of each individual. Through the 2006 data from a total of 29 353 trauma victims have been input the registry, approximately 3000 new cases are added each year. Since the introduction of the on-line version of the registry in 2002, the use of fresh frozen plasma (FFP) units is routinely documented. Between 2002 and 2006, 17 935 patients have been input the registry. Currently, there are 140 hospitals affiliated with the registry, mostly from Germany (n=90), of which 100 are actually contributing data into the database. Contributing hospitals are mostly level I trauma centers. The data are not dominated by single trauma centers but this does not exclude potential center effects due to different levels and strategies of care. The TR-DGU is not an obligatory registry. The participation is free of charge and data are contributed on a voluntary basis. It is estimated that from the total number of severe trauma cases in Germany, approximately 30% are covered by the registry. The trauma registry is approved by the review board of the DGU and is in compliance with the institutional requirements.
RESULTS
Frequency of ATC in multiple injuries upon ER admission
A retrospective analysis using the TR-DGU database was conducted to determine to what extent clinically relevant coagulopathy has already been established upon ER admission, and whether its presence was associated with the amount of intravenous fluids administered intravenously during the pre-hospital phase of care, with the severity of injury, and with impaired outcome and mortality.[9] Altogether 8 724 patients with complete data sets were screened. Coagulopathy was defined by the presence of abnormal coagulation parameters upon ER arrival of the patient, i.e. prothrombin time test (Quick's value) <70% and/ or platelets <100.000/l.[10] In Germany, the prothrombin time is preferentially reported and documented as Quick's value in percentage (%; 70-130% = normal [10]). A Quick's value of <70% is equivalent to a prothrombin time ratio of approximately 1.4.[11,12]
Acute traumatic coagulopathy upon ER admission was present in 2 989 (34.2%) of all patients. Males were more affected than females (72.5% vs. 27.5%) and in 96% the trauma mechanism was blunt. There was an increasing frequency of coagulopathy with increasing amounts of intravenous fluids administered during the pre-hospital phase of care (Figure 2). The incidence of ATC was also associated with the trauma load as reflected by injury severity scores (ISS). 2522 (84%) patients with coagulopathy had an ISS more than or equal to 16 upon hospital admission and the frequency of coagulopathy increased with higher ISS scores (Figure 2). The presence of acute ATC was associated with impaired outcome and increased mortality. Of all patients with coagulopathy, 29% developed multi-organ failure (MOF) in their later hospital stay. Early in-hospital mortality (<24 hr) was 13% in patients with coagulopathy versus 1.5% in patients without coagulopathy; the overall in-hospital mortality totalled 28% versus 8.4% (P<0.001). Mortality increased with injury severity but was generally higher in patients with coagulopathy across all severity grades studied. Figure 3 depicts mortality rates of patients with and without coagulopathy with respect to their severity of injury as reflected by ISS.
Figure 2.
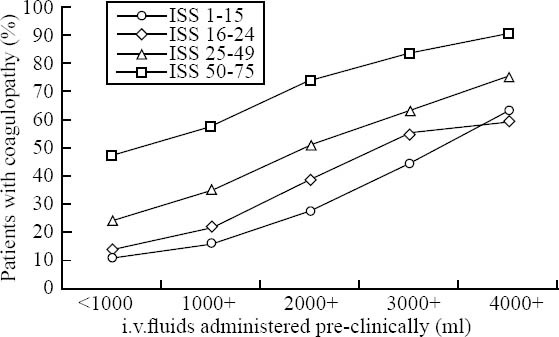
The incidence of ATC in subgroups according to ISS (4 subgroups) and intravenous fluids administered during the pre-hospital phase of care (5 subgroups). Each line represents a group of patients with a similar ISS score, while each vertical group represents patients who had received comparable amounts of intravenous fluids during the pre-hospital phase of care. Sample sizes for the groups ranged between 68 and 1439 patients.
Figure 3.
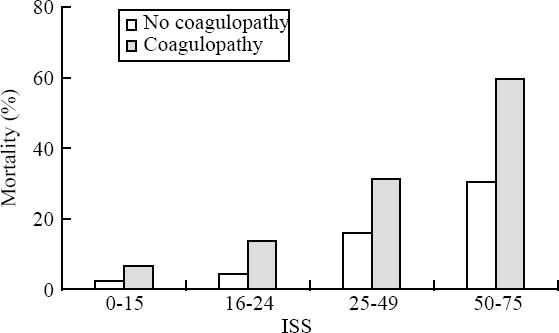
The mortality of patients with and without ATC on ER arrival according to the severity of injury as reflected by ISS.
ATC present on admission but independent of injury severity
Recently Brohi et al[5] introduced the role of hypoperfusion in the initiation of ATC by reporting their results from a prospective cohort study of major trauma patients (n=208) admitted to a single trauma center. In this cohort, patients without tissue hypoperfusion were not coagulopathic, irrespective of the amount of thrombin generated. Prolonged partial thromboplastin and prothrombin time was only observed with an increased base deficit (BD). An increasing BD was associated with high soluble thrombomodulin and low protein C levels. Low protein C levels were associated with prolonged partial thromboplastin and prothrombin time as well as hyperfibrinolysis with low levels of plasminogen activator inhibitor-1 and high d-dimer levels. Longer thrombomodulin time and low protein C level were significantly associated with increased mortality, blood transfusion requirements, acute renal injury, and reduced ventilator-free days. These researchers concluded that ATC occurs only in the presence of tissue hypoperfusion and appears to occur without significant consumption of coagulation factors. In an extension to this work, we analysed Base excess (BE) levels from severe multiply injured patients in respect to ATC frequencies upon ER admission. Also in our datasets, the frequency of ATC was strongly associated with BE level (Figure 4).
Figure 4.
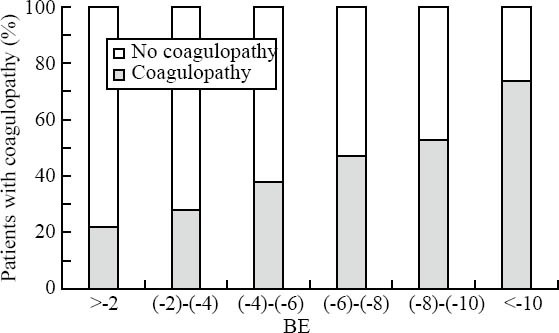
Incidence of ATC as a function of BE on ER arrival.
TASH-score: A simple scoring system to reliably predict the probability for massive MT after severe multiple injuries
Lack of reliable early indicators for the individual's risk for MT and thus persisting hemorrhage may result in underestimation of or failure to detect early surgical and coagulopathic bleeding, delayed intervention, and impaired outcome. Recently, our group presented a simple scoring system, i.e. the Trauma Associated Severe Hemorrhage (TASH) Score, that may easily and quickly (<15 minutes upon ER arrival) be applied in the ER to identify patients at high risk for MT after severe multiple injury.[13] Taken as a surrogate for severe ongoing bleeding, calculation of TASH-score can substantially impact strategies to control bleeding, to stabilize coagulation, and to improve infrastructure and logistics in acute trauma care.
To generate the score, potential clinical and laboratory variables documented in the TR-DGU database were subjected to uni-and multivariate logistic regression analysis to predict the probability for MT during early in-hospital resuscitation as a surrogate for post-traumatic life-threatening hemorrhage. MT was defined by a transfusion requirement of more than or equal to 10 units of packed red blood cell concentrates (pRBCs) between ER and ICU admission. Seven independent variables were identified to be significantly correlated with an increasing probability for MT and were incorporated into a risk score, the TASH-score (Figure 5). Detailed information about model building and validation has been published previously.[13] Increasing TASH-score is associated with an increased probability for MT. A clinical example of its application in acute clinical trauma care is demonstrated in Figure 6.
Figure 5.
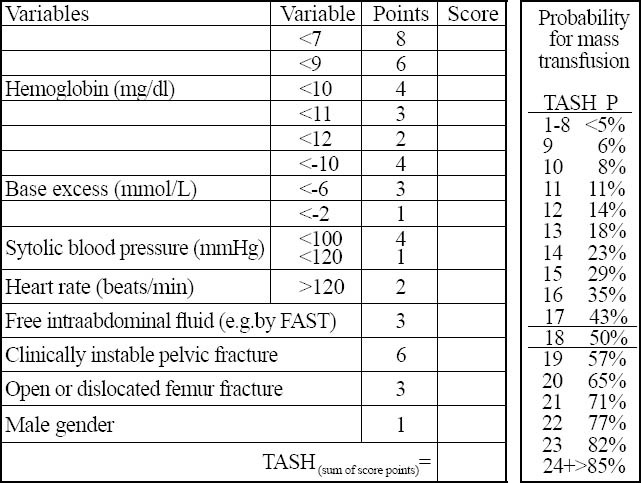
TASH-score. The TASH-score of 18 equals to a 50% risk for massive transfusion (MT) after severe multiple injuries.
Figure 6.
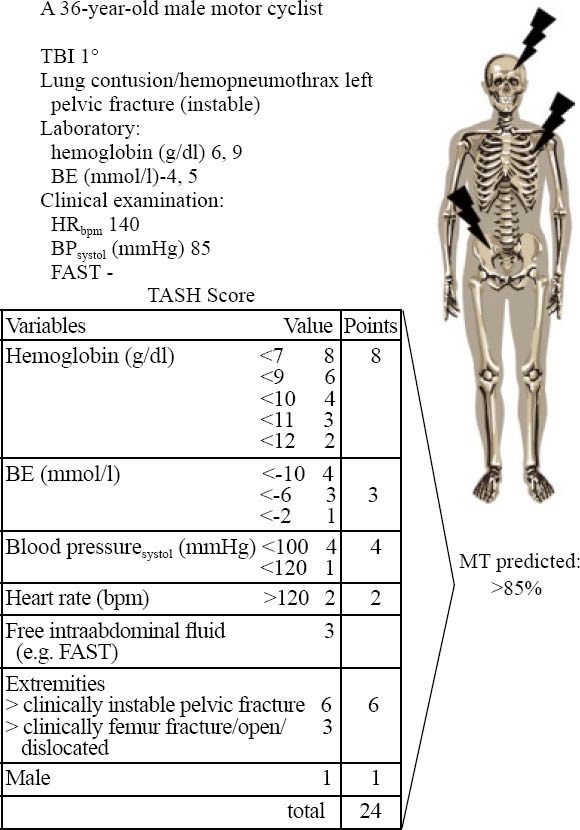
An example for the clinical application of the TASH-score to identify patients at risk for MT.
Current recommendations for coagulation management in acute care following major trauma
Early recognition together with adequate and aggressive management of acute early coagulopathy has been shown to reduce morbidity and mortality[7] and key recommendations for coagulation management in the acute phase after severe multiple injuries have been published.[14,15] Besides, general recommendations have been suggested for time management, monitoring, diagnostic procedures, surgical approaches for bleeding control including damage control surgery and embolization, and specific treatment strategies to support coagulation. These include physiological targets as well as the suggested use and dosing of blood-products, pharmacological agents, and coagulation factor replacement in the patient with acute bleeding trauma. Table 1 shows the current European guidelines for the management of bleeding following a major trauma.[15] A clinical strategy for blood volume replacement during the first 24 hours after injury is shown in Figure 7.
Table 1.
Synopsis of the current European guidelines for the management of bleeding after major trauma (adopted/modified from)[15]

Figure 7.
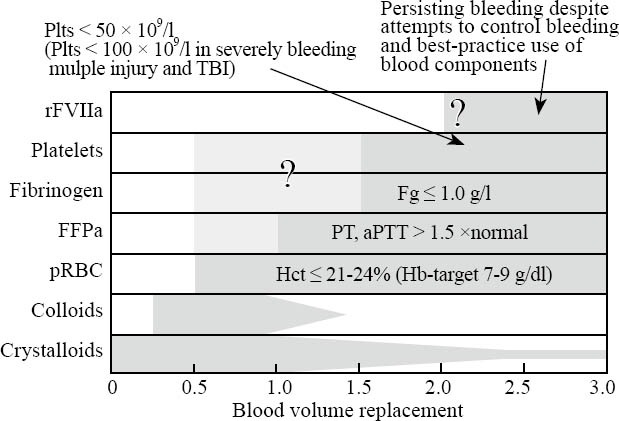
Clinical strategy for blood volume replacement during the first 24 hours after injury including triggering values for the administration of specific blood components. Recent studies have suggested a more aggressive coagulation management with different components administered early and in a balanced ratio. Antifibrinolytic agents (e.g. tranexamic acid, e-aminocaproic acid) should be considered in the treatment of the bleeding trauma patient but should be stopped once the bleeding is adequately controlled. PCC should be used for the emergency reversal of vitamin-K-dependent oral anticoagulants only; there is no place for the use of AT III in the treatment of the bleeding trauma patient. FFP:fresh frozen plasma; Fg: fibrinogen; Hb:haemoglobin; Hct:hematocrit; Plts:platelets; pRBC:packed red blood cells; PT:prothrombin time; aPTT:activated partial thromboplastin time; rFVIIA:recombinant factor Vila; TBI:traumatic brain injury (adopted/modified from[14])
Addressing an early and more balanced transfusion therapy in massively bleeding patients
Recent military experience suggests that the early use of fresh frozen plasma (FFP) together with packed red blood cell (pRBCs) concentration at a 1:1 ratio for casualties requiring MT (> 10 pRBC units/24 hr) is associated with improved outcome.[7,16,17] We retrospectively reviewed the datasets from the TR-DGU database for MT during the period of 2002 and 2006 to test the hypothesis whether an early aggressive transfusion at a pRBC:FFP ratio of 1:1 between ER arrival and ICU admission would be associated with reduced mortality.[18] Altogether 713 patients were identified for analysis and were divided into three groups according to the pRBC to FFP ratio transfused during immediate MT. One group had undergone MT with a pRBC:FFP ratio of more than 1.1, one with a pRBC:FFP ratio of 0.9-1.1 (1:1), and one with a pRBC:FFP ratio of less than 0.9. The mortality rates of patients were compared among the groups.
484/713 patients had undergone MT with a pRBC:FFP ratio of more than 1.1, 114 patients with a pRBC:FFP ratio of 0.9-1.1 (1:1), and 115 patients with a pRBC:FFP ratio of less than 0.9. There was no statistical difference between the three groups in injury characteristics and pathophysiological status at scene and upon ER arrival, laboratory findings, frequency of emergency interventions, and volume loading. The mortality rates at less than 6 hours, less than 24 hours, and 30 days in the three study groups are shown in Figure 8. 32.6% (158/484) of the patients with a pRBC:FFP ratio of more than 11 died at the first 24 hours after ER admission; the 30-day mortality rate of this group was 45.5% (220/484 patients). In contrast, the acute (<24 hours) and 30-day mortality rates were lower in patients who were transfused with a pRBC:FFP ratio of 0.9-1.1 (1:1) but lowest in those who had undergone MT with a pRBC:FFP ratio of <0.9. The acute (<24 hours) and 30-day mortality rates in this group were 11.3% (13/115 patients) and 24.3% (28/115 patients), respectively (Figure 8). The less than 6 hours mortality rates in the three groups were 24.6% (119/484 patients), 9.6% (11/114 patients), and 3.5% (4/115 patients).
Figure 8.
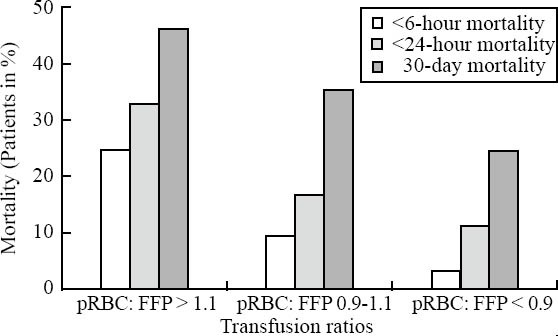
Mortality rates for patients transfused at different pRBC:FFP ratios during acute care. Early (< 6-hour and < 24-hour) and 30-day mortality rates in percent (%) for patients transfused at a pRBC:FFP ratio of > 1:1, a pRBC:FFP ratio of 0.9-1.1 (1:1), and a pRBC:FFP ratio of < 0.9 during immediate care.
DISCUSSION
The present study highlights the clinical relevance of ATC by providing recent data on its actual high frequency during acute trauma care, by suggesting a stratification tool to early identify patients at high risk for MT representing a surrogate for severe ongoing bleeding, and by providing current treatment strategies including novel approaches towards a more aggressive and a more balanced transfusion therapy. Early evidence suggests that the recognition accompanied by adequate and aggressive management of acute early coagulopathy would substantially reduce mortality and improve outcome in severely injured patients.[7]
ATC in one of four patients upon ER arrival has recently been identified to be associated with negative outcome.[19,20] Our data from the TR-DGU database revealed an incidence rate of 34.2%[9] and that the presence of ATC was associated with the amount of intravenous fluids administered during the pre-hospital care and the severity of injury.
Current literature suggests that ATC is multifactorial but there are certain mechanisms likely to be predominant while others are likely to manifest under specific circumstances only.[4] A recent review of mechanisms of ATC[4] proposed 6 key initiators in such patients: tissue trauma, shock, hemodilution, hypothermia, acidemia, and inflammation, with tissue trauma being the initiator and shock being the principal driver. In our datasets, the frequency of ATC was strongly associated with BE levels. Persisting shock leads to intravenous therapy and subsequent hemodilution that exacerbates the already established hemostatic disorder. The aggravation of the established coagulopathy is further triggered by hypothermia and acidemia if hemorrhage remains unchecked.
In general, trauma patients with ATC are at high risk to develop organ dysfunction and failure with prolonged ICU and overall hospital stay as well as increased mortality.[19-23] Some studies reported an up to 4-fold increase in mortality if coagulopathy is present upon ER arrival after severe multiple injuries.[19,20] MacLeod et al[20] reported coagulation abnormalities early after trauma to represent independent predictors of mortality even in the presence of other risk factors. Our data from the TR-DGU database presented here confirm these findings.
Although there is an increasing body of evidence for the frequency of ATC upon ER arrival, the identification of patients with active ongoing bleeding requiring MT is still unsatisfactory. Substantial problems include i.) the unavailability of global parameters within the very early in-hospital phase, i.e. PT and aPTT are usually not available until 30-40 minutes upon admission, and ii.) if available, their potential physiologic values.[19,24] Second, surgical relevant bleeding due to thoracic and/or retroperitoneal or intraperitoneal organ injury is difficult to detect and often requires time-consuming diagnostics.[25] Therefore, significant hemorrhage and coagulopathy may be underestimated or even missed during early resuscitation.[20,26] The awareness and recognition of a patient with a substantial bleeding problem and in potential need for MT at a very early stage after arrival would also be supportive in providing adequate logistics and infrastructure in tight collaboration with the local blood product supplier.
The TASH-score published previously by our group[13] is again recognized as an easy-to-calculate and valid scoring system to predict the individual's probability for MT and thus ongoing life-threatening hemorrhage at a very early stage after severe multiple injuries. All variables necessary to calculate the score are available within the first 15 minutes upon ER arrival. Furthermore, the variables are easily obtained not only in advanced trauma centers but also in less sophisticated facilities. The TASH-score was developed and validated in corresponding subsets on the basis of 6 044 severely injured patients from the TR-DGU database, and increasing TASH-scores equally corresponded to the increased probability for MT. To date, several predictive algorithms for MT have been suggested.[13,27-29] In comparing score performance reflected by ROC values, our TASH-score to date performed best with an area under the ROC curve of 0.892.[13] The score could be supportive in clinical decision-making for i.) early operative intervention in case of surgical bleeding, or ii.) early initiation of effective coagulation management. The score could also be employed in stratifying patients for trials of blood substitutes or other interventions, and in quantifying the success of such trials. One potential limitation of the TASH-score may be related to the fact that both development and validation of the score were performed on datasets from almost entirely blunt trauma patients (> 95%). It may be possible that the assumptions based upon datasets from those patients may not be appropriate for the penetrating trauma population.
The better understanding of the potential initiators and mechanisms that are responsible for the development of ATC has already led to some redirection in the management of bleeding patients after trauma with an emphasis on the early restoration of hemostasis. In this context, the concept of damage control resuscitation has been introduced[30] along with corresponding guidelines and recommendations to support coagulation in the acute phase after severe multiple injuries.[14,15] The current concepts and recommendations are based upon available strategies to aggressively correct ATC, to limit the duration of exposure to shock and hypoperfusion, and to reduce hemodilution, hypothermia and acidemia. The clinical role of inflammatory processes associated with ATC has not yet been fully elucidated.
A novel therapeutic approach includes the early administration of high-dose fresh frozen plasma to massively transfused trauma patients.[7] Since pRBCs also result in hemodilution and reduction of clotting ability,[31-34] mathematical modelling[35,36] as well as early clinical data[7,18,30,37] suggest that blood components should be administered in a fixed red blood cell: plasma: platelet ratio of 1:1:1 in order to avoid dilution and to substitute blood loss with a composition physiologically as similar as whole blood. In accordance, analysis of the data from the TR-DGU database in this study indicated a clear association between pRBC:FFP transfusion ratio and mortality after early aggressive FFP administration.[18]
The author is aware of the fact that the registry data like prospective observational studies share similar limitations in the comparability of therapeutic subgroups. Therefore, the observed associations do not necessarily represent causality. In addition, the retrospective nature of the present analyses eliminates the ability to determine whether the noted transfusion results reflect a proactive versus a reactive intervention. This, in turn, may be a limiting factor for assessing the role of transfusion in mitigating organ dysfunction.
There has been quite some discussion in the past on the potential gain attributed to early aggressive transfusion therapy. For data interpretation, additional information on treatment strategies used to control bleeding in the acute phase after trauma need to be considered as a delay in care could be a major determinant for increased transfusion requirement and outcome. This information, for example, includes the introduction of focused abdominal sonography for trauma (FAST),[38,39] the whole-body computed tomography[40-43] for prompt detection of bleeding, and the early use of interventional radiology[44] and surgical techniques such as damage control surgery[6,45,46] to control hemorrhage. Within the 1999-2005 TR-DGU database the time to completed radiography including chest and pelvic x-ray and FAST could be substantially reduced from 25 minutes (1999) to 15 minutes (2005).[47] Simultaneously, the use of whole-body computed tomography more than tripled from 1999 to 2005 while the rate of damage limiting orthopedic interventions for femur fractures in multiply injured patients increased to 53%.[47] The faster diagnostics translated into faster referral to both operation rooms for bleeding control in case of hemorrhagic shock and intensive care unit when no surgical intervention was necessary. The data from the same registry between 1993 and 2005 showed that the mortality decreased from 22.8% to 18.7%[47] as the percentage of patients requiring blood transfusion including massive transfusion and the number of blood concentrates transfused decreased.[48] Detailed information on the potential relationship of transfusion volume with surgical treatment is not available from the TR-DGU. There is no blood substitution during the pre-hospital phase of care among the hospitals or medical centers affiliated with the registry.
Despite the issues mentioned above, several experts have meanwhile revised their pre-ICU transfusion protocol emphasizing the early use of FFP in a pRBC:FFP ratio of 1:1.[7,16] This approach has recently been extended to the transfusion of an increased fibrinogen:pRBC ratio which was shown to be independently associated with improved survival to hospital discharge in patients with combat-related trauma requiring MT, primarily by decreasing death from hemorrhage.[49] By using a pro-active approach for an early balanced transfusion therapy in massively bleeding patients, Johannson and colleagues[50] introduced an acute transfusion package consisting of five units of pRBC, five unites of FFP, and two unites of PC (platelet concentration). A similar approach with a pre-defined ratio of the different blood products but based upon a validated guideline extended by timing of laboratory tests and a sound logistic protocol is urged by other authors.[51,52] However, further prospective studies are necessary to further elucidate this more balanced transfusion approach including the potential role of platelets and other therapeutic components.
Although key recommendations and guidelines for the management of bleeding following the major trauma have been issued,[14,15] the clinical management of ATC shows substantial differences in the speciality of physicians responsible for trauma management decisions. A recent qualitative international survey of clinical practice among senior physicians responsible for the treatment of patients with multiple injuries highlighted the variabilities of existing clinical practices around the world.[53] While blood loss, temperature, pH, platelets, prothrombin time/INR/activated partial thromboplastin time, and overall clinical assessment were the most common criteria for the assessment of coagulopathy, the management of hypothermia, acidosis, blood products, and adjuvant therapy showed a considerable amount of regional as well as institutional variability. Forty-five percent of the respondents claimed to follow a MT protocol in their institution, 19% reported inconsistent use, and 34% did not use a protocol at all for massive transfusion. Interestingly, only very few MT protocols specifically addressed the key issues recommended by the current guidelines. The results from this survey should draw more attention to the need for a more common definition of ATC and more standardized clinical protocols to ensure optimum patient care in the acute phase after severe multiple injuries.[53] These protocols should be based upon state-of-the art guidelines and recommendations being subject to updating and revision as important new evidence becomes available.
In conclusion, there is a high frequency of ATC upon ER admission which is associated with significant morbidity and mortality in multiply injured patients. The TASH-score is recognized as an easy-to-calculate and valid scoring system to predict the individual's probability for massive transfusion and thus ongoing life-threatening hemorrhage at a very early stage after severe multiple injuries. An early aggressive management of ATC including a more balanced administration of blood products to favor improved outcome should be proven in a well-controlled clinical trial.
Footnotes
Funding: None
Ethical approval: The TR-DGU is approved by the review board of the DGU and is in compliance with the institutional requirements.
Competing interest: There are no conflicts of interest associated with this article.
Contributors: Marc Maegele proposed the study and wrote the paper. TR-DGU and TR-DGU affiliated centers contributed to the study.
REFERENCES
- 1.Krug EG, Sharma GK, Lozano R. The global burden of injuries. Am J Public Health. 2000;90:523–526. doi: 10.2105/ajph.90.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 3.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 5.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion. Ann Surg. 2007;245:812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiber MA. Damage control surgery. Crit Care Clin. 2004;20:101–118. doi: 10.1016/s0749-0704(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 7.Borgman M, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 8.Arbeitsgemeinschaft “Scoring” der Deutschen Gesellschaft für Unfallchirurgie: Das Tramaregister der DGU. Unfallchirurg. 1994;97:230–237. [PubMed] [Google Scholar]
- 9.Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, et al. The AG Polytrauma of the German Trauma Society (DGU): Early coagulopathy in multiply injury: an analysis from the German Trauma Registry on ∔ patients. Injury. 2007;38:298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Thomas L. Labor und Diagnose. Indikation und Bewertung von Laborbefunden für die medizinische Diagnostik. 5. erweiterte Auflage, TH-Books Verlagsgesellschaft. 2000 [Google Scholar]
- 11.Lutze G. Mannheim: Roch Diagnostics GmbH (data on file); Useful facts about coagulation. [Google Scholar]
- 12.Wagner C, Dati F. Thomas L, editor. Thromboplastinzeit. Labor und Diagnose. TH-Books Verlagsgesellschaft. :613–616. [Google Scholar]
- 13.Yucel N, Lefering R, Maegele M, Vorweg M, Tjardes T, Ruchholtz S, et al. Trauma Associated Severe Haemorrhage (TASH)-Score: Probability of mass transfusion as surrogate for life threatening haemorrhage after multiple trauma. J Trauma. 2006;60:1228–1237. doi: 10.1097/01.ta.0000220386.84012.bf. [DOI] [PubMed] [Google Scholar]
- 14.Rossiant R, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Gordini G, et al. Key issues in advanced bleeding care. Shock. 2006;26:322–331. doi: 10.1097/01.shk.0000225403.15722.e9. [DOI] [PubMed] [Google Scholar]
- 15.Spahn D, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Gordini G, et al. Management of bleeding following major trauma. A European guideline. Crit Care. 2006:R17. doi: 10.1186/cc5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzales EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 17.Dann EJ, Michaelson M, Barzelay M, Hoffmann R, Bonstein L. Transfusion medicine during the summer of 2006: Lessons learned in Northern Isreal. Transfus Med Rev. 2008;22:70–76. doi: 10.1016/j.tmrv.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Maegele M, Lefering R, Paffrath T, Tjardes T, Simanski C, Bouillon B, et al. Red blood cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiply injury. A retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft für Unfallchirurgie. Vox Sang. 2008;95:112–119. doi: 10.1111/j.1423-0410.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 19.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 20.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 21.Blamback M. Blood coagulation and fibrinolytic factors as well as their inhibitors in trauma. Scand J Clin Lab Invest. 1985;178(Suppl):15–23. [PubMed] [Google Scholar]
- 22.Gando S, Kameune T, Nanzaki S, Hayakawa T, Nakanishi Y. Participation of tissue factor rand thrombin in posttraumatic systemic inflammatory syndrome. Crit Care Med. 1997;25:1820–1826. doi: 10.1097/00003246-199711000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Raum M, Bouillon B, Rixen D, Lefering R, Tiling T, Neugebauer E, et al. The prognostic value of prothrombin time in predicting survival after major trauma: a prospective analysis of 1,351 patients from the German Trauma Registry. Eur J Trauma. 2001;3:110–116. [Google Scholar]
- 24.Martinowitz U, Michaelson M. Guidelines for the use of recombinant activated factor VII (rFVIIa) in uncontrolled bleeding: a report by the Israeli Multidisciplinary rVIIa Task Force. J Thromb Haemost. 2005;3:640–648. doi: 10.1111/j.1538-7836.2005.01203.x. [DOI] [PubMed] [Google Scholar]
- 25.Advanced Trauma Life Support ATLS Student Manual. 7th Editions. Chicago, IL: American College of Surgeons; 2004. American College of Surgeons Abdominal trauma; p. 137. [Google Scholar]
- 26.Reed RL, Johnson TD, Hudson JD, Fischer RP. The disparity between hypothermic coagulopathy and clotting studies. J Trauma. 1992;33:465–470. doi: 10.1097/00005373-199209000-00022. [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin DF, Niles S, Salinas J, Perkins JG, Cox ED, Wade C, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008;64(Suppl 2):S57–S63. doi: 10.1097/TA.0b013e318160a566. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber MA, Perkins J, Kiraly L, Underwood S, Wade C, Holcomb JB. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;205:541–545. doi: 10.1016/j.jamcollsurg.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Moore F, McKinley B, Moore E, Nathens A, Rhee P, Puyana J, et al. Need for massive transfusion can be predicted early after trauma center arrival. J Trauma. 2007;62:270. [Google Scholar]
- 30.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 31.Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17:223–231. doi: 10.1016/s0887-7963(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 32.Ho AM, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation? Am J Surg. 2005;190:479–484. doi: 10.1016/j.amjsurg.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 33.Ketchum L, Hess JR, Hiippala S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma. 2006;60:S51–S58. doi: 10.1097/01.ta.0000199432.88847.0c. [DOI] [PubMed] [Google Scholar]
- 34.Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60:S91–S96. doi: 10.1097/01.ta.0000199549.80731.e6. [DOI] [PubMed] [Google Scholar]
- 35.Hirshberg A, Dugas M, Banez EI, Scott BG, Wall MR, Jr, Mattox KL. Minimizing dilutional coagulopathy in exsanguinating haemorrhage: a computer simulation. J Trauma. 2003;54:454–463. doi: 10.1097/01.TA.0000053245.08642.1F. [DOI] [PubMed] [Google Scholar]
- 36.Ho AM, Dion PW, Cheng CA, Karmakar MK, Cheng G, Peng Z, et al. A mathematical model for fresh frozen plasma transfusion strategies during major trauma resuscitation with ongoing haemorrhage. Can J Surg. 2005;48:470–478. [PMC free article] [PubMed] [Google Scholar]
- 37.Sperry JL, Ochoa JB, Gunn SR, Alarcon LH, Minei JP, Cuschieri J, et al. An FFP:PRBC transfusion ratio> 1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 38.Scalea TM, Rodriguez A, Chiu WC, Brenneman FD, Fallon WF, Jr, Kato K, et al. Focused Assessment with Sonography for Trauma (FAST): Results from an international consensus conference. J Trauma. 1999;46:466–472. doi: 10.1097/00005373-199903000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Heller K, Reardon R, Joing S. Ultrasound use in trauma: The FAST exam. Acad Emerg Med. 2007;14:525. doi: 10.1197/j.aem.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Linsenmaier U, Krötz M, Häuser H, Rock C, Rieger J, Bohndorf K, et al. Whole-body computed tomog.raphy in polytrauma: Techniques and management. Eur Radiol. 2002;12:1728–1740. doi: 10.1007/s00330-001-1225-x. [DOI] [PubMed] [Google Scholar]
- 41.Boehm T, Alkadhi H, Schertler T, Baumert B, Roos J, Marincek B, et al. Application of multislice spiral CT (MSCT) in multiple injured patients and its effect on diagnostic and therapeutic algorithms. Rofo. 2004;176:1734–1742. doi: 10.1055/s-2004-813740. [DOI] [PubMed] [Google Scholar]
- 42.Kanz KG, Körner M, Linsenmaier U, Kay MV, Huber-Wagner SM, Kreimeier U, et al. Priority-oriented shock trauma room management with the integration of multiple-view spiral computed tomography. Unfallchirurg. 2004;107:937–944. doi: 10.1007/s00113-004-0845-4. [DOI] [PubMed] [Google Scholar]
- 43.Geijer M, El-Khoury GY. MDCT in the evaluation of skeletal trauma: Principles, protocols, and clinical applications. Emerg Radiol. 2006;13:7–18. doi: 10.1007/s10140-006-0509-5. [DOI] [PubMed] [Google Scholar]
- 44.Arthurs ZM, Sohn VY, Starnes BW. Vascular trauma: Endovascular management and techniques. Surg Clin North Am. 2007;87:1179–1192. doi: 10.1016/j.suc.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 45.De Waele JJ, Vermassen FE. Coagulopathy, hypothermia and acidosis in trauma patients: The rationale for damage control surgery. Acta Chir Belg. 2002;102:313–316. doi: 10.1080/00015458.2002.11679322. [DOI] [PubMed] [Google Scholar]
- 46.Parr MJ, Alabdi T. Damage control surgery and intensive care. Injury. 2004;35:713–722. doi: 10.1016/j.injury.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Ruchholtz S, Lefering R, Paffrath T, Ostern HJ, Neugebauer E, Nast-Kolb D, et al. Rückgang der Traumaletalität [Reduction in mortality of severely injured patients in Germany] Dt Ärzteblatt. 2008;13:225–231. doi: 10.3238/arztebl.2008.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maegele M, Lefering R, Paffrath T, Simanski C, Wutzler S, Bouillon B, et al. Changes in transfusion practice in multiply injury between 1993 and 2006: A retrospective analysis on 5.389 patients from the German Trauma Registry. Transfusion Med. 2009;19:117–124. doi: 10.1111/j.1365-3148.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 49.Stinger HK, Spinella PC, Perkins JG, Grathwohl KW, Salinas J, Martini WZ, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64:S79–85. doi: 10.1097/TA.0b013e318160a57b. [DOI] [PubMed] [Google Scholar]
- 50.Johannson PI, Hansen MB, Sorensen H. Transfusion practice in massively bleeding patients: Time for a change? Vox Sang. 2005;89:92–96. doi: 10.1111/j.1423-0410.2005.00668.x. [DOI] [PubMed] [Google Scholar]
- 51.Kamphuisen PW, Geeraedts LMG, Demiral HM, Schaap NP, Frölke JPM. Transfusion of blood products in trauma patients with lethal hemorrhagic shock. J Thromb Haemostasis. 2005;3:S1397. [Google Scholar]
- 52.Geeraedts LM, Jr, Demiral H, Schaap NP, Kamphuisen PW, Pompe JC, Frölke JP. Blind” transfusion of blood products in exsanguinating trauma patients. Resuscitation. 2007;73:382–388. doi: 10.1016/j.resuscitation.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Hoyt DB, Dutton RP, Hauser CJ, Hess JR, Holcomb JB, Kluger Y, et al. Management of coagulopathy in the patients with multiple injuries: results from an international survey of clinical practice. J Trauma. 2008;65:755–765. doi: 10.1097/TA.0b013e318185fa9f. [DOI] [PubMed] [Google Scholar]


