Abstract
BACKGROUND:
In the management of critically ill patients, the assessment of volume responsiveness and the decision to administer a fluid bolus constitute a common dilemma for physicians. Static indices of cardiac preload are poor predictors of volume responsiveness. Passive leg raising (PLR) mimics an endogenous volume expansion (VE) that can be used to predict fluid responsiveness. This study was to assess the changes in stroke volume index (SVI) induced by PLR as an indicator of fluid responsiveness in mechanically ventilated patients with severe sepsis.
METHODS:
This was a prospective study. Thirty-two mechanically ventilated patients with severe sepsis were admitted for VE in ICU of the First Affiliated Hospital, Zhejiang University School of Medicine and Ningbo Medical Treatment Center Lihuili Hospital from May 2010 to December 2011. Patients with non-sinus rhythm or arrhythmia, parturients, and amputation of the lower limbs were excluded. Measurements of SVI were obtained in a semi-recumbent position (baseline) and during PLR by the technique of pulse indicator continuous cardiac output (PiCCO) system prior to VE. Measurements were repeated after VE (500 mL 6% hydroxyethyl starch infusion within 30 minutes) to classify patients as either volume responders or non-responders based on their changes in stroke volume index (ΔSVI) over 15%. Heart rate (HR), systolic artery blood pressure (ABPs), diastolic artery blood pressure (ABPd), mean arterial blood pressure (ABPm), mean central venous pressure (CVPm) and cardiac index (CI) were compared between the two groups. The changes of ABPs, ABPm, CVPm, and SVI after PLR and VE were compared with the indices at the baseline. The ROC curve was drawn to evaluate the value of ΔSVI and the change of CVPm (ΔCVPm) in predicting volume responsiveness. SPSS 17.0 software was used for statistical analysis.
RESULTS:
Among the 32 patients, 22 were responders and 10 were non-responders. After PLR among the responders, some hemodynamic variables (including ABPs, ABPd, ABPm and CVPm) were significantly elevated (101.2±17.6 vs.118.6±23.7, P=0.03; 52.8±10.7 vs. 64.8±10.7, P=0.006; 68.3±11.7 vs. 81.9±14.4, P=0.008; 6.8±3.2 vs. 11.9±4.0, P=0.001). After PLR, the area under curve (AUC) and the ROC curve of ΔSVI and ΔCVPm for predicting the responsiveness after VE were 0.882±0.061 (95%CI 0.759–1.000) and 0.805±0.079 (95%CI 0.650–0.959) when the cut-off levels of ΔSVI and ΔCVPm were 8.8% and 12.7%, the sensitivities were 72.7% and 72.7%, and the specificities were 80% and 80%.
CONCLUSION:
Changes in ΔSVI and ΔCVPm induced by PLR are accurate indices for predicting fluid responsiveness in mechanically ventilated patients with severe sepsis.
KEY WORDS: Passive leg raising, Volume resuscitation, Hemodynamic monitoring, Stroke volume index, Central venous pressure, Severe sepsis, Fluid responsiveness, ROC curve
INTRODUCTION
In critically ill patients with hypoperfusion, intravascular volume expansion (VE) is a cornerstone of hemodynamic therapy. Early resuscitation protocols including fluid therapy can be life-saving early in the course of sepsis.[1,2] However, VE may induce peripheral and pulmonary edema, and worsen microvascular perfusion and oxygen delivery in patients with right or left ventricular dysfunction.[3] In a preload unresponsive patient, large VE can exacerbate pulmonary edema, cause respiratory failure, prolong mechanical ventilation time, and contribute to the development of intra-abdominal hypertension.[4] Passive leg raising (PLR) was supposed to transfer venous blood from the legs toward the intrathoracic compartment, increasing the intrathoracic blood volume and the cardiac preload. The aim of the present study was to determine if SVI measurement could be used in conjunction with PLR to predict the hemodynamic response to VE.
METHODS
Patients
This study prospectively assessed consecutive patients admitted in the ICU (33 beds) of the First Affiliated Hospital, Zhejiang University School of Medicine and the ICU (26 beds) of Ningbo Medical Treatment Center Lihuili Hospital from May 2010 to December 2011. Thirty-two mechanically ventilated patients, defined septic chock[5] with acute circulatory failure, were eligible to participate in the study with written informed consent. Hemodynamic indices including SVI were monitored with the technique of pulse indicator continuous cardiac output (PiCCO) (Pulsion Medical Systems AG, Munich, Germany). Acute circulatory failure was defined as the presence of at least one clinical sign of inadequate tissue perfusion as follows: systolic blood pressure<90 mmHg (or a decrease of >40 mmHg in previously hypertensive patients) or the need for vasopressors (dopamine>5 μg/kg per minute or norepinephrine>0.1 μg/kg per minute) to maintain a systolic blood pressure>90 mmHg; urine output of <0.5 mL/kg per hour for at least 1 hour; tachycardia (heart rate>100/min); and mottled skin.[6] Non-sinus rhythm or arrhythmia ones and parturients were excluded.
Mechanical ventilation variables
The patients were sedated (Ramsay score 4) and ventilated in mode of volume control. The tidal volume was 10 mL/kg and the level of positive end-expiratory pressure was 5 cmH2O (1 cmH2O=0.098 kPa).
Measurements
A 4F thermistor-tipped arterial catheter (Pulsiocath thermodilution catheter; Pulsion Medical Systems, Munich, Germany) was inserted in the femoral artery, which connected to the PiCCO (Pulsion Medical Systems, Munich, Germany) and the bedside monitor (IntelliVue MP50/70: Philips Medical System, Boeblingen, Germany). Hemodynamic indices were determined using a triplicate injection of 15 mL ice-cold normal saline within 5 minutes through an additional 7 F central venous catheter introduced in the right subclavian vein. The bolus thermodilution measurements were made by the same observer to avoid interobserver variation.
Data collection
Study measurements were taken in four stages (Figure 1). In stage one, the patient was placed in a semi-recumbent position with the head elevated at 45 degrees, and hemodynamic indices were collected as the baseline. In stage two, the patient was placed in a supine position with the legs straight and elevated at 45 degrees for two minutes before hemodynamic indices were taken. In stage three, the patient was returned to the baseline position. In stage four, hemodynamic indices were immediately collected after VE (500 mL 6% hydroxyethyl starch infusion within 30 minutes).
Figure 1.
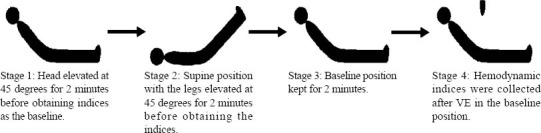
Patient position in the four stages of measurement.
Calibrated automatic bed elevation (using standard ICU beds) was used to move the patient between stages. Vasopressor doses and ventilator settings were not changed at any time while a patient was being studied. Patients were classified according to their hemodynamic response to VE. Responders were defined as the change of SVI (ΔSVI) not less than 15% in response to VE (ΔSVI from stage one to stage four), while non-responders were defined as ΔSVI less than 15%. A cutoff value of 15% was reported as a significant difference by thermodilution.[7]
Statistical analysis
Numerical data were expressed as mean±SD. Responder and nonresponder values were compared using an independent-sample Student’s t test. The values before and after PLR, before and after VE, and between stage 2 and stage 4 were compared using a paired-sample Student’s t test. Qualitative variables were reported as number and percentage and compared between the groups using Fisher’s exact test. The receiver-operating characteristic curves±SE were compared using the Hanley-McNeil test. Cut-off values for ΔSVI and for the change of CVPm (ΔCVPm) were chosen to correspond to the best respective Youden’s index calculated as follows: Youden’s index=sensitivity+specificity-1. Threshold indicator values such as sensitivity and specificity were calculated for each hemodynamic indicator tested. A P<0.05 was considered statistically significant. Statistical analysis was performed using SPSS 17.0 software (SPSS, Chicago, IL) for all tests.
RESULTS
Clinical data
Thirty two patients, 11 females and 21 males, aged 59.4±14.2 years, were included in the study. The cause of septic shock was pneumonia in 21 (65.6%) patients, bloodstream infection in 7 (21.9%), and abdominal infection in 4 (12.5%). ΔSVI increased by 15% or more in 22 (68.8%) patients (responders), and by less than 15% in 10 (31.2%) patients (non-responders). No statistical difference was found between responders and non-responders for age, sex, body mass index (BMI), days of ICU admission, days of mechanical ventilation, APACHEII scores and mortality (Table 1).
Table 1.
Descriptive clinical data
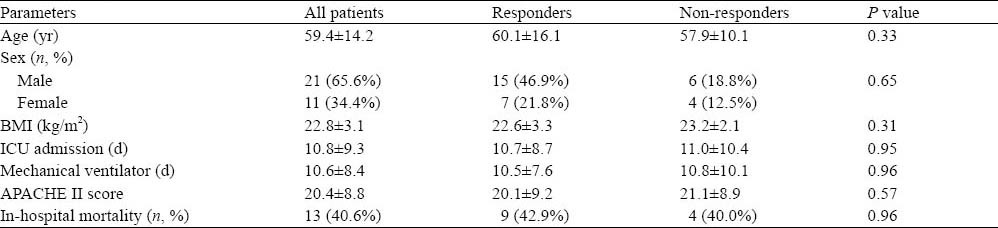
Baseline data
The responders had significantly lower initial HR, ABPs, ABPd, ABPm, CVPm and CI compared with the non-responders at the baseline (P<0.05) (Table 2).
Table 2.
Initial hemodynamic readings taken in stage one Table 3. Hemodynamic indices taken throughout the four stages of measurement
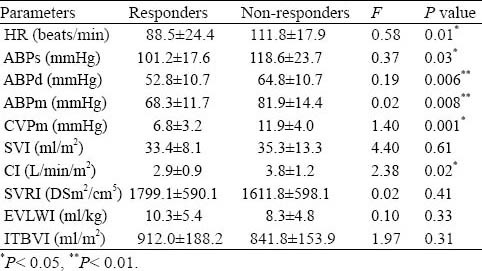
Differences between the two groups
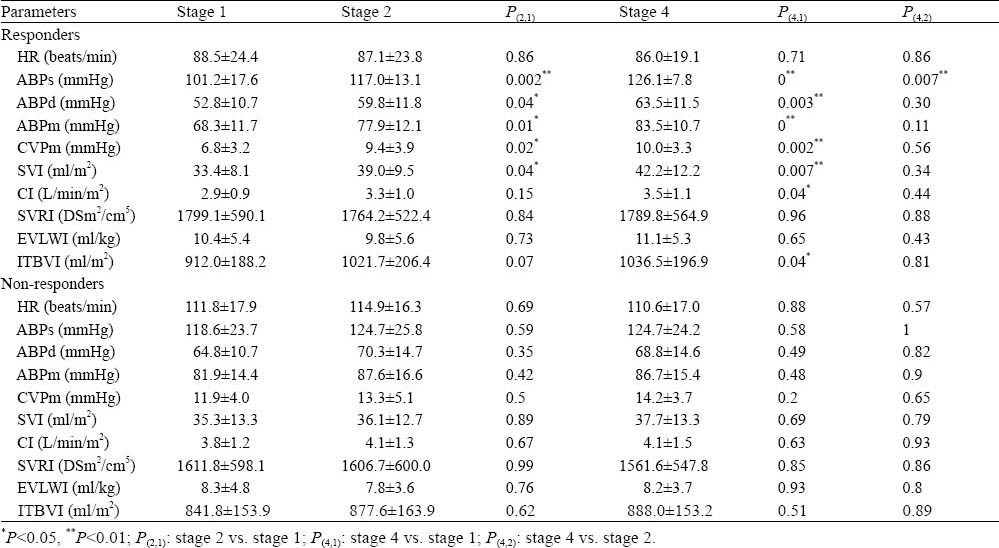
Changes in ABPs, ABPd, ABPm, CVPm and SVI compared with stage one induced by PLR and VE were significantly higher in the responders than in the non-responders (P<0.05). In the non-responders, neither PLR nor VE induced a significant change in any of the hemodynamic values measured (Table 3).
Table 3.
Hemodynamic indices taken throughout the four stages of measurement
ROC curve
The ΔSVI of 8.8% predicted fluid responsiveness with a sensitivity of 72.7% and a specificity of 80%, AUC (mean±SE)=0.882±0.061 (95%CI 0.759– 1.000); whereas the ΔCVPm of 12.7% predicted fluid responsiveness with a sensitivity of 72.7% and a specificity of 80%, AUC (mean±SE)=0.805±0.079 preload(95%CI 0.650–0.959) (Figure 2).
Figure 2.
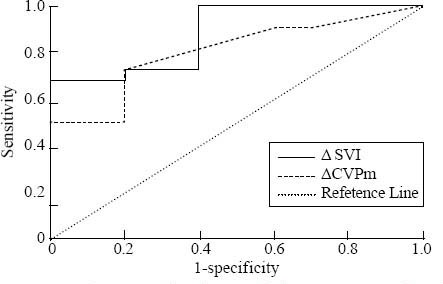
Receiver-operating characteristic curves comparing the capacity of changes induced by passive leg raising in ΔSVI and ΔCVPm to discriminate responders and non-responders regarding volume expansion in the overall population.
DISCUSSION
Blood volume plays an important role in the hemodynamic stability, which determines oxygen supplied to the tissues. Rapid infusion of crystalloids or colloids is the usual treatment for symptomatic hypovolemia.[8] Because VE does not always improve hemodynamic status, predictive parameters of fluid responsiveness are greatly needed. Blood volume is difficult to be measured at the bedside, so clinicians need to know whether left ventricular SVI increases after VE.[9] A simple, non-invasive bedside test for volume responsiveness determination which could assist clinicians in facing this daily dilemma might be of significant use.
PLR is a reversible maneuver that mimics rapid VE by shifting venous blood from the lower limbs toward the intrathoracic compartment.[10] The classic lower limb raising mimics a 300 mL VE.[11] Given that trunk lowering may induce a 150 mL increase in intrathoracic blood volume,[12] we suggest that the PLR maneuver used in our study may mimic a VE of approximately 450 mL. Thus, PLR increases the cardiac preload and, by definition increases SVI if the heart is preloaddependent. However, the effects of PLR on cardiac output are variable, probably depending on the degree of the existence of cardiac preload dependence.[13] The responders in the study had significantly lower initial HR, ABPs, ABPd, ABPm, CVPm and CI compared with the non-responders. This suggested that the preload of the responders at the baseline was lower than that of the non-responders. After PLR, the indices including ABPs, ABPd, ABPm, CVPm and SVI elevated in the responders (P<0.05) as the consequences after VE. While the indices mentioned were not different between baseline and after PLR or between baseline and after VE in the non-responders (P>0.05). The study demonstrated that a convenient ΔSVI measurement in conjunction with PLR could predict the hemodynamic response to VE. Changes in hemodynamic parameters such as SVI induced by PLR are accurate and interchangeable indices for predicting fluid responsiveness in non-intubated critically ill patients.[7,8,14] The changes in ΔSVI and ΔCVPm induced by PLR were also proved to be predictive of fluid responsiveness in ventilated septic shock patients in the study. When mechanical ventilation is proceeding, the volume of blood enclosed by the thoracic and splanchnic beds is stressed by positive airway pressure, and these vascular compartments are less compliant than when mechanical ventilation is not required. In these conditions, the increase in ΔSVI induced by PLR was expected to be higher in mechanically ventilated patients than in non-intubated patients. So, PLR is a reversible maneuver that mimics rapid VE which might be widely used to evaluate the hemodynamic changes.[13]
The usual static hemodynamic parameters are not reliable indexes of the cardiac preload, so VE with invasive measurement of cardiac output is widely used to detect cardiac preload dependence which may result in worsening pulmonary edema. The dilemma of which patients are subject to VE is encountered daily in the ICU. One of the principal uses for the pulmonary artery catheter (PAC) was to differentiate between various etiologies of hypotension and thereby guide therapy to optimize the hemodynamic status of a patient. However, with numerous clinical trials showing no benefit and concerns about safety, PAC is being used infrequently now in North American ICUs.[15] Transpulmonary thermodilution integrated in the PiCCO system does not require PAC placement and thus avoids the related risks, so it has been widely used for hemodynamic monitoring. Many physicians regarded CVP as a poor predictor of volume responsiveness and should not be used to make clinical decisions on fluid management.[16] But ΔCVPm induced by PLR was proved to be predictive of fluid responsiveness in this study. It suggested that dynamic changes in CVPm induced by PLR were more predictive of the preload than those static indicators on condition that the intrathoracic pressure, intra-abdominal pressure and ventricular compliance remaining unchanged. Thus the dynamic changes in CVPm induced by PLR provide a new method in preload estimation for wards without the condition of hemodynamic monitoring.
Sebastien et al[8] reported that SVI, measured by transthoracic echocardiography in conjunction with PLR, was an accurate index of fluid responsiveness by the research of the non-intubated septic shock patients. And ΔSVI>10% predicted fluid responsiveness with a sensitivity of 86% and a specificity of 90%. Lafanechère and colleagues[17] examined 22 intubated and fully sedated patients with an esophageal Doppler monitor in place. An increase in aortic blood flow of more than 8% during PLR predicted volume response with a sensitivity of 90% and a specificity of 83%, somewhat higher than the sensitivity and specificity in this study. It might be due to the measurement of PiCCO. But the feasibility of transthoracic echocardiography is variable which depends on hospital equipment, patient echogenicity, and physicians’ skills. However, use of the PiCCO method to manage critically ill patients has been proved to decrease the need for vasopressors and mechanical ventilation despite a larger infused volume.[18] The advantage of PiCCO method is that it can be conveniently used to predict the volume responsiveness in conjunction with PLR in intubated patients with septic shock.
Catecholamines with α-adrenergic properties, by their venous vasoconstrictor effects, could have affected the results. They might have shifted venous blood from an unstressed to a stressed volume and might have amplified the preload augmentation effect of PLR in the patients. However, this phenomenon did not affect the interpretation of the results since the dosages of the catecholamines kept unchanged during the study.
The results of this study suggest that in septic shock patients receiving mechanical ventilation, the hemodynamic response to VE could be predicted by simply measuring ΔCVPm and ΔSVI in conjunction with PLR. It may have two practical implications: the existence of a cardiac preload dependence could be detected without the use of a PAC; and a potentially harmful fluid-loading procedure could be avoided when unnecessary.
Footnotes
Funding: None.
Ethical approval: The study was approved by the Ethical Committee of Ningbo Medical Treatment Center Lihuili Hospital and the First Affiliated Hospital, Zhejiang University School of Medicine.
Conflicts of interest: There are no competing interests involving this study.
Contributors: Dong ZZ proposed and wrote the paper. Zheng X and Shi H provided technical support. All authors approved the final version.
REFERENCES
- 1.Micek ST, Roubinian N, Heuring T, Bode M, Williams J, Harrison C, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2006;34:2707–2713. doi: 10.1097/01.CCM.0000241151.25426.D7. [DOI] [PubMed] [Google Scholar]
- 2.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson ND, Meade MO, Hallett DC, Stewart TE. High values of the pulmonary artery wedge pressure in patients with acute lung injury and acute respiratory distress syndrome. Intensive Care Med. 2002;28:1073–1077. doi: 10.1007/s00134-002-1354-y. [DOI] [PubMed] [Google Scholar]
- 4.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, et al. National Heart Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 5.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM / ESICM / ACCP / ATS / SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 6.Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34:1402–1407. doi: 10.1097/01.CCM.0000215453.11735.06. [DOI] [PubMed] [Google Scholar]
- 7.Maizel J, Airapetian N, Lorne E, Tribouilloy C, Massy Z, Slama M. Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med. 2007;33:1133–1138. doi: 10.1007/s00134-007-0642-y. [DOI] [PubMed] [Google Scholar]
- 8.Préau S, Saulnier F, Dewavrin F, Durocher A, Chagnon JL. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med. 2010;38:819–825. doi: 10.1097/CCM.0b013e3181c8fe7a. [DOI] [PubMed] [Google Scholar]
- 9.Antonelli M, Levy M, Andrews PJ, Chastre J, Hudson LD, Manthous C, et al. Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, 27–28 April 2006. Intensive Care Med. 2007;33:575–590. doi: 10.1007/s00134-007-0531-4. [DOI] [PubMed] [Google Scholar]
- 10.Jabot J, Teboul JL, Richard C, Monnet X. Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med. 2009;35:85–90. doi: 10.1007/s00134-008-1293-3. [DOI] [PubMed] [Google Scholar]
- 11.Boulain T, Achard JM, Teboul JL, Richard C, Perrotin D, Ginies G. Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest. 2002;121:1245–1252. doi: 10.1378/chest.121.4.1245. [DOI] [PubMed] [Google Scholar]
- 12.Buhre W, Weyland A, Buhre K, Kazmaier S, Mursch K, Schmidt M, et al. Effects of the sitting position on the distribution of blood volume in patients undergoing neurosurgical procedures. Br J Anaesth. 2000;84:354–357. doi: 10.1093/oxfordjournals.bja.a013439. [DOI] [PubMed] [Google Scholar]
- 13.De Hert SG, Robert D, Cromheecke S, Michard F, Nijs J, Rodrigus IE. Evaluation of left ventricular function in anesthetized patients using femoral artery dP/dt (max) J Cardiothorac Vascanesth. 2006;20:325–330. doi: 10.1053/j.jvca.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33:1125–1132. doi: 10.1007/s00134-007-0646-7. [DOI] [PubMed] [Google Scholar]
- 15.Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet. 2005;366:472–477. doi: 10.1016/S0140-6736(05)67061-4. [DOI] [PubMed] [Google Scholar]
- 16.Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64–68. doi: 10.1097/01.CCM.0000249851.94101.4F. [DOI] [PubMed] [Google Scholar]
- 17.Lafanechère A, Pène F, Goulenok C, Delahaye A, Mallet V, Choukroun G, et al. Changes in aortic blood flow induced by passive leg raising predict fluid responsiveness in critically ill patients. Critical Care. 2006;10:R132. doi: 10.1186/cc5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uchino S, Bellomo R, Morimatsu H, Sugihara M, French C, Stephens D, et al. Pulmonary artery catheter versus pulse contour analysis: a prospective epidemiological study. Crit Care. 2006;10:R174. doi: 10.1186/cc5126. [DOI] [PMC free article] [PubMed] [Google Scholar]


