Abstract
BACKGROUND:
Induction of hypothermia (a 4 °C decrease from baseline) improves outcomes in adult cardiac arrest and neonatal hypoxic ischemic encephalopathy, and may benefit other conditions as well. Methods used to implement or prevent hypothermia typically require skin contact with blankets or pads or intravascular access with catheter devices. The study was to evaluate the potential to induce mild therapeutic hypothermia via an esophageal route in a porcine model.
METHODS:
Single-animal proof-of-concept study of a prototype esophageal device in a 70 kg Yorkshire swine. We measured the rate of temperature change after placement of a prototype device to induce hypothermia via the esophagus, and compared this rate to known temperature changes that occur under similar laboratory conditions without a hypothermic device.
RESULTS:
Swine temperature decreased from a starting temperature of 37.8 °C to 33.8 °C (achieving the goal of a 4 °C decrease) in 175 minutes, resulting in a cooling rate of 1.37 °C/h. Histopathology of the esophagus showed normal tissue without evidence of injury.
CONCLUSION:
A prototype of an esophageal cooling device induced hypothermia effectively in a large single-swine model.
KEY WORDS: Mild therapeutic hypothermia, Esophagus, Swine, Cardiac arrest
INTRODUCTION
Induction of hypothermia (a 4 °C decrease from baseline) appears to improve outcomes in at least two clinical conditions (adults resuscitated from cardiac arrest and neonates suffering from hypoxic ischemic encephalopathy), and may be of benefit in others, such as spinal cord injury, stroke, meningitis and some subsets of traumatic brain injury.1-8] Nevertheless, the overall use of therapeutic hypothermia in eligible patients has remained low.[9-11]
Methods currently used to induce hypothermia typically require skin contact with blankets or pads, or intravascular access with catheter devices. These methods involve some limitations inherent in their approach, and surveys suggest that the technical difficulties involved in some methods may contribute to the underuse of treatment.[9] Specific challenges with intravascular devices include the need to invasively access central circulation via needle puncture, the requirement for sterility in the placement of catheters, and the need for physician time and expertise for placement. Specific challenges with skin contact devices include the need to cover large critical areas of the skin and inefficiency in heat exchange across the skin which results in longer times to achieve goal temperature. Alternative approaches may be useful in helping to increase the number of treatment options available to physicians. The aim of this study was to evaluate a new esophageal device prototype in a swine model in order to determine if this approach could successfully induce mild therapeutic hypothermia.
METHODS
Study design and setting
This was a single-animal proof-of-concept study performed under a protocol approved by the hospital’s Institutional Animal Care and Use Committee (IACUC). The study utilized methods consistent with current veterinary and USDA standards, with a state-of-the-art, Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International-accredited vivarium. Animal care and handling was in accord with the Office of Laboratory Animal Welfare guidance for humane care and use of animals and with regulations outlined in the USDA Animal Welfare Act (9 CFR Parts 1, 2 and 3) and the conditions specified in the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington DC, 1996).
Interventions
One Yorkshire swine (Sus scrofa) weighing 70 kg was selected for this study. After acclimation, the swine was medicated intramuscularly with a pre-anesthetic mix of Telazol (tiletamine/zolazepam) 4.4 mg/kg and xylazine 2.0 mg/kg. The swine was endotracheally intubated and anesthetized with 3% inhalational isoflurane. Because the minimum alveolar concentration (MAC) of isoflurane for this size and species of swine is below 2%, and because reductions in body temperature further reduce the MAC, a concentration of 2% inhalational isoflurane was used for maintenance. Shivering was further prevented with intravenous pancuronium.
The experimental device prototype was designed to be placed through the mouth into the esophagus. The prototype is made of extruded medical-grade silicone, and is similar in size and shape to a standard orogastric tube (70 cm length and 1.2 cm diameter). Within the device are chambers to provide pathways for the flow of cold water from an external heat exchanger, and a central channel to allow suction and decompression of the stomach. The chambers are arranged concentrically, so that cold water flows from the inlet distally along the outer chamber, then returns proximally through the middle chamber. The central channel remains open to gastric contents, with the proximal end connected to wall suction.
After endotracheal intubation and achievement of deep anesthesia, the experimental device was placed in the esophagus and connected to an external heat exchange unit (Gaymar Medi-Therm, Gaymar industries, Inc., Orchard Park, New York) which uses distilled water as the coolant. The length of insertion of the device was determined by external measurement and subsequent marking of the appropriate distance on the device with surgical tape. The tip of the device was lubricated with a water soluble lubricant and inserted through the oropharynx into the esophagus to the marked depth. Confirmation of adequate placement was confirmed by auscultation of stomach sounds during insufflation of 20 mL of air via a syringe, followed by successful withdrawal of stomach contents through the central gastric access pathway within the device. Intermittent wall suction was then connected to the gastric outlet to provide continuous gastric decompression. Continuous cardiac monitoring was performed with a 3-lead EKG rhythm recorder. Room temperature was 22 °C. Intravenous access was obtained by cannulation of an ear vein, and normal saline was administered at a maintenance rate.
Outcome measures
Our primary outcome measure was swine temperature. Temperature was recorded with a YSI 400 series compatible rectal temperature probe which was connected to the input patient probe connector on the external heat exchange unit, providing a continuous digital display of swine temperature. Swine temperature was recorded manually at five-minute intervals throughout the study. The resulting temperature change was then compared to existing published data describing temperature profiles under similar control laboratory conditions in which no device to induce hypothermia was used.[12-14]
RESULTS
After initiation of inhalational anesthesia, the starting swine temperature was 37.8 °C. With the temperature of the coolant in the heat exchange unit set to 4 °C, swine temperature decreased to 33.8 °C (achieving the goal of a 4 °C decrease) in 175 minutes (Figure 1), equating to a drop of 1.37 °C per hour. Measurement of coolant flow through the device demonstrated a rate of 740 mL/min of distilled water. Comparison to existing data on the temperature profile of swine under similar conditions of anesthesia, but without attempting to induce hypothermia, shows temperature drops of less than 1 °C over a similar time frame (or approximately 0.3 °C per hour).[12]
Figure 1.
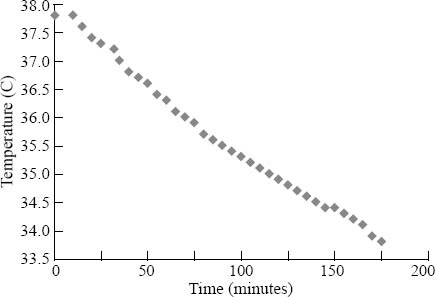
Plot of swine temperature over time.
Cardiac monitoring showed some bradycardia (pulse less than 60 beats per minute, but greater than 40 beats per minute, without hemodynamic changes) without ectopy. Histopathology of the esophagus using standard microscopic evaluation of thin sections of tissues was observed after necropsy by a certified veterinary pathologist immediately after device removal. The findings revealed normal tissue with mild hyperkeratosis considered to be within the range or normal, without evidence of injury.
DISCUSSION
This study demonstrates that a non-invasive approach to reducing patient temperature via contact with the esophagus is feasible. Existing methods used to induce mild therapeutic hypothermia typically involve surface contact devices or intravascular devices. Surface contact devices can be somewhat cumbersome to place, and may obstruct access to the patient by providers when attempting to perform procedures or cardiopulmonary examination. Because resistance to heat transfer across skin may increase as body temperature decreases and blood is shunted away from skin via vascular smooth muscle contraction, the time to attainment of goal temperature can be prolonged. Intravascular devices are fairly invasive, technically challenging to place, require sterility, and are costly.
An esophageal device to induce hypothermia would overcome many limitations of existing approaches to induce mild therapeutic hypothermia, and has been proposed as feasible based on mathematical modeling.[15] Because the esophagus is surrounded by a large volume of blood flow, the available heat transfer capacity (via conduction across the mucosa and subsequent convection through surrounding blood flow) is large and not likely to be diminished with temperature reduction. Placement of an esophageal cooling device does not require sterility or surgical skill, does not require vascular access, and is relatively unobtrusive to patient access, simply replacing the nasogastric or orogastric tube often placed in patients receiving mild therapeutic hypothermia. This device is also not accompanied by the risk of needlestick injuries to healthcare providers.
Early previous attempts of patient temperature reduction via the gastrointestinal tract met with inconsistent success due to device configurations, approaches, and materials available at the time.[16-19] The prototype device presented in this work provides an approach to induce hypothermia via an esophageal route while maintaining gastric access for suctioning and decompression, which may provide improved contact between the heat exchange surface and the esophageal mucosa by minimizing or preventing gaseous distention of the esophageal wall. Moreover, the configuration of the coolant flow pathway of the device (using concentric chambers with counter-current flow of coolant) allows for substantial volume of coolant to pass through the device, further increasing the heat transfer capacity. The cooling rate achieved in this experiment (1.37 °C per hour) compares favorably to other devices, with reported rates ranging from 0.31 °C/h using a saline infusion and application of ice packs to 1.5 °C/h using intravascular catheters, with a multi-modality approach recently reported to achieve a 2.6 °C/h cooling rate.[20,21]
Limitations
This study provides initial data suggesting that induction of hypothermia via an esophageal route is feasible; however, confirmation of these results in more than one animal may be warranted. Although histopathologic analysis of the esophagus was performed on a total of eight sections of the esophagus, endoscopic analysis of the length of the esophageal mucosa, which may identify unexpected changes, was not performed. Additionally, the histopathologic analysis was performed on the tissue obtained immediately after study termination and swine euthanasia; longer term monitoring will be required to exclude any delayed manifestations of esophageal changes that may result from this approach to inducing hypothermia. In accord with principles to ensure the smallest possible number of subjects, and because ample data exist demonstrating that temperature typically decreases by less than 1 °C during anesthesia with provision of maintenance IV fluids, we did not include a control animal to measure temperature under conditions without the use of the cooling device. For example, existing published data on swine undergoing anesthesia and surgical procedures under control conditions with inhalational anesthesia and infusion of maintenance IV fluids show a mean temperature drop of less than 1 °C over 180 minutes of monitoring.[12-14] We did not perform invasive intravascular temperature measurements, but rather relied on rectal temperature as a surrogate of core temperature. Although rectal temperature may lag that of the pulmonary artery by up to 5 minutes,[22] rectal monitoring is a generally accepted substitute.[23,24] It was felt to be adequate for the present proof-of-concept study.
In conclusion, a prototype of an esophageal device designed to modify body temperature was able to induce hypothermia effectively in a large single-swine model. Further investigation is warranted in determining the safety and efficacy of this approach in a larger number of animals over the longer treatment times typically recommended for mild therapeutic hypothermia after cardiac arrest and neonatal hypoxic ischemic encephalopathy.
Footnotes
Funding: The Advanced Cooling Therapy, LLC provided funding for this study.
Ethical approval: This study was approved by the Feinberg School of Medicine Administration Panel of Laboratory Animal Care, Chicago, USA.
Conflicts of interest: Dr. Kulstad is an equity owner of a company, Advanced Cooling Therapy, LLC, that provided funding for this study. The authors were solely responsible for study design, collection, analysis, and interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication.
Contributors: All authors have made substantive contributions to the study, and all authors endorse the data and conclusions.
REFERENCES
- 1.HACA. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 2.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S, Laptook AR, Poole WK. Hypothermia for perinatal asphyxial encephalopathy. N Engl J Med. 2010;362:1051–1052. doi: 10.1056/NEJMc0912848. author reply 1052. [DOI] [PubMed] [Google Scholar]
- 4.Sinclair HL, Andrews PJ. Bench-to-bedside review: Hypothermia in traumatic brain injury. Crit Care. 2010;14:204. doi: 10.1186/cc8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froehler MT, Ovbiagele B. Therapeutic hypothermia for acute ischemic stroke. Expert Rev Cardiovasc Ther. 2010;8:593–603. doi: 10.1586/erc.09.129. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich WD, Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7:43–50. doi: 10.1016/j.nurt.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mourvilliers B. IHPOTOTAM: Induced HyPOthermia TO Treat Adult Meningitis. ClinicalTrials.gov Identifier: NCT00774631. [Accessed March 23, 2011]. Available at: http://clinicaltrials.gov/ct2/show/study/NCT00774631 .
- 8.Kelly FE, Nolan JP. The effects of mild induced hypothermia on the myocardium: a systematic review. Anaesthesia. 2010;65:505–515. doi: 10.1111/j.1365-2044.2009.06237.x. [DOI] [PubMed] [Google Scholar]
- 9.Abella BS, Rhee JW, Huang KN, Vanden Hoek TL, Becker LB. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64:181–186. doi: 10.1016/j.resuscitation.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Brooks SC, Morrison LJ. Implementation of therapeutic hypothermia guidelines for post-cardiac arrest syndrome at a glacial pace: Seeking guidance from the knowledge translation literature. Resuscitation. 2008;77:286–292. doi: 10.1016/j.resuscitation.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Merchant RM, Becker LB, Abella BS, Asch DA, Groeneveld PW. Cost-effectiveness of therapeutic hypothermia after cardiac arrest. Circ Cardiovasc Qual Outcomes. 2009;2:421–428. doi: 10.1161/CIRCOUTCOMES.108.839605. [DOI] [PubMed] [Google Scholar]
- 12.Lima-Rodriguez JR, Garcia-Gil FA, Garcia-Garcia JJ, Rocha-Camarero G, Martin-ancho MF, Luis-Fernandez L, et al. Effects of premedication with tiletamine/zolazepam/ medetomidine during general anesthesia using sevoflurane/ fentanyl in swine undergoing pancreas transplantation. Transplant Proc. 2008;40:3001–3006. doi: 10.1016/j.transproceed.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 13.Wenzel V, Padosch SA, Voelckel WG, Idris AH, Krismer AC, Bettschart-Wolfensberger R, et al. Survey of effects of anesthesia protocols on hemodynamic variables in porcine cardiopulmonary resuscitation laboratory models before induction of cardiac arrest. Comp Med. 2000;50:644–648. [PubMed] [Google Scholar]
- 14.Greene SA, Benson GJ, Tranquilli WJ, Grimm KA. Effect of isoflurane, atracurium, fentanyl, and noxious stimulation on bispectral index in pigs. Comp Med. 2004;54:397–403. [PubMed] [Google Scholar]
- 15.Vaicys V, Eason A, Schieber JD, Kulstad EB. Therapeutic hypothermia induction via an esophageal route-a computer simulation. Am J Emerg Med. 2011 Jun 10; doi: 10.1016/j.ajem.2011.04.026. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen YH, Leikersfeldt G, Drenck NE. Forced-air surface warming versus oesophageal heat exchanger in the prevention of peroperative hypothermia. Acta Anaesthesiol Scand. 1998;42:348–352. doi: 10.1111/j.1399-6576.1998.tb04928.x. [DOI] [PubMed] [Google Scholar]
- 17.Bräuer A, Weyland W. Oesophageal heat exchanger in the prevention of perioperative hypothermia. Acta Anaesthesiologica Scandinavica. 1998;42:1232–1233. doi: 10.1111/j.1399-6576.1998.tb05286.x. [DOI] [PubMed] [Google Scholar]
- 18.Dunn JE, Williams LF. Esophageal cooling as a technic of selective brain hypothermia. Techn Docum Rep Sam-Tdr-63-19. Technical documentary report United States Air Force Systems Command. 1963;94:1–7. [PubMed] [Google Scholar]
- 19.Rodgers JB, Older TM, Stabler EV. Gastric hypothermia: a critical evaluation of its use in massive upper gastrointestinal bleeding. Ann Surg. 1966;163:367–372. doi: 10.1097/00000658-196603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoedemaekers CW, Ezzahti M, Gerritsen A, van der Hoeven JG. Comparison of cooling methods to induce and maintain normo-and hypothermia in intensive care unit patients: a prospective intervention study. Crit Care. 2007;11:R91. doi: 10.1186/cc6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kory P, Weiner J, Mathew JP, Fukunaga M, Palmero V, Singh B, et al. A rapid, safe, and low-cost technique for the induction of mild therapeutic hypothermia in post-cardiac arrest patients. Resuscitation. 2011;82:15–20. doi: 10.1016/j.resuscitation.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Hanneman SK, Jesurum-Urbaitis JT, Bickel DR. Comparison of methods of temperature measurement in swine. Lab Anim. 2004;38:297–306. doi: 10.1258/002367704323133682. [DOI] [PubMed] [Google Scholar]
- 23.Lefrant JY, Muller L, de La Coussaye JE, Benbabaali M, Lebris C, Zeitoun N, et al. Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med. 2003;29:414–418. doi: 10.1007/s00134-002-1619-5. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz T, Bair N, Falk M, Levine C. A comparison of five methods of temperature measurement in febrile intensive care patients. Am J Crit Care. 1995;4:286–292. [PubMed] [Google Scholar]


