Abstract
BACKGROUND:
Paraquat (PQ) is an effective herbicide and is widely used in agricultural production, but PQ poisoning is frequently seen in humans with the lung as the target organ. Currently, there are many studies on lung injury after PQ poisoning. But the kidney as the main excretory organ after PQ poisoning is rarely studied and the mechanisms of this poisoning is not very clear. In this study, we observed the expression of caspase-3 and livin protein in rat renal tissue after PQ poisoning as well as the therapeutic effects of ulinastatin.
METHODS:
Fifty-four Sprague-Dawley (SD) rats were randomly divided into three experimental groups: control group (group A), paraquat poisoning group (group B) and ulinastatin group (group C), with 18 rats in each group. Rats in group B and group C were administered intragastrically with 80 mg/kg PQ, rats in group C were injected peritoneally with 100 000 U/kg ulinastatin once a day, while rats in group A were administered intragastrically with the same volume of saline as PQ. At 24, 48, 72 hours after poisoning, the expression of livin in renal tissue was detected by Westen blotting, the expression of caspase-3 was detected by immunohistochemistry, and the rate of renal cell apoptosis was tested by TUNEL detection. The histopathological changes were observed at the same time.
RESULTS:
Compared to group A, the expression of caspase-3 in the renal tissue of rats in groups B and C increased significantly at any time point. Compared with group B, the expression of caspase-3 in renal tissue of rats in group C decreased. Compared with group A, the expression of livin in renal tissue in rats of groups B and C increased significantly at any time point (P<0.01), especially in group C (P<0.01). TUNEL method showed that the rate of renal cell apoptosis index was higher in group B at corresponding time points than in group A (P<0.01), and was lower in group C at corresponding time points than in group B (P<0.01).
CONCLUSION:
UTI has a protective effect on the renal tissue of rats after paraquat poisoning through up-regulating the expression of livin and down-regulating the expression of caspase-3, but the regulation path still needs a further research.
KEY WORDS: Paraquat, Ulinastatin, Renal, Apoptosis, Livin, Caspase-3
INTRODUCTION
PQ poisoning can lead to dysfunction of multiple organs, especially the lung. The death rate of patients after PQ poisoning is high, but there are no effective therapeutic methods. Currently, there are many basic and clinical studies on lung injury after PQ poisoning, but the kidney as the main excretory organ after PQ poisoning is rarely studied and the mechanism of its injury is not clear. Some studies suggest that the imbalance of production and clearance of oxygen radicals and lipid peroxides may lead to cell injury, and this is important in kidney injury after PQ poisoning.[1–6] In this study, we observed the expression of apoptosis associated proteinslivin and caspase-3 in renal tissue of rats after PQ poisoning, and the effect of ulinastatin (UTI) on the rats.
METHODS
Main experimental reagents
20% PQ solution was produced by Shanghai Xianzhengda Company. Ulinastatin was purchased from Tianpu Biochemistry Medical Limited Company; horseradish peroxidase tagged goat anti-rabbit/rabbit anti mouse IgG and Western blot kits were purchased from American Upstate Biotechnology Company; mouse anti-rat β-actin antibody, livin and caspase-3 rabbit polyclonal antibody, ABC kit, S-P immunohistochemisty dyeing kits were provided by Beijing Boaosen Biotechnologies Limited Company.
Grouping of animals and model establishment
A total of 54 clean Sprague-Dawley rats, body weight 220±40 g, were provided by the Department of Animal Science, Medical College of Nanchang University. The rats were randomly divided into a control group (group A), a poisoning group (group B) and an ulinastatin group (group C). Models of acute PQ poisoning were established according to Tian et al.[7] The rats in groups B and C were intragastrically administered with PQ (80mg/kg), while the rats in group A were intragastrically administered with the same volume of stroke-physiological saline solution. The rats in group C were peritoneally injected with 100 000 U/kg ulinastatin per day at 30 minutes after poisoning, and the rats in groups A and B were peritonealy injected with the same volume of stroke-physiological saline solution. At 24, 48, 72 hours after poisoning, the rats were anesthetized peritonealy with 3% pentobarbital (35 mg/kg) and executed through bloodletting from the abdominal aorta.
Tissue specimens
Apparatuses were sterilized in advance, left kidney was scissored, freezed in liquid nitrogen and stored in a –80 ºC refrigerator to test the expression of livin; right kidney was scissored, washed by PBS damping fluid, put in 10% formol and stored in a 4 ºC refrigerator to test the expression of caspase-3 by immunohistochemical method under a light microscope.
Measurement of the expreession of renal tissue caspase-3, renal apoptotic index
Immunohistochemical streptomycin avidin biotin labeling was used to dye the tissue. The expression of caspase-3 was observed through buffy particles in cell membrane and hyalomitome under a light microscope. Each sample was prepared for five slices, five integrated and nonoverlapping high power fields were chosed for each slice under a light microscope, and HIS was used to analyze these slices. A was defined as positive cell number and B as positive cell chromogenic intension, HIS=A×B. Renal cell apoptosis was detected on paraffin sections by TUNEL according to the manufacturer’s instructions, and the renal apoptotic index was calculated.
Measurement of the expression of renal tissue livin protein
Western blot was manipulated with routine methods. Renal tissue was clearaged for 30 minutes, and lysate was shifted to a 1.5 mL centrifuge tube by a transferpettor, then centrifuged at 4 °C, 12 000 r/min for 4 minutes. The supernatant was collected and stored at –80 °C, and total protein was assayed by the Lowry method. The quantity of sample was 20 μg protein in each hole, and damping fluid was added in prepared sample, mixed equally, boiled for 5 minutes, centrifuged in 10 000 r/min for 10 minutes. Then sample was added equally on 7.5% polyacrylamide gel to undertake electrophoresis. After electrophoresis, protein was transferred to PVDE membrane and closed with freshly prepared PBS containing 3% ungrease milk powder. Livin rabbit polyclonal antibody (1:200) and anti-β-actin antibody (1:500) were added, one antibody was washed three times with freshly prepared PBS containing 3% ungrease milk powder, then was bred with horseradish peroxidase tagged two antibody (goat anti-rabbit IgG), finally results were recorded with X-polished section by the chemoluminescence method. Protein hybridization straps were scanned and analyzed.
Statistical analysis
Experimental data were analyzed with SPSS 12.0 software. Quantitative data were demonstrated in the form of mean±SD, and group comparison was made with one-way analysis of variance and two samples t test, Pearson’s product-moment correlation coefficient was used to analyze correlation. The difference was considered statistically significant when P<0.05.
RESULTS
Histopathological observation
The kidneys of rats in group A were marron, and the boundary between cortex and medulla was clear in the incision surface. The structure of renal tissue was clear, and no obvious hyperemia, edema and vacuolar degeneration were observed under a light microscope (Figure 1).
Figure 1.
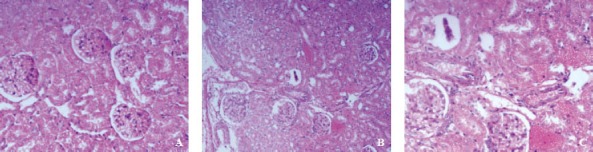
Pathological section of renal tissue (HE×100).
The kidneys of rats in group B were slightly edematous and peplos was tense 12 hours after PQ poisoning. As time extended, hyperemia and edema worsened, and hemorrhagic spots, even sheets of hemostasis appeared on the surface. The boundary between the cortex and medulla was vague on incision surface. Glomerular capillaries were ecchymotic, renal tubular epithelial cells were swelling under a light microscope 12 hours after PQ poisoning. Then, hemostasis and swelling worsened gradually, scattered or foliated necrosis appeared, cell boundary was vague, lumens were narrowed or occluded, and protein cast and inflammatory cell infiltration were observed. Homogen cast and red cell cast were observed in renal tubules (Figure 1).
Hyperemia and edema were more obvious in the kidneys of rats in group C than in group A. Peplos was tense, proximal convoluted tubule epithelial cells were swelling, and lumens were narrowed. As time extended, these changes were worsened, and vacuolar degeneration and lumens occlusion appeared; but compared to group B pathological changes lessened significantly (Figure 1).
Expression of renal tissue caspase-3, renal apoptotic index and effect of ulinastatin
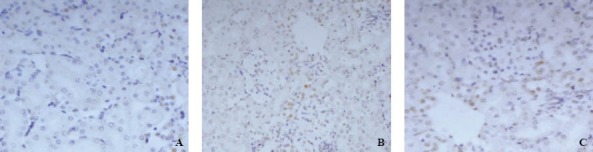
The expression of renal caspase-3 was weak in glomerular epithelial cells in rats of group A. The expression of renal caspase-3 was positive in glomerular epithelial cells, renal tubular epithelial cell membrane and hyalomitome 24 hours after PQ poisoning. There was statistical significance at all time points between group B and group A (P<0.01). The expression of renal caspase-3 decreased in group C compared with group A (Table 1, Figure 2).
Table 1.
Changes of caspase-3 in renal tissue detected by immuneohistochemistry (mean±SD)
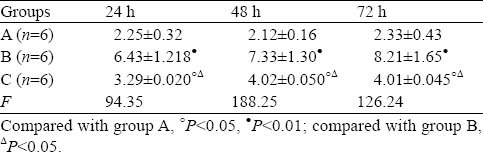
Figure 2.
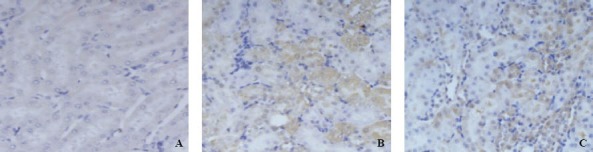
Immunohistochemistry of caspase-3 in renal tissue (SP×400).
No apoptotic renal cells were observed in either group before poisoning. At 24 hours after poisoning, a small number of apoptotic renal cells were observed in group B and increased significantly at 48 and 72 hours. Renal apoptotic index was significantly lower in group C than in group B (Table 2, Figure 3).
Table 2.
Changes of renal apoptotic index detected by TUNEL (mean±SD)
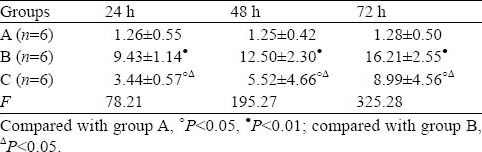
Figure 3.
Changes in apoptosis of renal tissue (TUNEL, 400×).
Expression of renal tissue livin and effect of ulinastatin
The expression of renal livin was low in group A. There was no statistical significance in the three subgroups (P>0.05). The expression of renal livin increased gradually in group B compared with group A, and increased further in group C compared with group B (Table 3, Figure 4).
Table 3.
Changes of livin in renal tissue detected by Western blotting (mean±SD)
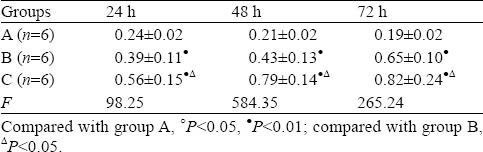
Figure 4.
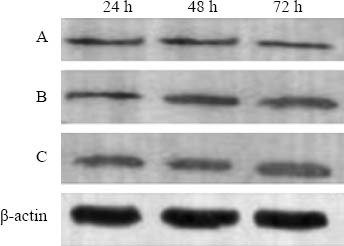
Expression of renal tissue livin protein in groups A, B, C detected by Western blotting.
Pearson’s prouduct-moment correlation coefficient analysis
Spearman’s rank-order correlation coefficient test was used to analyze the expression of livin and number of caspase-3 positive cells in all groups. Results demonstrated that the expression of livin and caspase-3 in group B was positively correlated, coefficient of rank correlation was r=0.442, P=0.014; the expression of livin and caspase-3 in group C was negatively correlated, and coefficient of rank correlation was r=–0.638, P=0.000.
DISCUSSION
The lung is the major target organ of PQ poisoning. Acute lung jnjury, multiple organ dysfunctions, and pulmonary interstitial fibrosis are the main causes of death for PQ poisoning.[8–9] As the kidney is the main excretory organ of PQ, studies suggest that PQ clearence is diphasic (fast phase and slow phase), and PQ is excreted by the kidney through original form and initiative secretion.[10–11] The degree of kidney injury is confirmed as the important factor of prognosis.[12–15] PQ content is higher in the kidney than in the lung in the early phase of PQ poisoning, and when disappearing in the lung after 21 days, PQ can still be detected in the kidney.[16] We found that renal pathological changes included swelling, denaturation and mortification of proximal convoluted tubule epithelium, mesenchymal hyperemia and edema. These changes worsened as time extended, and were similar as reported elsewhere.[17–19]
UTI can protect damaged body through inhibition of the activity of proteolytic enzyme, release of inflammatory mediators, production of oxygen radicals, and apoptosis.[20–22] It was reported that UTI can alleviate PQ-induced injury through inhibiting the release of NFκB, TNF-α, IL-1β, IL-2, SOD, MDA, and MMP-9 and enhancing synthesis of heat shock protein.[23–25] Meanwhile, UTI can up-regulate the expression of FLICE-likeinhibitory-protein in the pulmonary tissue of septic rat to alleviate injury.[26]
Apoptosis is the main mechanism of organ injury after PQ poisoning. Caspase is the main executant of apoptosis. And caspase-3 is the key enzyme of apoptosis, and caspase-3 mediated signal transduction is needed in most apoptosis. So caspase-3 activation is seen as the marker of apoptosis.[27] Dinis-Oliveira et al[28] found that apoptotic cells in the lung increased gradually after PQ poisoning. Conzalez et al[29] cultivated cerebellar granulocyte in light concentration PQ and found increased activity of aminopeptodrate enzymes and caspase-3 in mitochondria and enhanced apoptosis. Livin is a new member of inhibitors of the apoptosis protein family, a new kind of anti-apoptosis protein independent of bcl-2, and can inhibit apoptosis through suppressing caspase prolease activation cascade.[30] This study demonstrated that compared to the control group, the expression of renal caspase-3 increased with time after PQ poisoning. Hence we supposed PQ poisoning started apoptosis cascade, and apoptosis participated in the process of kidney injury combining with pathological changes. After UTI intervention, the expression of renal caspase-3 decreased and livin increased. Correlation analysis demonstrated negative correlation, suggesting UTI can alleviate PQ-induced kidney injury through enhancing the expression of livin and inhibiting the expression of caspase-3. These results indicate a new view point for investigating the mechanism of UTI. But what position the effect of UTI on livin possesses, which way UTI affects the activation of caspase-3, and how long can upregulation of livin by UTI sustain? These questions need further studies.
Footnotes
Funding: None.
Ethical approval: Not needed.
Conflicts of interest: No benefits in any form have been received or will be received from a commercial party related dircctly or indirectly to the subject of this article.
Contributors: Zhang ZJ wrote the main body of the article. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Adachi J, Tomita M, Yamakawa S, Asano M, Naito T, Ueno Y. 7-Hydroperoxycholesterol as a marker of oxidative stress in rat kidney induced by paraquat. Free Radic Res. 2000;33:321–327. doi: 10.1080/10715760000301491. [DOI] [PubMed] [Google Scholar]
- 2.Kurisaki E, Hiraiwa K. Western blot analysis for 4-hydroxy-2nonenal(HNE)-modified proteins in paraquat-treated mice. Leg Med (Tokyo) 2009;11:s431–433. doi: 10.1016/j.legalmed.2009.01.082. [DOI] [PubMed] [Google Scholar]
- 3.Welford SM, Dorie MJ, Li X, Haase VH, Giaccia AJ. Renal oxygenation suppresses VHL loss-induced senescence that is caused by increased sensitivity to oxidative stress. Mol Cell Biol. 2010;30:4595–4603. doi: 10.1128/MCB.01618-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samai M, Haque T, Naughton DP, Gard PR, Chatterjee PK. Reduction of paraquat-induced renal cytotoxicity by manganese and copper complexes of EGTA and EHPG. Free Radic Biol Med. 2008;44:711–721. doi: 10.1016/j.freeradbiomed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Mølck AM, Friis C. The cytotoxic effect of paraquat to isolated renal proximal tubular segments from rabbits. Toxicology. 1997;122:123–132. doi: 10.1016/s0300-483x(97)00088-7. [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Gil HW, Yang JO, Lee EY, Hong SY. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol Dial Transplant. 2009;24:1226–1232. doi: 10.1093/ndt/gfn615. [DOI] [PubMed] [Google Scholar]
- 7.Tian YP, Liu FR, Tong F, Shi HW, Yao DQ. Pathologic changes and expression of Heme oxygenase-1 in paraquat-induced renal injury. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2009;27:468–471. [PubMed] [Google Scholar]
- 8.Satomi Y, Sakaguchi K, Kasahara Y, Akahori F. Novel and extensive aspects of paraquat-induced pulmonary fibrogenesis: comparative and time-course microarray analyses in fibrogenic and non-fibrogenic rats. J Toxicol Sci. 2007;32:529–553. doi: 10.2131/jts.32.529. [DOI] [PubMed] [Google Scholar]
- 9.Bertsias GK, Katonis P, Tzanakakis G, Tsatsakis AM. Review of clinical and toxicological features of acute pesticide poisonings in Grate (Greece) during the period 1991-2001. Med Sci Monit. 2004;10:622–627. [PubMed] [Google Scholar]
- 10.Machaalani R, Lazzaro V, Duggin GG. The characterization and uptake of paraquat in cultured baboon kidney proximal tubule cells (bPTC) Hum Exp Toxicol. 2001;20:90–99. doi: 10.1191/096032701672136818. [DOI] [PubMed] [Google Scholar]
- 11.Houzé P, Baud FJ, Mouy R, Bismuth C, Bourdon R, Scherrmann JM. Toxicokinetics of paraquat in human. Hum Exp Toxicol. 1990;9:5–12. doi: 10.1177/096032719000900103. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Shun HC, Shao DB, Tang WJ, Liu HM, Li W, et al. Analysis of clinical classification and outcome of patients with acute paraquat poisoning. Chin J Memerg Med. 2010;19:128–131. [Google Scholar]
- 13.Roberts DM, Wilks MF, Roberts MS, Swaminathan R, Mohamed F, Dawson AH, et al. Changes in the concentrations of creatinine, cystatin C and NGAL in patients with acute paraquat self-poisoning. Toxicol Lett. 2011;202:69–74. doi: 10.1016/j.toxlet.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao YF, Shi J. Analysis of factors related to sudden death of patients with acute paraquat poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2010;28:525–526. [PubMed] [Google Scholar]
- 15.Hong SY, Yang DH, Hwang KY. Associations between laboratory parameters and outcome of paraquat poisoning. Toxicol Lett. 2000;118:53–59. doi: 10.1016/s0378-4274(00)00264-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhao B, Jia XD, Zhang ZC. Relationship between paraquat tissue content and organ injury in paraquat poisoning rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2010;28:220–223. [PubMed] [Google Scholar]
- 17.Gil HW, Yang JO, Lee EY, Hong SY. Paraquat-induced Fanconi syndrome. Nephrology (Carlton) 2005;10:430–432. doi: 10.1111/j.1440-1797.2005.00437.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang BL, Tu YY, Zhong YX, Cao YZ, Fu GQ, Tian XX, et al. Study of multiple organ failure induced by paraquat in rats. Chin J Emerg Med. 2010;19:1296–1299. [Google Scholar]
- 19.Ramírez-Zambrano E, Zambrano E, Rojas G, Zambrano M, Teneud L. Protective effect of melatonin and sodium thiosulphate on histopathology and ultrastructure of the kidney in rats with acute paraquat poisoning. Invest Clin. 2007;48:81–89. [PubMed] [Google Scholar]
- 20.Li FR, Qiu L, Yang XF, Qi H, Ren LL, Zhou HX. The protective effects of ulinastatin and adenosine triphosphate on islet function during islet isolation and after islet transplantation. Pancreas. 2009;38:227–230. doi: 10.1097/MPA.0b013e3181788e3d. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Xie K, Zhang J, Dang Y, Qiong Z. Prospective clinical and experimental studies on the cardioprotective effect of ulinastatin following severe burns. Burns. 2008;34:674–680. doi: 10.1016/j.burns.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Su BH, Chiu HY, Soga T, Lin KJ, Hsu CT. Ulinastatin alone does not reduce caspase 3-mediated apoptosis in protease-positive Aeromonas hydrophilia-induced sepsis. J Formos Med Assoc. 2007;106:97–104. doi: 10.1016/S0929-6646(09)60224-2. [DOI] [PubMed] [Google Scholar]
- 23.Chen BA, Liang DM, Yuan Y, Wang G, Hu Q, Zhao QY, et al. Effects of ulinastatin to pro-inflammatory mediators in rats with acute paraquat intoxication. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009;21:371–372. [PubMed] [Google Scholar]
- 24.Zhang ZJ, Zhou CY, Luo YJ, Xiong HW. Expression of heat shock protein 70 in lung tissues of acute paraquat poisoned rats and intervenetion of ulinastatin. World J Emerg Med. 2010;1:229–233. [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu QM, He F, Hong GL, Lu ZQ, He XY, Liang H. Expression of angiotensin converting enzyme and angiotensin converting enzyme 2 gene in lung of paraquat poisoning rats and protection of sodium dimercaptopropane sulfonate. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2010;28:275–279. [PubMed] [Google Scholar]
- 26.Shen L, Shun ZD, Chen Q, Cao TW, Zhu HC. The changes of c-FLIP in the lung of rats with sepsis and the influence of Ulinastatin. Chin J Emerg Med. 2010;19:128–131. [Google Scholar]
- 27.Xu J, Wei C, Xu C, Bennett MC, Zhang G, Li F, et al. Rifampicin protects PC12 cells against MPP+-induced apoptosis and inhibits the expression of an alpha-Synuclein multimer. Brain Res. 2007;30:220–225. doi: 10.1016/j.brainres.2006.12.074. [DOI] [PubMed] [Google Scholar]
- 28.Dinis-Oliveira RJ, Sousa C, Remião F, Duarte JA, Ferreira R, Sánchez Navarro A, et al. Effects of sodium salicylate in the paraquat-induced apoptotic events in rat lungs. Free Radical Biol Med. 2007;43:48–61. doi: 10.1016/j.freeradbiomed.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 29.González-Polo RA, Rodríguez-Martín A, Morán JM, Niso M, Soler G, Fuentes JM. Paraquat-induced apoptotic cell death in cerebellar granule cells. Brain Res. 2004;1011:170–176. doi: 10.1016/j.brainres.2004.02.078. [DOI] [PubMed] [Google Scholar]
- 30.Chang H, Schimmer AD. Livin/melanoma inhibitor of apoptosis protein as a potential therapeutic target for the treatment of malignancy. Mol Cancer Ther. 2007;6:24–30. doi: 10.1158/1535-7163.MCT-06-0443. [DOI] [PubMed] [Google Scholar]


